Abstract
Clinicians in hematology practice commonly encounter anemia, hypercalcemia and renal failure, which when present in combination evoke a diagnostic workup for multiple myeloma. We report a 71-years old lady who presented to our hematology clinic with fever and easy fatiguability of 3 months duration and on investigations was found to have anemia and hypercalcemia. Direct Coomb’s test characterized the anemia as complement mediated (anti-C3d) hemolysis. Biochemical investigations revealed normal 25(OH) Vitamin D3 and suppressed Parathormone levels and a negative workup for plasma cell dyscrasias, sarcoidosis and autoimmune disorders. CT scan revealed a paravertebral mass with cervical, supraclavicular and abdominal lymphadenopathy along with splenomegaly and left pleural effusion. Biopsy from the paravertebral mass confirmed the diagnosis of Hodgkin’s disease (nodular sclerosis) using immunohistochemistry. Bone marrow examination suggested infiltration by lymphoma. Hypercalcemia was managed with saline and zoledronic acid. Administration of prednisolone (1 mg/kg/day) along with chemotherapy (ABVD regimen) led to normalization of calcium and hemoglobin levels. However, hemolysis recurred 2 weeks later and hence, Rituximab (375 mg/m2) was administered on a weekly schedule for 4 doses and ABVD (2 weekly) was continued, which brought hemolysis under control. Co-occurrence of two paraneoplastic manifestations (complement mediated hemolytic anemia and hypercalcemia) in Hodgkin’s lymphoma is very unusual. Present report aims not only to highlight a rare presentation of Hodgkin’s lymphoma but also focus on the role of Rituximab in controlling hemolysis associated with this disease.
Keywords: Autoimmune hemolytic anemia, Cold antibody, Humoral hypercalcemia, Rituximab, Hodgkin’s disease
Introduction
Immune mediated hemolysis and hypercalcemia are rare in Hodgkin’s lymphoma (HL), with a reported incidence of 2.7 and 0.6–5.4 % respectively [1, 2]. Interestingly, both of these complications are associated with an advanced and active disease and tend to resolve after treatment of the underlying disease. Presence of either of them in HL has not been found to confer an additional poorer outcome [3, 4]. We report an elderly lady who presented to us with anemia and hypercalcemia that was later on diagnosed as a case of HL with cold antibody mediated hemolytic anemia. Administration of Rituximab along with chemotherapy led to the resolution of hemolysis.
Case Report
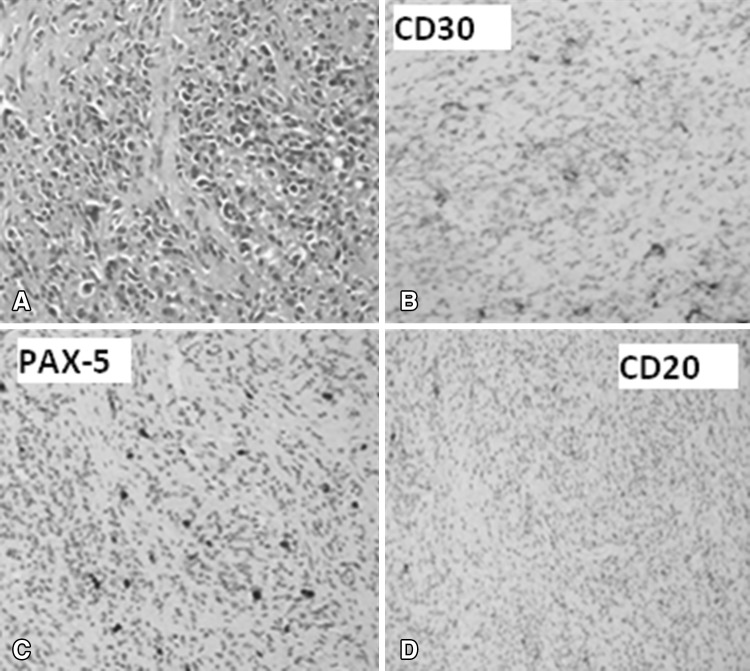
We herein report a 71-years old lady of Indian origin who presented to our hematology clinic with complaints of fever and easy fatiguability of 3 months duration. Fever was documented to be 101 °F, without any diurnal variation and was not associated with any localizing symptoms. On examination, she was conscious and oriented to time, place and person. She was febrile to touch, had blood pressure: 134/80 mmHg and pulse: 112/min. General examination was remarkable for the presence of pallor and there was no icterus or palpable lymphadenopathy. Liver (4 cm below) and spleen (2 cm) were palpable on per abdomen examination. Her investigations revealed Hb: 54 g/l, white cell count: 7 × 109/l, platelet counts: 300 × 109/l, mean corpuscular volume: 105.2 fl, red cell differential width: 23.4 %, reticulocyte count: 10.2 %, sedimentation rate: 60 mm/h and agglutination in peripheral smear, a picture consistent with hemolytic anemia. Hemolytic work up revealed plasma Hb: 30 mg% (0–15 mg%) and a negative urine hemoglobin. Patient’s serum tested positive for monospecific antibodies against C3d (IgG–C3d, DCT) suggestive of cold antibody mediated hemolytic anemia. Cryoglobulins were not detectable. Biochemical