Abstract
Background
Type 2 diabetes (T2D) and obesity are associated with nonalcoholic fatty liver disease, cardiomyopathy, and cardiovascular mortality. Both show stronger links between ectopic and visceral fat deposition, and an increased cardiometabolic risk compared with subcutaneous fat.
Objectives
This study investigated whether lean patients (Ln) with T2D exhibit increased ectopic and visceral fat deposition and whether these are linked to cardiac and hepatic changes.
Methods
Twenty-seven obese patients (Ob) with T2D, 15 Ln-T2D, and 12 normal-weight control subjects were studied. Subjects underwent cardiac computed tomography, cardiac magnetic resonance imaging (MRI), proton and phosphorus MR spectroscopy, and multiparametric liver MR, including hepatic proton MRS, T1- and T2*-mapping yielding “iron-corrected T1” [cT1].
Results
Diabetes, with or without obesity, was associated with increased myocardial triglyceride content (p = 0.01), increased hepatic triglyceride content (p = 0.04), and impaired myocardial energetics (p = 0.04). Although cardiac structural changes, steatosis, and energetics were similar between the T2D groups, epicardial fat (p = 0.04), hepatic triglyceride (p = 0.01), and insulin resistance (p = 0.03) were higher in Ob-T2D. Epicardial fat, hepatic triglyceride, and insulin resistance correlated negatively with systolic strain and diastolic strain rates, which were only significantly impaired in Ob-T2D (p < 0.001 and p = 0.006, respectively). Fibroinflammatory liver disease (elevated cT1) was only evident in Ob-T2D patients. cT1 correlated with hepatic and epicardial fat (p < 0.001 and p = 0.01, respectively).
Conclusions
Irrespective of body mass index, diabetes is related to significant abnormalities in cardiac structure, energetics, and cardiac and hepatic steatosis. Obese patients with T2D show a greater propensity for ectopic and visceral fat deposition.
Key Words: diabetic cardiomyopathy, epicardial fat deposition, fatty liver disease, magnetic resonance imaging, magnetic resonance spectroscopy
Abbreviations and Acronyms: 1H-MRS, proton magnetic resonance spectroscopy; 31P-MRS, phosphorus magnetic resonance spectroscopy; ATP, adenosine triphosphate; BMI, body mass index; BP, blood pressure; CT, computed tomography; cT1, iron-corrected T1; HOMA-IR, homeostasis model assessment of insulin resistance; Ln-T2D, lean patients with type 2 diabetes; LV, left ventricular; MR, magnetic resonance; MRI, magnetic resonance imaging; NAFLD, nonalcoholic fatty liver disease; Ob-T2D, obese patients with type 2 diabetes; PCr, phosphocreatine; T2D, type 2 diabetes
Type 2 diabetes (T2D) and obesity are both associated with nonalcoholic fatty liver disease (NAFLD), cardiomyopathy 1, 2, and increased cardiovascular mortality 3, 4. The incidence of T2D continues to increase, driven predominantly by the obesity epidemic. Although obesity is likely to be a strong contributor to diabetic cardiomyopathy (5), many patients with diabetic cardiomyopathy have normal body mass index (BMI), suggesting that diabetes and obesity may have different mechanisms by which they mediate cardiovascular change and that diabetic cardiomyopathy may occur in patients with T2D without obesity. Furthermore, evidence suggests that distribution of excess fat is an important determinant of cardiovascular risk, and ectopic and visceral adiposity confer a much higher risk than subcutaneous adiposity 6, 7.
Ectopic and visceral fat storage may be linked to insulin resistance, and it is widely known that insulin resistance is the strongest predictor of development of diabetes (8). Increasing evidence points to a strong association between insulin resistance and nonischemic heart failure (9), although there are differing opinions regarding whether this relationship is of a protective or pathological nature 10, 11, 12. Thus, the presence of ectopic and visceral fat deposition in patients with T2D even in the absence of a global increase in total body fat may potentially play a significant role in this association. Assessing body composition is, therefore, likely to be more important in patients with T2D than simple metrics of obesity. Liver fat is considered a key feature of ectopic fat associated with dysfunctional adipose tissue and visceral fat deposition (13), and there is also growing interest in the imaging of epicardial adipose tissue as a proxy measure of visceral fat.
Epicardial adipose tissue, a form of visceral fat, may affect the underlying myocardium by secreting a wide range of adipokines (14). Furthermore, excess liver fat has been shown to be accompanied by cardiac structural and functional changes (15). Computed tomography (CT) allows quantification of epicardial fat volume, and proton magnetic resonance spectroscopy (1H-MRS) allows quantification of lipid content in the liver and the heart. Multiparametric magnetic resonance (MR) of the liver, including 1H-MRS for assessment of steatosis and T1 and T2* mapping (yielding iron corrected T1 [cT1]) (16), allows noninvasive quantification of liver fat and identification of the presence of hepatic fibroinflammatory disease with a high diagnostic accuracy (16).
Myocardial energetic compromise is an important feature of both the diabetic (17) and the nondiabetic obese heart (5). However, changes in cardiac energy metabolism in lean patients with diabetes have not been previously studied. Myocardial phosphocreatine to adenosine triphosphate concentration ratio (PCr/ATP) is a sensitive indicator of the myocardial energy status, and phosphorus magnetic resonance spectroscopy (31P-MRS) allows noninvasive assessment of the PCr/ATP.
Our primary aim was to test the hypothesis that lean patients (Ln) with T2D exhibit increased ectopic and visceral fat deposition. Our secondary aim was to test whether or not ectopic and visceral adiposity in diabetes is associated with insulin resistance and cardiac and hepatic changes. We used cardiac CT, multiparametric liver magnetic resonance imaging (MRI), cardiac MRI, 1H-MRS, and 31P-MRS to assess and compare epicardial, hepatic, and myocardial fat deposition; hepatic fibroinflammatory changes; and cardiac structure, function, and energetics in lean and obese patients (Ob) with T2D and in control subjects without diabetes.
