Abstract
Classic “BCR-ABL1-negative” MPN is an operational sub-category of MPN that includes polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) harboring JAK2V617F as the most common mutation. JAK2V617F can be detected in about 95 % of patients with PV while remaining 5 % of PV patients carry a somatic mutation of JAK2 exon 12. Approximately one-third of patients with ET or PMF do not carry any mutation in JAK2 or MPL. In December 2013, mutations were described in calreticulin (CALR) gene in 67–71 and 56–88 % of JAK2V617F and MPL negative patients with ET and PMF, respectively. Since this discovery CALR mutations have been reported to be mutually exclusive with JAK2V617F or MPL mutations. However recently few studies (eleven published reports) reported the coexistence of JAK2V617F and CALR in MPN. In the present study we are reporting JAK2V617F positive ET patient from our center with coexisting CALR exon 9 mutation type c.1214_1225del12 (p.E405_D408del) that was never reported before as a coexisting mutation and describing in detail the clinical outcomes.
Keywords: Calreticulin, Coexistence, Essential thrombocythemia, JAK2V617F, Myeloproliferative neoplasms
Introduction
Myeloproliferative Neoplasms (MPN) are a heterogeneous group of clonal disorders derived from multipotent hematopoietic myeloid progenitors. Among MPN, polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are operational sub-category communally called classic “BCR-ABL1-negative” MPN. These three disorders are characterized by stem cell-derived clonal myeloproliferation whereas at molecular level JAK2V617F is the most common mutation [1, 2]. JAK2V617F mutation is present in 95 % of patients with PV while remaining 5 % of PV patients carry a somatic mutation of JAK2 exon 12. Amongst JAK2V617F negative ET and MF patients, 5–10 % carries mutation in exon 10 of MPL gene whereas approximately one-third do not carry any mutation in JAK2 or MPL [3]. In December 2013, calreticulin (CALR) gene mutations were described in 67–71 and 56–88 % of JAK2V617F and MPL negative patients with ET and PMF, respectively [4, 5].
CALR is located on chromosome 19p13.2 and consists of 9 exons spanning 4.2 kb. More than 50 types of CALR mutations have been reported so far in MPNs and all are somatic insertions or deletions in exon 9. Two mutation variants type 1 (L367fs*46) resulted from 52 bp deletion and type 2 (K385fs*47) resulted from 5-bp TTGTC insertion are the most frequent. Several other distinct variants have been seen infrequently [1]. All CALR mutations currently described in MPNs results in one base pair reading frame shift that leads to protein product with an altered C terminal. Calreticulin is a functionally complex Ca2+ binding protein localized primarily in the endoplasmic reticulum (ER) but is also found in the nucleus, cell membranes and extracellular matrix. Calreticulin protein consists of three domains: amino terminal N-domain (residues 1–180), central proline-rich P domain (residues 181–290) and carboxyl terminal C-domain (residues 291–400). Functionally, calreticulin is believed to participate in Ca2+ homeostasis and handling proper glycoprotein folding inside ER whereas influences a variety of process including immune response to cancer, apoptosis and proliferation, cell phagocytosis, wound healing and fibrosis outside ER. Frame shift due to mutations causes loss of most of the acidic domain and KDEL signal (ER retention motif) at C terminus and C terminus becomes positively charged in comparison to wild type protein which is largely negatively charged [1–3, 6–8].
CALR mutations have been recommended to be included in the diagnostic algorithm for MPNs because of its clinical utility. It is reported that CALR mutated patients have a better outcome than JAK2V617F positive patients [7, 9]. Majorly CALR mutations have been reported to be mutually exclusive with JAK2V617F or MPL mutations [4, 5, 10, 11] and it is suggestive in diagnostic workup of MPN that CALR mutations should not be studied in those MPN patients who are already known to carry JAK2 or MPL mutations [7]. However there are few recent studies reporting coexistence of JAK2V617F with CALR or MPL. According to our knowledge so far there have been only eleven publications reporting twenty-five ET, three PMF, one PV and one MPN-U [2, 3, 12–21] yet very few discussing the phenotype and clinical outcomes with respect to this coexistence [15, 16, 19, 20].
Case Report
In July 2011, a 55-year-old female patient with average built and height was referred to our hospital with a history of gradual elevation of platelet counts accompanied with pain in right hypochondriac region and feet for thrombocytosis workup. She was taking Warfarin at the time of referral. She had complaints of pain in left upper abdomen and constipation. She is a known case of hypertension for last 4–5 years. On examination she was unremarkable. Her peripheral blood count showed a platelets count of 1086 × 109/L, RBC count 5.12 × 1012/L, total leukocyte count 17.7 × 109/L (absolute neutrophils 13.8 %) and hemoglobin 12.3 g/dL with MCV and MCH 78 fl and 25 pg, respectively. Her coagulation profile had prothrombin time 17.1 s (control 12 s) and INR 1.4. Her PT time was slightly prolonged as she was on Warfarin. Ultrasound abdomen showed 3.0 cm wedge shape area in spleen, small shrunken left kidney otherwise normal. After persistent thrombocytosis, bone marrow aspirate and trephine biopsy was planned.