investigations revealed blood urea: 52.6 mg/dl, Creatinine: 1.65 mg/dl, total calcium: 12.2 mg/dl, phosphorous: 3.7 mg/dl, alkaline phosphatase: 139 U/l (42–128 U/l), albumin: 3.23 g/dl, proteins: 7.27 g/dl, total bilirubin-2.1 mg/dl (direct fraction-0.4 mg/dl), and lactate dehydrogenase: 789 U/l (240–480 U/l). Workup for multiple myeloma (in view of anemia, hypercalcemia and renal failure) including Serum and urine protein electrophoresis, serum free light chain assay and abdominal fat pad biopsy were negative. Serum immunoglobulin profile was normal [IgG: 1755 mg/dl (800–1800 mg/dl), IgA: 342 mg/dl (110–410 mg/dl) and IgM: 130 mg/dl (35–220 mg/dl)]. Skeletal survey didn’t reveal any Lytic areas and bone scan was unrevealing. Intact Parathormone levels (iPTH) were suppressed: 13.44 pg/ml (15–65 pg/ml) and 25(OH) Vitamin D3 was 26.54 ng/ml (1.1–42.9 ng/ml). Serum Angiotensin converting enzyme (ACE) level was 90 U/l (66–114 U/l) and thyroid function test was normal. Autoimmune workup including antinuclear antibody and double stranded antibody was negative. Procalcitonin level was normal and viral serologies (Australia antigen, anti-HCV, EBV, CMV and HIV) were negative. Contrast enhanced CT revealed a lobulated sheet like soft tissue (7.6 × 3.7 × 9 cm) in the prevertebral and right paravertebral location with extension superiorly up to lower border of L4 to the lower border of S3 vertebra inferiorly along with splenomegaly, cervical, mediastinal and abdominal lymphadenopathy and left sided pleural effusion. PET–CT revealed FDG avid left supraclavicular, mediastinal and abdominal lymphadenopathy, prevertebral and paravertebral soft tissue and FDG avid left sided pleural effusion. CT guided biopsy from the paravertebral mass revealed large atypical cells positive for CD15, CD30 and PAX-5 and negative for CD20 and CD3 with background cells being positive for CD3. Hence, a diagnosis of Hodgkin’s lymphoma, nodular sclerosis was made (Fig. 1). Bone marrow biopsy also showed presence of large atypical cells, some of these were binucleated. These cells showed expression of CD30 and hence the marrow was infiltrated by CD30+ lymphoma (Fig. 2). Pleural fluid analysis revealed a lymphocyte rich effusion (total cells-800, 80 % lymphocytes and 20 % neutrophils, sugar-70 mg/dl, proteins-4.5 g/dl and Polymerase chain reaction for Mycobacterium tuberculosis was negative). Flow cytometry of pleural fluid revealed CD30+ malignant cells. Patient was diagnosed as a case of Hodgkin’s lymphoma, nodular sclerosis subtype, Ann Arbor stage IVBEX, Hasenclever index-4 with complement mediated hemolysis and hypercalcemia of malignancy. 2D-Echocardiography and pulmonary function tests were normal. She was hydrated with normal saline and administered zoledronic acid (4 mg). Prednisolone (1 mg/kg/day) was started and simultaneously, she was administered first cycle of ABVD (Adriamycin: 25 mg/m2, Bleomycin: 10 U/m2, Vinblastine: 6 mg/m2 and Dacarbazine: 375 mg/m2), which led to normalisation of serum calcium levels over 4 days and improvement in her hemoglobin levels (Hb: 104 gm/l) over a 2 weeks period. However, she again experienced a gradual fall in hemoglobin level over next 2 weeks (45 g/l) along with agglutination in the peripheral smear and a raised reticulocyte count (8.9 %). Hence, Rituximab (375 mg/m2) was administered to the patient along with ABVD in the next cycle and was then given weekly Rituximab for a total of 4 doses and 2 weekly ABVD was continued. Administration of two doses of Rituximab led to resolution of hemolysis and patient was discharged in a stable condition. Patient is still under our follow up and she is planned for interim PET–CT after 4 cycles of ABVD for response assessment.