Methods
The study was approved by the National Research Ethics Committee (Ref 13/SW/0257), and informed written consent was obtained from each participant. Patients were recruited from general practice surgeries in Oxfordshire, United Kingdom. A total of 27 Ob-T2D, 15 Ln-T2D, and 12 healthy normal weight control subjects were recruited to the study. We have previously reported changes in myocardial energetics, triglyceride content, and left ventricular (LV) structure and function in patients with diabetes compared with healthy volunteers 18, 19. Using this database, and expanding the data with novel recruitment of 12 healthy volunteers to the study, here we report a comparison of the changes in these cardiac features in 2 subgroups of patients with diabetes (obese and lean) compared with healthy volunteers. Additionally, we report an analysis of epicardial fat volumes, liver triglyceride content, and liver fibroinflammatory changes.
Exclusion criteria
Subjects were excluded if they had a previous diagnosis of cardiovascular or liver disease, hypertension (resting systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg), contraindications to MRI, ischemic changes on 12-lead electrocardiography, or renal impairment (estimated glomerular filtration rate <30 ml/min/1.73 m2); if they were tobacco smokers; if their alcohol intake was above 21 units in a week for men or 14 units for women; or if they were insulin dependent.
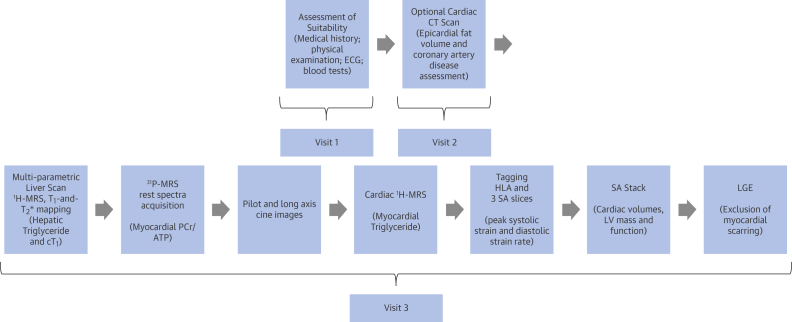
Control subjects had no history of heart disease, diabetes mellitus (fasting glucose level ≥6.7 mmol), or hypertension and were not taking any medications. Study assessments were carried out on a single visit for the healthy control subjects and over 2 or 3 visits for patients with T2D, depending on individuals’ consent for attending cardiac CT assessments (Figure 1).
Figure 1.
Study Protocol for Patients With T2D
Suitability of patients with type 2 diabetes (T2D) was assessed during the first hospital visit. Those patients who consented to have a cardiac computed tomography (CT) scan were then invited for the second hospital visit. The third hospital visit included magnetic resonance imaging (MRI) and magnetic resonance spectroscopy scans (3T). Multiparametric liver MRI included proton magnetic resonance spectroscopy (1H-MRS) for hepatic triglyceride; T1 and T2* mapping yielded iron-corrected T1 (cT1). This was followed by cardiac magnetic resonance, which included cine imaging to assess left ventricular (LV) volumes, mass, and ejection fraction; myocardial tagging for assessment of peak circumferential systolic strain and diastolic strain rate; cardiac 1H-MRS for myocardial triglyceride; and late gadolinium enhancement (LGE) imaging for exclusion of myocardial scarring. Control subjects underwent identical MRI protocols. 31P-MRS = phosphorus magnetic resonance spectroscopy; ECG = electrocardiogram; HLA = horizontal long axis; PCr/ATP = myocardial phosphocreatine to adenosine triphosphate concentration ratio; SA = short axis.
Anthropometric measurements
Height and weight were recorded and BMI was calculated. Brachial blood pressure was recorded as an average of 3 supine measures taken over 10 min (DINAMAP-1846-SX, Critikon Corp., Tampa, Florida). Fasting venous blood was drawn for glucose, insulin, hemoglobin A1c (HbA1c), triglycerides, renal function, liver function, and free fatty acids. Insulin levels and HbA1c were checked in the patients with diabetes, but not in control subjects. Homeostasis model assessment of insulin resistance (HOMA-IR) was used to evaluate insulin resistance using the following equation: (fasting serum insulin [μU/l] × fasting plasma glucose [mmol·l−1])/22.5 (20).
Cardiac CT
Coronary CT
An optional scan of coronary computed tomographic angiography (CCTA) was offered to patients with diabetes to exclude obstructive coronary artery disease (>50% of luminal stenosis) and for assessment of epicardial fat volumes. CCTA was performed with a GE VCT 64 slice scanner (GE Healthcare, Little Chalfont, United Kingdom) and a Snapshot Pulse protocol with prospective electrocardiographic triggering. Participants received beta-blockade (intravenous metoprolol) and sublingual glyceryl trinitrate prior to the scan to achieve a heart rate of <65 beats/min. During the CCTA acquisition, 70 ml of iodinated contrast (Niopam 370, Bracco, High Wycombe, United Kingdom) was injected at a rate of 6 ml/s followed by a 50-ml saline flush. The scan covered a region from 1 to 2 cm above the left main coronary artery to 1 to 2 cm below the myocardial apex in a single breath hold.
Epicardial fat volume quantification
CT images were reconstructed using medium-soft kernel (standard) with slice thickness of 0.625 mm and then transferred to a dedicated workstation for image processing (TeraRecon Aquarius iNtuition version 4.4.11, TeraRecon Inc., San Mateo, California). The adipose tissue volume was quantified using contrast-enhanced CT images. The layer of the epicardium was manually traced, and a 3-dimensional image was constructed using a semiautomated method. The volume was then calculated by a blinded operator (S.T.) and defined as the tissue with attenuation of −190 to −30 HU.
Cardiac MR
All LV imaging was performed on a 3.0-T MR system (Siemens, Erlangen, Germany). Images for LV volumes and diastolic function were acquired using a steady-state free precession sequence and analyzed using cmr42 (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada). To determine midventricular systolic circumferential strain and diastolic strain rate, myocardial tagging was performed 21, 22. Tagged images were analyzed using Cardiac Image Modeller software (CimTag2D version 7, Auckland Medical Research, Auckland, New Zealand). Semiautomated analysis was performed by aligning a grid to the myocardial tagging planes at end-diastole. A more detailed description of the MRI methods and MR acquisition parameters is included in the Online Appendix.