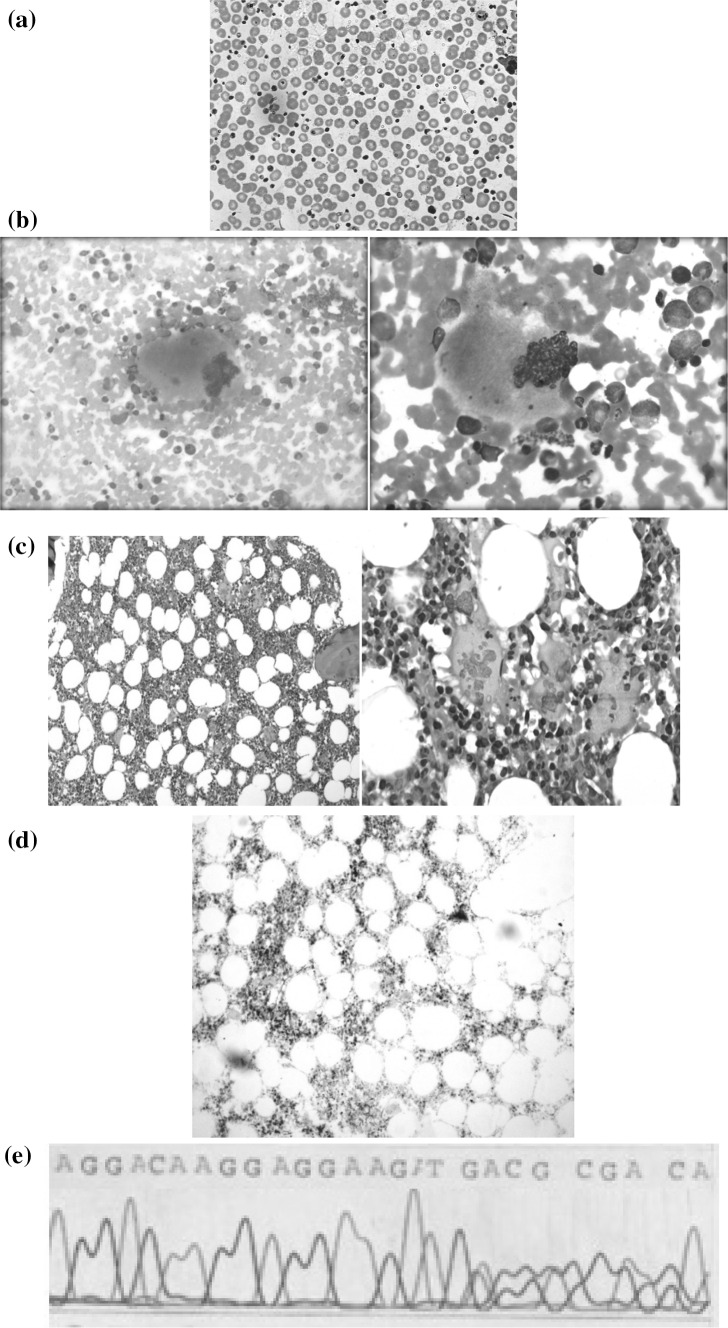
Peripheral smear morphology was normochromic normocytic having hypersegmented neutrophils with platelets anisocytosis (Fig. 1a). Bone marrow aspirate consisted of normocellular fragments and trails. Hyperplastic erythropoiesis showed megaloblastoid/dyserythropoietic features. Myelopoeisis showed all stages of maturation and differentiation with prominent giant metamyelocytes and band forms. Megakaryocytes were increased having “Staghorn appearance” exhibiting hyperlobulation and multilobulation. Multiple platelets aggregates and islands were seen throughout the aspirate smear (Fig. 1b). Adequate length trephine core biopsy sections, attained with hematoxylin and eosin (H&E), showed cellularity around 60–65 % with preserved bony architecture and intact fat spaces, having normoplastic erythropoiesis and active maturation and differentiation in myelopoiesis. Megakarocytes were increased and seen in clusters at few places exhibiting hyperlobulation. Bone marrow biopsy stained with H&E showed an increase in megakaryocytes in loose aggregates. Many megakaryocytes were usually large with abundant mature cytoplasm and deeply lobulated Staghorn Nuclei (Fig. 1c). A reticulin stain showed the absence of relavent reticulin fibrosis (Fig. 1d). At molecular level JAK2V617F mutation was analyzed by DNA tetra-primer amplification refractory mutation system (ARMS-PCR). CALR mutations were identified by bi-directional Sanger sequencing [4] later in a cohort of research. ARMS-PCR for JAK2V617F mutation was positive whereas sequencing exhibited c.1214_1225;p.E405_D408del mutation pattern (Fig. 1e).