Fig. 1.
Biopsy specimen from the paravertebral mass showing presence of large atypical cells in a background of sclerosis (a, Hematoxylin and eosin, ×200). These atypical cells are immunopositive for CD30 and PAX 5 (b, c, ×200) and immunonegative for CD20 (d, ×200)
Fig. 2.
Bone marrow biopsy smear showing presence of large atypical cells in a reactive background (a, Hematoxylin and eosin stain, ×200). Higher magnification showed presence of Reed Sternberg cell (b, Hematoxylin and eosin stain, ×1000). These cells are immunopositive for CD 30 and are immunonegative for CD20 (c, d, Immunohistochemistry for CD30, ×400 and CD20, ×2000 respectively)
Discussion
Etiology of anemia in Hodgkin’s lymphoma is multifactorial. Anemia of chronic disease, decreased red cell survival, infiltration of bone marrow by tumor and marrow suppression by chemotherapy/radiotherapy are the common mechanisms [5]. In 1967, Eisner et al. [1] first reported the presence of immune mediated hemolysis in HL with an incidence for Coomb’s positivity to be 2.7 %. Levine et al. reviewed 71 cases of HL and found that seven patients had Coomb’s positivity. All of the cases with a positive DCT were males and had an active (six had ‘B’ symptoms) and advanced disease (stage III/IV) and only 3 had overt hemolysis. Three cases had a positive DCT at diagnosis and 4 had it at the time of relapse. Histology revealed nodular sclerosis subtype in 3 and mixed cellularity in 4 cases. Type of antibody was characterized as IgG against It antigen on red cells in three cases and all the cases responded to treatment of HL [3]. However, our patient had a cold antibody mediated hemolysis and we could not find any case reported in the English literature. Our case had only a transient response to steroids and chemotherapy. Therefore, she was treated with Rituximab which brought hemolysis under control.
Plimpton and Gellhorn first reported the occurrence of hypercalcemia in HL in 1956 [6]. Hypercalcemia in HL is rare and its incidence has been reported as 0.9, 1.6 and 5.4 % in different series depending on the cut-off levels for high serum calcium [4]. Hypercalcemia of malignancy involves three mechanisms: 1. Humoral hypercalcemia mediated by PTHrP—seen in solid tumors like breast cancer and adult T cell leukemia/lymphoma (ATLL), 2. Direct osteoclast mediated bone resorption due to bony metastasis—seen in solid tumors and multiple myeloma, 3. Calcitriol mediated hypercalcemia—seen in Hodgkin’s and non-Hodgkin’s lymphoma as well as granulomatous disorders like tuberculosis, sarcoidosis, leprosy and disseminated Candidiasis [4, 7]. Mechanism of hypercalcemia in HL has long been suggested to involve extra-renal activation of 1α-hydroxylase leading to production of 1, 25(OD)2 Vitamin D3 or Calcitriol, an active metabolite of Vitamin D, which leads to increased re-absorption of calcium and phosphate from intestine, increased osteoclast activation and bone resorption as well as increased phosphate re-absorption in renal tubules [8]. The source of 1α-hydroxylase in HL has been postulated as monocytes and macrophages infiltrating the tumor akin to tuberculosis or sarcoidosis and is stimulated by IFN-γ secreted by T-lymphocytes. However, it has also been realized that Calcitriol alone is insufficient to mediate hypercalcemia in HL and other humoral mediators are also possibly involved. In this regard, IL-1 and TNF have been found to up regulate IFN-γ receptors on macrophages [9]. Like sarcoidosis, patients with HL exhibit increased sensitivity to Vitamin D supplements and sunlight, which have been found to precipitate hypercalcemia in these patients [7]. Classical biochemical profile in Calcitriol mediated hypercalcemia include: an elevated calcium, normal/slightly elevated phosphate, normal 25(OH) Vitamin D, suppressed PTHrP and PTH, elevated Calcitriol and a normal/increased tubular reabsorption of phosphate [10]. Seymour et al. [4] reviewed 38 cases of hypercalcemia in HL and summarized that an old age, male dominance, lower incidence of nodular sclerosis subtype, advanced stage (75 % had stage III/IV), active disease (68 % had ‘B’ symptoms) and an infra-diaphragmatic presentation characterized these patients. Remarkably, both hemolytic anemia and hypercalcemia in HL are seen with advanced and active disease and presence of both in HL has not been associated with a poorer prognosis and tends to subside after treatment of the underlying disease [3, 4].