31P-magnetic resonance spectroscopy
31P-MRS was performed to obtain the rest PCr/ATP from a voxel placed in the midventricular septum, with the subjects lying prone with their heart over the center of the 31P heart/liver coil in the isocenter of the magnet. 31P-MRS post-processing analysis was performed using in-house software within Matlab version R2012a (Mathworks, Natick, Massachusetts). A more detailed description of the cardiac 31P-MRS acquisition parameters is included in the Online Appendix.
Cardiac and liver 1H-MRS
Myocardial 1H-MRS was obtained from the midinterventricular septum. Liver triglyceride content was measured using 1H-MRS, avoiding vascular and biliary structures. Spectroscopic acquisitions were performed using electrocardiographic trigger. Water-suppressed spectra were acquired to measure myocardial and liver triglyceride content, and spectra without water suppression were acquired and used as an internal standard. Spectra were analyzed using Matlab and the AMARES algorithm in the Java-based Magnetic Resonance User Interface. Myocardial and liver triglyceride contents were calculated as a percentage relative to water: (signal amplitude of lipid/signal amplitude of water) × 100. A more detailed description of the 1H-MRS acquisition parameters is included in the Online Appendix.
Liver MRI
The liver multiparametric MR protocol has been previously described (16). MR scans were performed using a 3-T scanner (Tim Trio, Siemens). Transverse abdominal T1 and T2* MR maps were acquired for the estimation of extracellular fluid and liver iron, respectively. Patients fasted overnight prior to their MRI scans.
cT1 and fibroinflammatory liver disease
T1 relaxation time increases with increases in extracellular fluid, such as in fibrosis and inflammation. However, the presence of iron, which can be accurately measured from T2* maps, has an opposing effect on the T1. An algorithm has been created that allows for the bias introduced by elevated iron to be removed from the T1 measurements, yielding the cT1.
LiverMultiScan (Perspectum Diagnostics, Oxford, United Kingdom) is a software product specifically developed to measure cT1 from T1 and T2* maps. For this study, the LiverMultiScan was used to analyze anonymized images by investigators blinded to the clinical data (C.K., M.P.). cT1 was measured in a single, operator-defined region of interest away from vascular and biliary structures.
Statistical analysis
All statistical analysis was performed with commercially available software packages (SPSS Statistics version 20, IBM, Armonk, New York). All data were checked for normality using the Kolmogorov-Smirnov test and presented as mean ± SD. Comparisons between the 3 groups were performed by 1-way analysis of variance with post hoc Bonferroni corrections. Bivariate correlations were performed using the Pearson or Spearman method as appropriate. The Student t test was used for comparison of normally distributed datasets where data were obtained for only 2 T2D groups. Significance was defined as p < 0.05.
Results
Participant characteristics
Demographic, clinical, and biochemical data are shown in Table 1. A total of 27 Ob-T2D patients (14 males, age 56 ± 8 years, BMI 33 ± 3 kg/m2, mean diabetes duration 6.1 ± 4.7 years, mean HbA1c 7.7 ± 1.4%), 15 Ln-T2D patients (9 males, age 56 ± 9 years, BMI 23 ± 2 kg/m2, mean diabetes duration 6.6 ± 6.5 years, mean HbA1c 7.4 ± 0.9%), and 12 healthy volunteers (8 males, age 50 ± 10 years, BMI 23 ± 2 kg/m2) were recruited. Participants in all groups were of similar age and sex, and there were no significant differences in blood pressure, diabetes duration, diabetes treatment, or metabolic profile between the 2 diabetes groups (Table 1). Systolic blood pressure was statistically higher in participants with T2D compared with control subjects, although it remained within normal limits. A total of 77% of the patients with diabetes were on statin therapy, and patients with diabetes therefore had lower low-density lipoprotein cholesterol levels compared with control subjects.
Table 1.
Clinical and Biochemical Characteristics
| Normal Control Subjects (n = 12) |
Lean T2D Patients (n = 15) |
Obese T2D Patients (n = 27) |
p Value | |
|---|---|---|---|---|
| Age, yrs | 50 ± 10 | 56 ± 9 | 56 ± 8 | 0.163 |
| BMI, kg/m2 | 23 ± 3 | 23 ± 2 | 33 ± 3∗ | <0.001 |
| Male | 58 | 60 | 40 | 0.35 |
| Diabetes duration, yrs | — | 6.1 ± 4.7 | 6.6 ± 6.5 | 0.78 |
| Heart rate, beats/min | 66 ± 10 | 65 ± 7 | 69 ± 7 | 0.34 |
| Systolic blood pressure, mm Hg | 118 ± 14 | 131 ± 7† | 130 ± 9† | 0.002 |
| Diastolic blood pressure, mm Hg | 70 ± 8 | 76 ± 7 | 76 ± 7 | 0.05 |
| Plasma fasting glucose, mmol/l | 5.0 ± 0.5 | 8.1 ± 3.0† | 9.5 ± 3.3† | 0.001 |
| Glycated hemoglobin, % | — | 7.4 ± 0.9 | 7.7 ± 1.4 | 0.22 |
| Hematocrit, % | 43 ± 3 | 42 ± 3 | 43 ± 3 | 0.81 |
| Insulin, pmol/l | — | 107 ± 142 | 218 ± 255 | 0.03 |
| HOMA-IR, % | — | 1.26 ± 0.70 | 5.45 ± 5.6 | 0.03 |
| Plasma triglycerides, mmol/l | 0.92 ± 0.38 | 1.87 ± 1.81 | 1.75 ± 0.81 | 0.15 |
| Plasma free fatty acids, mmol/l | 0.59 ± 0.42 | 0.61 ± 0.20 | 0.67 ± 0.43 | 0.82 |
| Total cholesterol, mmol/l | 4.7 ± 1.0 | 3.8 ± 0.8 | 4.1 ± 1.0 | 0.10 |
| HDL, mmol/l | 1.55 ± 0.56 | 1.24 ± 0.29 | 1.20 ± 0.31† | 0.03 |
| LDL, mmol/l | 2.93 ± 0.46 | 1.85 ± 0.59† | 2.12 ± 0.82† | 0.002 |
| Medications | ||||
| Metformin | — | 14 (93) | 23 (85) | 0.45 |
| Sulfonylurea | — | 4 (27) | 12 (44) | 0.27 |
| Aspirin | — | 2 (13) | 7 (26) | 0.35 |
| Statin | — | 8 (60) | 19 (70) | 0.51 |
| ACE-I | — | 7 (47) | 10 (37) | 0.56 |
Values are mean ± SD, %, or n (%).