Fig. 1.
a Peripheral blood smear stained with leishman shows thrombocytosis. b Aspirate stained with leishman shows enlarged and mature megakaryocytes. c Bone marrow biopsy shows staghorn nuclei. d Reticulin stain shows the absence of relavent reticulin fibrosis. e Sanger sequencing of CALR exon 9 showing mutation pattern
On the basis of laboratory, bone marrow and molecular findings she was diagnosed with ET. She was treated with Capsule Hydroxyurea from 2011 to 2012 whereas treatment with Warfarin continued. The patient was in complete remission without cytoreductive therapy in her last visit in 2014.
Discussion
Co-occurrence of JAK2V617F and CALR mutations has been reported in few MPN cases across different ethnic groups with frequency below 1 % except one study [20] reporting it 6.8 %. However unfortunately there are not many reports on the phenotype and clinical course of patients positive for these double mutations. Xu et al. [16] provided the first data in detail regarding the phenotype and clinical outcomes in CALR and JAK2V617F positive patients and they have more patients with both mutations under observation. Both patients reported a positive response to interferon alfa therapy. Patient reported in the study of McGaffin et al. [15] was a 79 year old female with a history of sustained thrombocytosis and anisothrombia in the absence of any reactive causes at the time of referral. A diagnosis of ET was made on the basis of the clinical details, morphology and JAK2V617F mutation status. CALR mutation detected was c.1094_1139del;p.Q365fs*50. The patient is well without any history of bleeding, thrombosis, cerebrovascular disease or any other occlusive symptoms. Xu et al. [16] reported one ET patient, a 63-year-old female who was admitted to hospital in July, 2003 with a history of gradual elevation of platelet counts accompanied by intermittent dizziness for 3 years. The patient is well and without history of bleeding, thrombosis, cerebrovascular disease, or any other occlusive symptoms including hepatosplenomegaly and neurological issues. Heterozygous JAK2V617F and c.1092_1143del;p.L367fs*46 in CALR exon 9 were detected without cytogenetic abnormalities and BCR-ABL fusion gene. The patient was in complete hematologic remission at the time of publication. The other patient was a 65-year-old male with PV who presented in August, 2012 with a year-long history of increased weakness and fatigue with splenomegaly and no adenopathy. No cytogenetic abnormalities or BCR-ABL and FIPL-PDGFRα fusion genes were detected. Homozygous JAK2V617F and an uncommon variant c.1095-1097del;p.E371fs*49 in CALR exon 9 were detected. This patient was still in complete remission without cytoreductive therapy as of July 2014. In the study of Zmora et al. [19] the double positive patient was 86 year old male with CALR mutation c.1142_1144del; E380del. A diagnosis of PMF was made on the basis of the clinical characteristics, morphology and JAK2V617F mutation status. The patient died of acute myocardial infarction in October 2011. Lim et al. [20] reported the high frequency (6.8 %) until now of patients with both mutations. They studied 92 ET patients and found 13 patients with both mutations. CALR and JAK2-mutated ET patients in their study were found to be associated with oldest age, higher thrombotic events and higher major arterial thrombotic events after diagnosis and more patients were in the high-risk group for thrombohemorrhagic complications.
It has been observed that phenotypic manifestations of CALR and JAK2V617F differ in ET and PMF that define subtypes with different prognosis [10, 12, 14, 18, 22] however comparatively the pathogenetic contribution of CALR/JAK2V617F double mutation is still unrevealed and it is not known if this would be another subgroup of MPN in future. The occurrence of JAK2V617F/CALR double mutants may be under-reported because many groups follow the initial findings of mutual exclusiveness for both genes [15]. Double-mutant patients might have a different phenotype and clinical course from the JAK2-positive or CALR-positive subgroups and identification of the true frequency of these patients may be an important factor for defining the prognosis, risk factors and outcomes for MPN patients [15, 19, 20].
Conclusion
It has been proposed to include CALR mutation screening in WHO diagnostic criteria of MPN due to its important diagnostic and prognostic significance [9]. Until today 24 CALR mutation types have been reported to coexist with JAK2V617F in ET, PMF, PV and MPN-U and among them the frequency of type I and type II is 20.8 and 8.3 %, respectively. Coexistence of CALR exon 9 mutation c.1214_1225;p.E405_D408del with JAK2V617F in our patient is an addition in already reported few studies on this topic. It is important to identify the double mutant MPN patients for these two mutations and study the influence of this coexistence on phenotype and clinical course to better understand the diagnosis and prognosis in these MPN patients.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from the participant included in this study.