In conclusion, we report complement mediated hemolysis and hypercalcemia as an initial presentation of Hodgkin’s disease in an elderly lady. Although, hypercalcemia in our case resolved with the treatment of lymphoma, hemolytic anemia persisted. Whether presence of cold antibodies in Hodgkin’s lymphoma is incidental or truly paraneoplastic needs to be validated in further studies. Presence of both hemolysis and hypercalcemia in a patient of Hodgkin’s lymphoma indicates an advanced and active disease and needs immediate therapy. However, clinicians should be aware that they are themselves not poor prognostic markers in a given case.
Compliance with Ethical Standards
Conflicts of interest
The authors declare no conflicts of interests.
Ethical statement
The authors declare that the manuscript submitted is in accordance with the ethical guidelines as under Helsinki Declaration 1976.
Consent for publication
The authors hereby declare that appropriate written and informed consent was obtained from the patient prior to publication of any material.
References
- 1.Eisner E, Ley AB, Mayer K. Coomb’s-positive hemolytic anemia in Hodgkin’s disease. Ann Intern Med. 1967;66:258–273. doi: 10.7326/0003-4819-66-2-258. [DOI] [PubMed] [Google Scholar]
- 2.Linde R, Basso L. Hodgkin’s disease with hypercalcemia detected by thallium-201 scintigraphy. J Nucl Med. 1987;28:112–115. [PubMed] [Google Scholar]
- 3.Levine AM, Thornton P, Forman SJ, Van Hale P, Holdorf D, Rouault CL, Powars D, et al. Positive Coombs test in Hodgkin’s disease: significance and implications. Blood. 1980;55:607–611. [PubMed] [Google Scholar]
- 4.Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non Hodgkin’s lymphomas. Blood. 1993;82:1383–1394. [PubMed] [Google Scholar]
- 5.Giannopoulos P, Bergsagel DE. The mechanism of the anemia associated with Hodgkin’s disease. Blood. 1959;14:856–869. [PubMed] [Google Scholar]
- 6.Gellhorn A, Plimpton CH. Hypercalcemia in malignant disease without evidence of bone destruction. Am J Med. 1956;21:750–759. doi: 10.1016/0002-9343(56)90081-X. [DOI] [PubMed] [Google Scholar]
- 7.Karmali R, Barker S, Hewison M, Fraher L, Katz DR, O’Riordan JL. Intermittent hypercalcemia and vitamin D sensitivity in Hodgkin’s disease. Postgrad Med J. 1990;66:757–760. doi: 10.1136/pgmj.66.779.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JS. Vitamin D metabolite-mediated hypercalcemia. Endocrinol Metab Clin N Am. 1989;18:765–778. [PubMed] [Google Scholar]
- 9.Krakauer T, Oppenhaim JJ. IL-1 and tumor necrosis factor α each up-regulate both the expression of IFN-γ receptors and enhance IFN-γ induced HLA-DR expression on human monocytes and a human monocytic cell line (THP-1) J Immunol. 1993;150:1205–1211. [PubMed] [Google Scholar]
- 10.Adams ND, Gray RW, Lemann J, Jr, Cheung HS. Effects of calcitriol administration on calcium metabolism in healthy men. Kidney Int. 1982;21:90–97. doi: 10.1038/ki.1982.13. [DOI] [PubMed] [Google Scholar]