ACE-I = angiotensin-converting enzyme inhibitors; BMI = body mass index; HDL = high-density lipoprotein; HOMA-IR = homeostasis model assessment of insulin resistance; LDL = low-density lipoprotein; T2D = type 2 diabetes.
p < 0.05 versus lean T2D and control subjects with Bonferroni correction.
p < 0.05 versus control subjects with Bonferroni correction.
Cardiac geometry and function
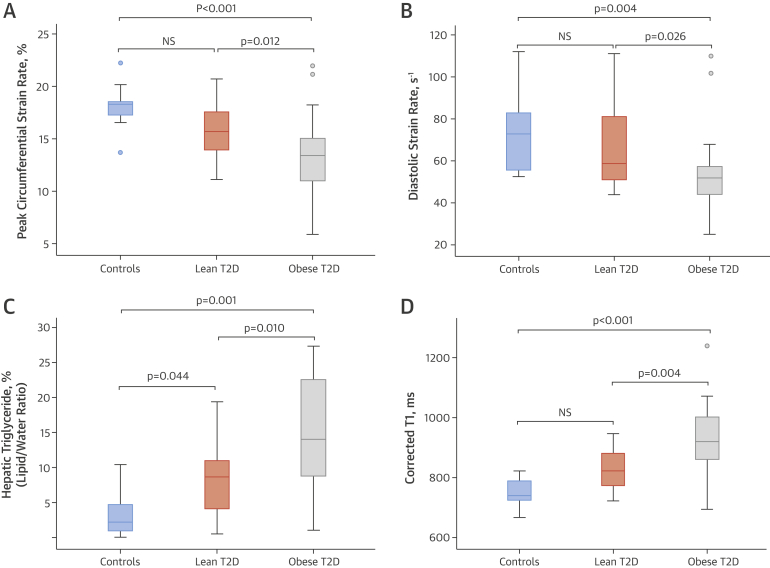
Cardiac MR results for LV volumes and function are summarized in Table 2. LV volumes and ejection fraction were similar between the Ln- and Ob-T2D groups and control subjects. However, although LV ejection fractions were not significantly different across the groups, more subtle functional changes with impairment in peak circumferential systolic strain and diastolic strain rates were evident in Ob-T2D compared with control subjects (p = 0.001 and p = 0.006, respectively) and also compared with Ln-T2D (p = 0.015 and p = 0.026, respectively). As we have previously shown, diabetes was associated with LV concentric hypertrophy (19), characterized by increased LV mass to volume ratio and increased LV mass, in both diabetes groups compared with control subjects.
Table 2.
CMR and Cardiac MRS Findings
| Control Subjects (n = 12) |
Lean T2D Patients (n = 15) |
Obese T2D Patients (n = 27) |
p Value | |
|---|---|---|---|---|
| LV end-diastolic volume, ml | 145 ± 40 | 124 ± 33 | 126 ± 25 | 0.15 |
| LV ejection fraction, % | 68 ± 5 | 73 ± 7 | 68 ± 8 | 0.11 |
| LV mass, g | 91 ± 30 | 123 ± 33∗ | 119 ± 28∗ | 0.01 |
| LV mass index, g/m2 | 48 ± 11 | 66 ± 15∗ | 57 ± 10 | 0.001 |
| LV mass to LV end-diastolic volume, g/ml | 0.63 ± 0.13 | 0.95 ± 0.26∗ | 0.90 ± 0.20∗ | <0.001 |
| Peak systolic circumferential strain, negative (−), % | 18.1 ± 2.1 | 16.5 ± 2.6 | 13.4 ± 3.6† | <0.001 |
| Peak circumferential diastolic strain rate, s−1 | 74 ± 20 | 68 ± 19 | 56 ± 26† | 0.006 |
| Myocardial PCr/ATP ratio | 2.08 ± 0.40 | 1.75 ± 0.29∗ | 1.64 ± 0.32∗ | 0.003 |
| Myocardial triglyceride, % (lipid/water ratio) | 0.48 ± 0.28 | 1.14 ± 0.66∗ | 1.22 ± 0.91∗ | 0.004 |
Values are mean ± SD.
CMR = cardiac magnetic resonance; LV = left ventricular; MRS = magnetic resonance spectroscopy; PCr/ATP = phosphocreatine to adenosine triphosphate concentration ratio; T2D = type 2 diabetes.
p < 0.05 versus control subjects with Bonferroni correction.
p < 0.05 versus lean patients with T2D and control subjects with Bonferroni correction.
Cardiac metabolic phenotype
Cardiac 1H- and 31P-MRS results for myocardial triglyceride and energetics are summarized in Table 2. Diabetes was associated with cardiac steatosis even in the absence of obesity (Ln-T2D vs. control subjects; p = 0.01), and there was no significant difference in myocardial triglyceride levels between the Ob- and Ln-T2D groups. PCr/ATP was significantly reduced in both T2D groups compared with control subjects (Ob-T2D vs. control subjects; p = 0.002; Ln-T2D vs. control subjects; p = 0.043). There was no significant difference in myocardial PCr/ATP ratio between the Ob- and Ln-T2D groups (p = 0.92). There were no significant correlations between the myocardial PCr/ATP ratio and the markers of ectopic and visceral adiposity, such as hepatic triglyceride content (r = −0.17; p = 0.36), or with epicardial fat volume (r = −0.23; p = 0.27).