Footnotes
Munazza Rashid and Rifat Zubair Ahmed contributed equally to this study and should be considered as co-first authors.
References
- 1.Luo W, Yu Z. Calreticulin (CALR) mutation in myeloproliferative neoplasms (MPNs) Stem Cell Investig. 2015 doi: 10.3978/j.issn.2306-9759.2015.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123:2220–2228. doi: 10.1182/blood-2013-11-537167. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Liu E, Sun Q, et al. The prevalence of JAK2, MPL, and CALR mutations in Chinese patients with BCR-ABL1-negative myeloproliferative neoplasms. Am J Clin Path. 2015;144:165–171. doi: 10.1309/AJCPALP51XDIXDDV. [DOI] [PubMed] [Google Scholar]
- 4.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of Calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 5.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi J, Nicolaou KA, Nicolaidou V, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014;28:1152–1154. doi: 10.1038/leu.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tafferi A, Pardanani A. CALR mutations and a new diagnostic algorithm for MPN. Nat Rev Clin Oncol. 2014;11:125–126. doi: 10.1038/nrclinonc.2014.16. [DOI] [PubMed] [Google Scholar]
- 8.Guglielmelli P, Nangalia J, Green AR, Vannucchi M. CALR mutations in myeloproliferative neoplasms: hidden behind the reticulum. Am J Hematol. 2014;89:453–456. doi: 10.1002/ajh.23678. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Thiele J, Vannucchi AM, et al. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia. 2014;28:1407–1413. doi: 10.1038/leu.2014.35. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, Kim SY, Lee SM, et al. Incidence, clinical features, and prognostic impact of CALR exon 9 mutations in essential thrombocythemia and primary myelofibrosis: an experience of single tertiary hospital in Korea. Ann Lab Med. 2014;35:233–237. doi: 10.3343/alm.2015.35.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado-Neto JA, de Melo Campos P, de Albuquerque DM, et al. Somatic mutations of calreticulin in a Brazilian cohort of patients with myeloproliferative neoplasms. Rev Bras Hemat Hemot. 2015;37:211–214. doi: 10.1016/j.bjhh.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–1477. doi: 10.1038/leu.2014.3. [DOI] [PubMed] [Google Scholar]
- 13.Broséus J, Lippert E, Harutyunyan AS, et al. Low rate of calreticulin mutations in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2014;28:1374–1376. doi: 10.1038/leu.2014.49. [DOI] [PubMed] [Google Scholar]
- 14.Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia. 2014;28:1912–1914. doi: 10.1038/leu.2014.138. [DOI] [PubMed] [Google Scholar]
- 15.McGaffin G, Harper K, Stirling D, McLintock L. JAK2V617F and CALR mutations are not mutually exclusive; findings from retrospective analysis of a small patient cohort. Br J Haematol. 2014;167:276–278. doi: 10.1111/bjh.12969. [DOI] [PubMed] [Google Scholar]
- 16.Xu N, Ding L, Yin C, et al. A report on the co-occurrence of JAK2V617F and CALR mutations in myeloproliferative neoplasm patients. Ann Hematol. 2015;94:865–867. doi: 10.1007/s00277-014-2248-0. [DOI] [PubMed] [Google Scholar]
- 17.Shirane S, Araki M, Morishita S, et al. JAK2, CALR, and MPL mutation spectrum in Japanese patients with myeloproliferative neoplasms. Haematologica. 2015;100:e46–e48. doi: 10.3324/haematol.2014.115113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha JS, Kim YK. Calreticulin exon 9 mutations in myeloproliferative neoplasms. Ann Lab Med. 2015;35:22–27. doi: 10.3343/alm.2015.35.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamora L, Xicoy B, Cabezón M, et al. Co-existence of JAK2 V617F and CALR mutations in primary myelofibrosis. Leuk Lym. 2015;11:1–2. doi: 10.1080/15502724.2014.1001685. [DOI] [PubMed] [Google Scholar]
- 20.Lim KH, Chang YC, Gon-Shen Chen C, et al. Frequent CALR exon 9 alterations in JAK2V617F-mutated essential thrombocythemia detected by high-resolution melting analysis. Blood Cancer J. 2015;5:e295. doi: 10.1038/bcj.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Assaf C, Van Obbergh F, Billiet J, et al. Analysis of phenotype and outcome in essential thrombocythemia with CALR or JAK2 mutations. Haematologica. 2015;100:893–897. doi: 10.3324/haematol.2014.118299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tefferi A, Lasho TL, Tischer A, et al. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood. 2014;124:2465–2466. doi: 10.1182/blood-2014-07-588426. [DOI] [PMC free article] [PubMed] [Google Scholar]