Epicardial fat
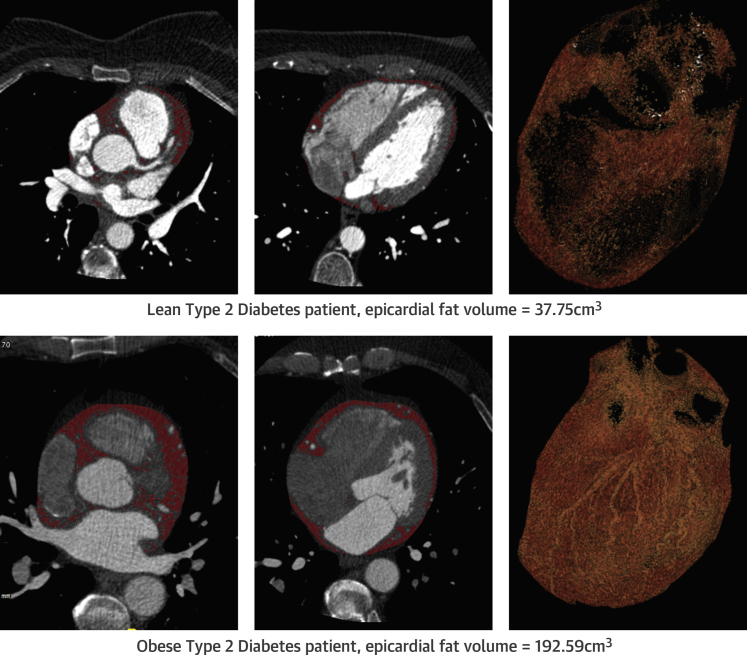
Epicardial fat volume assessment was carried out in 33 patients (79% of the study patients) who have opted for CCTA. The Ob-T2D group had higher epicardial fat volumes compared with the Ln-T2D group (96 ± 40 cm3 vs. 71 ± 21 cm3; p = 0.04). Figure 2 shows representative images of epicardial fat volume in an LN- and an Ob-T2D patient.
Figure 2.
Representative Examples of CT Epicardial Fat Volumes in a Lean and an Obese Patient With T2D
(Top) Lean patient with T2D with epicardial fat volume 37.75 cm3. (Bottom) Obese patient with T2D with epicardial fat volume 192.59 cm3. Abbreviations as in Figure 1.
Hepatic steatosis, iron content, fibrosis, and inflammation
Liver enzymes and multiparametric liver MRI results for hepatic steatosis, fibrosis, and hemosiderosis are summarized in Table 3. Similar to cardiac steatosis, diabetes, even in the absence of obesity, was associated with hepatic steatosis (hepatic triglyceride content in Ln-T2D vs. control subjects; p = 0.044); however, hepatic steatosis was most marked in the Ob-T2D group: approximately 2-fold higher than in the Ln-T2D group (Ob-T2D vs. Ln-T2D; p = 0.012) and approximately 4-fold higher than in control subjects (Ob-T2D vs. control subjects; p = 0.005). Iron levels were normal across the groups.
Table 3.
Liver Assessments
| Control Subjects | Lean T2D Patients (n = 15) |
Obese T2D Patients (n = 27) |
p Value | |
|---|---|---|---|---|
| Liver enzymes | ||||
| Bilirubin, umol/l | 12 ± 4 | 12 ± 6 | 11 ± 4 | 0.48 |
| ALT, IU/l | 22 ± 9 | 30 ± 22 | 36 ± 17 | 0.18 |
| ALP, IU/l | 145 ± 29 | 150 ± 50 | 163 ± 46 | 0.47 |
| Albumin, g/l | 44 ± 3 | 45 ± 2 | 46 ± 3 | 0.53 |
| Multiparametric liver MRI | ||||
| cT1, ms | 753 ± 45 | 821 ± 67 | 924 ± 116∗ | <0.001 |
| Hepatic triglyceride, % (lipid/water ratio) | 3.8 ± 3.6 | 7.6 ± 4.6† | 14.8 ± 8.4∗ | <0.001 |
| T2*, ms | 20 ± 4 | 20 ± 4 | 18 ± 5 | 0.41 |
| Liver iron, mg/g | 1.3 ± 0.12 | 1.34 ± 0.13 | 1.33 ± 0.19 | 0.99 |
Values are mean ± SD.
ALP = alkaline phosphatase; ALT = alanine aminotransferase; cT1 = corrected T1; LIF = liver inflammation and fibrosis score; MRI = magnetic resonance imaging; other abbreviations as in Table 2.
p < 0.05 versus lean patients with T2D and control subjects with Bonferroni correction.
p < 0.05 versus control subjects with Bonferroni correction.
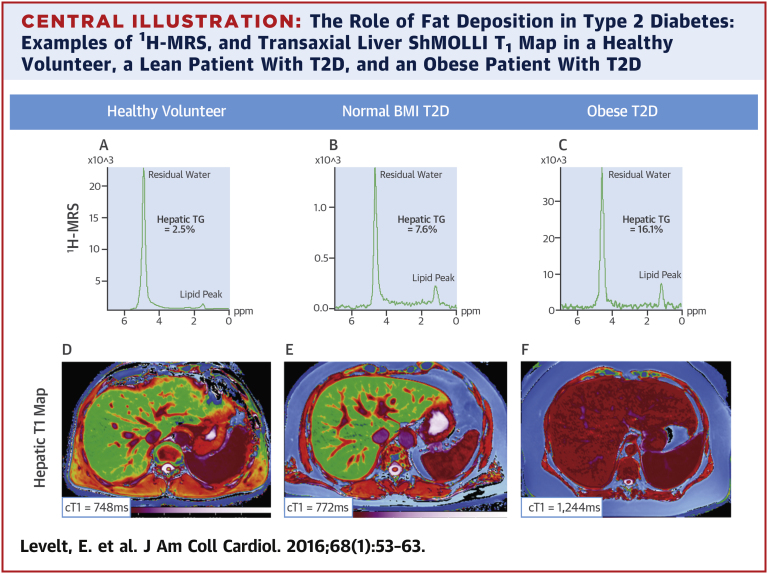
Mean cT1 was highest in the Ob-T2D group, where the highest levels of hepatic triglyceride content were detected. The numeric differences in mean cT1 between Ln-T2D and control subjects did not reach statistical significance (p = 0.245), whereas cT1 in the Ob-T2D group was significantly increased compared with the Ln-T2D group (p = 0.004) and control subjects (p < 0.001), indicating significant fibroinflammatory liver disease in this group. Figure 3 represents differences in cardiac function, hepatic steatosis, and hepatic cT1 across the study cohorts. There was a positive correlation between the hepatic cT1 and hepatic triglyceride content (r = 0.71; p < 0.001). Importantly, despite the presence of hepatic steatosis and fibroinflammatory changes in the Ob-T2D group, there was no significant difference in liver enzymes compared with control subjects, and there was no association between liver cT1 and liver enzymes. Alanine aminotransferase levels were only minimally elevated (>45 to <80 IU/l) in 5 Ob-T2D patients and were normal in all other patients. The Central Illustration shows representative liver 1H-MR spectra, and a liver T1 map in a volunteer and in a lean and an obese patient with T2D.
Figure 3.
Differences in Cardiac Function, Hepatic Steatosis, and Hepatic cT1 Among the Study Cohorts
(A) Peak circumferential systolic strain; (B) diastolic strain rate; (C) hepatic triglyceride content (%); and (D) hepatic corrected T1 map (ms). The dots indicate values outside the interquartile range.
Abbreviations as in Figure 1.
Central Illustration.
The Role of Fat Deposition in Type 2 Diabetes: Examples of 1H-MRS, and Transaxial Liver ShMOLLI T1 Map in a Healthy Volunteer, a Lean Patient With T2D, and an Obese Patient With T2D
(A) Proton magnetic resonance spectroscopy (1H-MRS) of healthy volunteer with hepatic triglyceride (TG) 2.5%. (B)1H-MRS of lean patient with type 2 diabetes (T2D) with hepatic TG 7.6%. (C) Obese patient with T2D with hepatic TG 16.1%. (D) Healthy volunteer with liver Shortened Modified Look-Locker Inversion recovery (ShMOLLI) T1 map with corrected T1 (cT1) 748 ms. (E) Lean patient with T2D with liver ShMOLLI T1 map with cT1 772 MS. (F) Obese patient with T2D with liver ShMOLLI T1 map with cT1 1244 ms. BMI = body mass index.
Relationship of insulin resistance, ectopic fat accumulation, and cardiac function
Insulin resistance, measured by HOMA-IR, was significantly higher in the Ob-T2D compared with the Ln-T2D group (p = 0.03). When investigating all T2D subjects, there was a positive correlation between the HOMA-IR and epicardial fat volumes (r = 0.47; p = 0.029), hepatic triglyceride (r = 0.39; p = 0.046), and hepatic cT1 (r = 0.58; p = 0.001), and there was a negative correlation between HOMA-IR and peak circumferential systolic strain (r = −0.52; p = 0.003). Furthermore, peak circumferential systolic strain also correlated negatively with the hepatic triglyceride (r = −0.49; p = 0.001) and epicardial fat volumes (r = −0.53; p = 0.004). Similarly, diastolic strain rate correlated negatively with hepatic triglyceride (r = −0.54; p < 0.001) and epicardial fat volumes (r = −0.59; p = 0.001). Myocardial triglyceride did not correlate with epicardial fat volumes (r = 0.36; p = 0.103) or with hepatic triglyceride (r = 0.23; p = 0.168).
Discussion
This study demonstrates for the first time that diabetes, even in the absence of obesity, is associated with significant cardiac structural and metabolic abnormalities, whereas significant functional changes, such as reductions in peak systolic strain and diastolic strain rates, are only evident in obese patients with diabetes. Furthermore, we show that those patients with diabetes who are also obese have higher epicardial fat volumes, significant NAFLD, and higher insulin resistance. Importantly, we demonstrate here that the degree of hepatic and epicardial fat accumulation is associated with cardiac contractile dysfunction in diabetes. We confirm the findings of previous studies showing the association between epicardial fat deposition and insulin resistance 23, 24; moreover, we also demonstrate that there is an association between fibroinflammatory liver disease and insulin resistance in patients with diabetes. The correlation of systolic strain and diastolic strain rates with hepatic and epicardial fat and insulin resistance suggests a link between these in patients with diabetes. However, the causality of these relationships will need to be investigated in future studies.
Finally, as is widely known, the spectrum of NAFLD ranges from fatty liver alone to nonalcoholic steatohepatitis (25). We show here that diabetes, even in the absence of obesity, is associated with hepatic steatosis at the mild end of the liver disease spectrum, but not with significant fibroinflammatory liver disease. Importantly, using multiparametric liver imaging, we show that significant NAFLD and nonalcoholic steatohepatitis are present in asymptomatic patients with T2D with minor or no alanine aminotransferase elevation. This technique promises to answer a pressing need for a reliable, quick, and noninvasive screening, staging, and monitoring tool for diabetic liver disease.
Ectopic and visceral fat, insulin resistance, and the heart
Our results suggest that Ln-T2D patients are likely to have less pronounced insulin resistance, lower levels of epicardial and hepatic fat accumulation, and better cardiac function than Ob-T2D patients. It is now widely accepted that adipose tissue is a dominant regulator of lipid and glucose metabolism (26). Multiple studies support the concept that insulin resistance is prompted, and sustained by, dysregulated fat tissue 27, 28, 29. In addition, insulin resistance and ectopic adiposity are associated with an even greater cardiovascular risk 30, 31, and obese subjects with T2D are at high risk of developing ectopic adiposity (32). There are many molecular mechanisms that may contribute to the association between insulin resistance and nonischemic cardiomyopathy (9). These include metabolic inefficiency (33), impaired vascular function (34), inflammation, and mitogenic actions of insulin on myocardium leading to changes of left ventricular geometry (35).
Epicardial adipose tissue has dichotomous functional characteristics, both adverse and protective, interacting locally with the coronary arteries and the myocardium through paracrine and vasocrine pathways. Under physiological conditions, epicardial fat supplies heat to the myocardium and exerts a protective effect on the coronary arteries 23, 36. Its pathological increase, and the coexistence of other metabolic and hemodynamic abnormalities, turn it into an adverse lipotoxic, prothrombotic, and proinflammatory organ (31).
In our study, the dissociation of myocardial steatosis from epicardial and liver fat is in keeping with a previous study in patients without diabetes and supports the hypothesis that myocardial lipid accumulation may represent a separate entity that is influenced by factors beyond visceral adiposity (37). Rijzewijk et al. (38) and McGavock et al. (39) previously demonstrated myocardial steatosis in patients with T2D. Furthermore, McGavock et al. (39) performed 1H-MRS in a large cohort of patients with T2D and were the first to show that hepatic triglyceride was not predictive of myocardial triglyceride (38). Thus, elevated levels of intracellular triglyceride in hepatocytes do not necessarily reflect elevated triglyceride levels in cardiac myocytes in T2D, which we confirm here.
Ectopic fat and the liver
Our results suggest that Ln-T2D patients are more likely to have simple steatosis and Ob-T2D patients are more likely to have steatohepatitis. Our study is the first to date to noninvasively assess the severity of liver damage using a multiparametric MRI protocol in Ln- and Ob-T2D patients and to determine the effect of fibroinflammatory liver disease on the cardiac phenotype. We have demonstrated that asymptomatic Ob-T2D patients have significantly higher liver cT1 compared with Ln-T2D patients and healthy volunteers. This would indicate a greater burden of fibroinflammatory liver disease in this group of patients who should be prioritized for NAFLD screening in clinical practice. Importantly, these differences were present on imaging but not on alanine aminotransferase levels, suggesting that alanine aminotransferase alone is not a sensitive screening test for the presence of NAFLD in these patients. It has previously been shown that liver cT1 is associated with fibrosis (16) and also that it can differentiate simple steatosis from steatohepatitis 40, 41, 42.
NAFLD is defined as excessive fat accumulation in the liver (>5.6%) (43); it is among the leading causes of death in T2D (44) and is linked to hepatic insulin resistance (45). Despite this strong association and the emergence of NAFLD as a novel cardiovascular risk factor, only a few studies have addressed the presence of myocardial structural and functional changes in patients with NAFLD. Specifically, NAFLD, diagnosed either by ultrasonography or by liver biopsy, was shown to be associated with a higher prevalence of reduced coronary flow reserve (46), coronary calcification (47), impairment in diastolic function (48), concentric LV remodeling, and reduced longitudinal shortening (49).
Study limitations
This study is limited by a relatively small sample size. Of 42 patients with T2D, 9 patients (21%) did not consent to have CCTA performed, and it is possible that occult coronary artery disease could be present in this minority of patients. CCTA was not performed in normal volunteers to avoid unnecessary radiation exposure. Significant coronary artery disease was deemed to be unlikely in this normal cohort, and epicardial fat volumes were therefore only assessed and compared in Ob- and Ln-T2D patients.
For assessment of liver disease, we have not used liver biopsy, the current gold standard, because it is invasive and limited by sampling and observer-dependent variability (50). Instead, we used a recently established, noninvasive, multiparametric scanning method, which has demonstrated a high diagnostic accuracy for the assessment of liver fibrosis, steatosis, and hemosiderosis (16).
Although the differences in mean peak systolic strain and diastolic strain rates in Ln-T2D compared with control subjects did not reach statistical significance, this may be due to the relatively small sample size. Larger studies of Ln-T2D patients are needed to confirm this. Although the release of adipokines including adiponectin and leptin has been considered among the important actions of adipocytes, we did not assess circulating levels of adiponectin or leptin.
There is evidence of a role for the sympathetic nervous system in the relationship between insulin and hypertension in obese patients with hypertension (51). We did not demonstrate any significant difference in resting heart rates or the systolic blood pressure between the 2 diabetes groups to suggest an enhanced adrenergic drive in the obese group, but we did not assess circulating catecholamine levels.
Finally, the observational nature of our findings precludes inferences of causality. Additional research is necessary to further delineate the relationship between ectopic and visceral adiposity with potential systemic effects such as insulin resistance and their role in the development of cardiac dysfunction in patients with T2D.
Conclusions
Ob-T2D patients show a greater propensity than Ln-T2D patients for ectopic and visceral fat deposition that is associated with cardiac contractile dysfunction and fibroinflammatory liver disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Diabetes is associated with abnormalities of cardiac structure, energetics, and steatosis irrespective of BMI. Ob-T2D patients have a greater propensity for ectopic and visceral fat deposition, cardiac dysfunction, fibroinflammatory liver disease, and insulin resistance.
TRANSLATIONAL OUTLOOK: Further studies are needed to delineate the mechanistic relationships between ectopic and visceral adiposity, insulin resistance, and cardiac dysfunction in patients with T2D.
Footnotes
The study was supported by the Oxford Partnership Comprehensive Biomedical Research Centre, with funding from the Department of Health’s National Institute for Health Research Biomedical Research Centers funding plan. Drs. Pavlides, Kelly, Robson, and Neubauer are shareholders in Perspectum Diagnostics and have patents in the field of MR for the assessment of liver disease. Dr. Banerjee is an employee of, company director for, and stockholder in Perspectum Diagnostics. Dr. Rodgers is supported by a Sir Henry Dale Fellowship jointly funded by the Welcome Trust and the Royal Society (grant number 098436/Z/12/Z). Dr. Antoniades has received an unrestricted grant from Sanofi. Drs. Rider and Neubauer have received support from the Oxford British Heart Foundation Centre of Research Excellence. Dr. Neubauer is a non-executive director of and consultant for Perspectum Diagnostics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
For an expanded Methods section, please see the online version of this article.
Appendix
References
- 1.Garcia M.J., McNamara P.M., Gordon T., Kannel W.B. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S., Evans J.C., Levy D. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Kannel W.B., McGee D.L. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 4.Jiang J., Ahn J., Huang W.-Y., Hayes R.B. Association of obesity with cardiovascular disease mortality in the PLCO trial. Prev Med. 2013;57:60–64. doi: 10.1016/j.ypmed.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rider O.J., Francis J.M., Tyler D. Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging. 2013;29:1043–1050. doi: 10.1007/s10554-012-0174-6. [DOI] [PubMed] [Google Scholar]
- 6.Okura T., Nakata Y., Yamabuki K., Tanaka K. Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol. 2004;24:923–929. doi: 10.1161/01.ATV.0000125702.26272.f6. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi G., Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 8.Lillioja S., Mott D.M., Howard B.V. Impaired glucose tolerance as a disorder of insulin action. N Engl J Med. 1988;318:1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- 9.Witteles R.M., Fowler M.B. Insulin-resistant cardiomyopathy: clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Taegtmeyer H., Beauloye C., Harmancey R., Hue L. Insulin resistance protects the heart from fuel overload in dysregulated metabolic states. Am J Physiol Heart Circ Physiol. 2013;305:H1693–H1697. doi: 10.1152/ajpheart.00854.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan C.J., Ruderman N.B., Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol. 2013;1:9–10. doi: 10.1016/S2213-8587(13)70027-5. [DOI] [PubMed] [Google Scholar]
- 12.Nolan C.J., Ruderman N.B., Kahn S.E. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64:673–686. doi: 10.2337/db14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britton K.A., Fox C.S. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 14.Antonopoulos A.S., Sabharwal N., Shirodaria C. Epicardial adipose tissue volume selectively predicts myocardial redox state in patients with ischemic heart disease [abstr] Circulation. 2014;130:A19182. [Google Scholar]
- 15.Petta S., Argano C., Colomba D. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928–933. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee R., Pavlides M., Tunnicliffe E.M. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neubauer S., Horn M., Cramer M. Myocardial phosphocreatine-to-ATP ratio as a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 18.Levelt E., Rodgers C.T., Clarke W.T. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J. 2015 Sept 20 doi: 10.1093/eurheartj/ehv442. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levelt E., Mahmod M., Piechnik S.K. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes. 2016;65:44–45. doi: 10.2337/db15-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews D.R., Hosker J.P., Rudenski A.S. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Lawton J.S., Cupps B.P., Knutsen A.K. Magnetic resonance imaging detects significant sex differences in human myocardial strain. Biomedical Eng Online. 2011;10:76. doi: 10.1186/1475-925X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuber M., Spiegel M.A., Fischer S.E. Single breath-hold slice-following CSPAMM myocardial tagging. MAGMA. 1999;9:85–91. doi: 10.1007/BF02634597. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G., Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 24.Rijzewijk L.J., Jonker J.T., van der Meer R.W. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. J Am Coll Cardiol. 2010;56:225–233. doi: 10.1016/j.jacc.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Mehta R., Younossi Z.M. Natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2012;1:112–113. doi: 10.1002/cld.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young M.E., McNulty P., Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 27.Nelson M.D., Victor R.G., Szczepaniak E.W. Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. Am J Cardiol. 2013;112:1019–1024. doi: 10.1016/j.amjcard.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johannsen D.L., Tchoukalova Y., Tam C.S. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes Care. 2014;37:2789–2797. doi: 10.2337/dc14-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitman M.L. Metabolic lessons from genetically lean mice. Annu Rev Nutr. 2002;22:459–482. doi: 10.1146/annurev.nutr.22.010402.102849. [DOI] [PubMed] [Google Scholar]
- 30.Thakur M.L., Sharma S., Kumar A. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223:507–511. doi: 10.1016/j.atherosclerosis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Mazurek T., Zhang L., Zalewski A. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 32.Dubois S.G., Heilbronn L.K., Smith S.R. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity. 2006;14:1543–1552. doi: 10.1038/oby.2006.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taegtmeyer H., McNulty P., Young M.E. Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q.J., Holland W.L., Wilson L. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poornima I.G., Parikh P., Shannon R.P. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 36.Greenstein A.S., Khavandi K., Withers S.B. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 37.Granér M., Siren R., Nyman K. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98:1189–1197. doi: 10.1210/jc.2012-3190. [DOI] [PubMed] [Google Scholar]
- 38.Rijzewijk L.J., van der Meer R.W., Smit J.W.A. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 39.McGavock J.M., Lingvay I., Zib I. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 40.Pavlides M., Tunnicliffe E.M., Collier J. Multi-parametric MRI can diagnose steatohepatitis and cirrhosis in patients with NAFLD (abstr) Hepatology. 2014;60:728A. [Google Scholar]
- 41.Hoad C.L., Palanlyappan N., Kaye P. A study of T₁ relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 2015;28:706–714. doi: 10.1002/nbm.3299. [DOI] [PubMed] [Google Scholar]
- 42.Pavlides M., Banerjee R., Sellwood J. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308–315. doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szczepaniak L.S., Nurenberg P., Leonard D. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 44.de Marco R., Locatelli F., Zoppini G. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756–761. doi: 10.2337/diacare.22.5.756. [DOI] [PubMed] [Google Scholar]
- 45.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yilmaz Y., Kurt R., Yonal O. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211:182–186. doi: 10.1016/j.atherosclerosis.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 47.Kim D., Choi S.-Y., Park E.H. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goland S., Shimoni S., Zornitzki T. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–955. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 49.Hallsworth K., Hollingsworth K.G., Thoma C. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J Hepatol. 2013;58:757–762. doi: 10.1016/j.jhep.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Sebastiani G., Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682–3694. doi: 10.3748/wjg.v12.i23.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reaven G.M., Lithell H., Landsberg L. Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.