Abstract
Objective
The optimization of medical exposure is one of the major issues regarding radiation protection in the world, and The International Committee of Radiological Protection and the International Atomic Energy Agency recommend establishing diagnostic reference levels (DRLs) as tools for dose optimization. Therefore, the development of DRLs based on the latest survey has been required for nuclear medicine-related societies and organizations. This prompted us to conduct a nationwide survey on the actual administered radioactivity to adults for the purpose of developing DRLs in nuclear medicine.
Methods
A nationwide survey was conducted from November 25, 2014 to January 16, 2015. The questionnaire was sent to all of the 1249 nuclear medicine facilities in Japan, and the responses were collected on a website using an answered form.
Results
Responses were obtained from 516 facilities, for a response rate of 41 %. 75th percentile of 99mTc-MDP and 99mTc-HMDP: bone scintigraphy, 99mTc-HM-PAO, 99mTc-ECD and 123I-IMP: cerebral blood flow scintigraphy, 99mTc-Tetrofosmin, 99mTc-MIBI and 201Tl-Cl; myocardial perfusion scintigraphy and 18F-FDG: oncology PET (in-house-produced or delivery) in representative diagnostic nuclear medicine scans were 932, 937, 763, 775, 200, 831, 818, 180, 235 and 252, respectively. More than 90 % of the facilities were within the range of 50 % from the median of these survey results in representative diagnostic nuclear medicine facilities in Japan. Responses of the administered radioactivities recommended by the package insert, texts and guidelines such as 740 MBq (99mTc-MDP and 99mTc-HMDP: bone scintigraphy), 740 MBq (99mTc-ECD and 99mTc-HM-PAO: cerebral blood flow scintigraphy) and 740 MBq (99mTc-Tetrofosmin and 99mTc-MIBI: myocardial perfusion scintigraphy), etc. were numerous. The administered activity of many radiopharmaceuticals of bone scintigraphy (99mTc-MDP and 99mTc-HMDP), cerebral blood flow scintigraphy (99mTc-HM-PAO) and myocardial perfusion scintigraphy (99mTc-Tetrofosmin and 99mTc-MIBI), etc. were within the range of the EU DRLs and almost none of the administered radioactivity in Japan exceeded the upper limit of SNMMI standard administered radioactivity.
Conclusions
This survey indicated that the administered radioactivity in diagnostic nuclear medicine in Japan had been in the convergence zone and nuclear medicine facilities in Japan show a strong tendency to adhere to the texts and guidelines. Furthermore, the administered radioactivities in Japan were within the range of variation of the EU and the SNMMI administered radioactivities.
Keywords: Survey, Diagnostic reference level, Radiopharmaceutical, Radioactivity, Optimization of dose
Introduction
The International Committee of Radiological Protection (ICRP) recommended three fundamental principles (justification, optimization of protection, and application of dose limits) for radiation protection. It should be noted that with regard to medical exposure of patients, it is not appropriate to apply dose limits or dose constraints, because such limits would often do more harm than good [1, 2]. Therefore, the justification and optimization of protection are very important in clinical practice. However, with the development of radiation medical technology increases in the medical exposure dose are of concern. The optimization of medical exposure is one of the major issues regarding radiation protection in the world, and the ICRP and the International Atomic Energy Agency (IAEA) recommended establishing diagnostic reference levels (DRLs) as tools for dose optimization [3, 4]. In Europe, the European Union (EU) required establishment of DRLs by Council Directive 97/43/Euratom in 1996 [5]. It is suggested that DRLs should be set by countries, regions, academic societies or associations and they have been defined in Europe and North America [6–12]. On the other hand, in Japan the Japanese Society of Nuclear Medicine (JSNM) or other research groups have recommended the standard administration radioactivity dose [13, 14]. The Japan Association of Radiological Technologists (JART) recommended the reduction target dose [15, 16] and the JART conducted a nationwide survey of radiopharmaceutical doses [17]. Unfortunately this survey was not strictly limited to “actual” administered doses but included radioactivity doses determined by the time and date of assay of radiopharmaceuticals. Until 2015, neither a nationwide survey of “actual” administered doses had been conducted nor had DRLs been proposed by any nuclear medicine-related societies or organizations.
Concerning pediatric nuclear medicine, the European Association of Nuclear Medicine (EANM) dosage card has been proposed and developed by the Pediatric Task Group EANM in Europe [18–21] and consensus guidelines have been proposed and developed by the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in North America [22–26]. In 2014, the Japanese consensus guidelines for pediatric nuclear medicine were provided by JSNM in Japan [27].
The Japan Network for Research and Information on Medical Exposures (J-RIME) was established in 2010 with the cooperation of related academic societies [28]. The J-RIME decided to establish the first DRLs (Japan DRLs) of common modality as all medical radiation-related societies and organizations at the annual meeting held in 2013. Therefore, the establishment of DRLs based on the latest survey results was required by nuclear medicine-related societies and organizations. This survey was performed voluntarily by medical radiation-related societies and organizations but was not forced by national offices.
The JSNM and JSNMT conducted a nationwide survey on the actual administered radioactivity in adults for the purpose of establishing DRL in nuclear medicine.
In Japan there is a unique system for delivered radiopharmaceuticals. When radiopharmaceuticals are provided from radiopharmaceutical manufacturers to nuclear medicine facilities, the radioactivity dose has been determined by the time and date of assay of radiopharmaceuticals. For example, in the case of 99mTc and 123I agents, the radiopharmaceutical to be delivered to the nuclear medicine facility has been assayed as the assay radioactivity (radioactivity at 12 am) of the delivery date (examination date). In addition, in the case of 201Tl and 67Ga agents, radioactivity in the two days after the delivery date is delivered (radioactivity at the delivery day is about 1.6 times the assay radioactivity). That is, the assay radioactivity does not actually mean the true administered radioactivity.
Methods
Distribution, collection, and contents of the questionnaire
A nationwide survey on the actual administered radioactivity of adults for the purpose of providing DRLs in nuclear medicine was conducted from November 25, 2014 to January 16, 2015. The questionnaire was sent to all 1249 facilities where nuclear medicine examinations are performed in Japan, and the responses were sent to a website.
The questionnaire included items such as the average administered radioactivity dose of an adult for each diagnostic nuclear medicine examination, number of scanners, number of staff members, number of board certified nuclear medicine physicians and nuclear medicine radiological technicians.
How to calculate or evaluate the average administered radioactivity in each facility
The average administered radioactivity was obtained from the responses following this questionnaire.
The average value of the actually measured doses at the administered time or the average value of the assay dose that was corrected for the administered time.
The average administered radioactivity per week or the average administered radioactivity of several dozen times.
When the administered time is set at the facility, the average dose at that time.
The target administered radioactivity.
In the case of rare nuclear medicine examinations, the average administered radioactivity for several months or 1 year, or, the administered radioactivity in standard procedures.
For positron emission tomography (PET), the above 2 or 3 are used as a reference. The estimated radioactivity when using an automatic injecting machine for 18F-FDG.
When calculating the average doses, responses that appeared clearly erroneous were excluded.
Results and discussion
Response rate and the distribution of administered radioactivity
Replies were obtained from 516 facilities (response rate 41 %). The average, 75th, 80th and 90th percentile of each administered radioactivity are shown in Table 1.
Table 1.
Nationwide survey results and diagnostic reference levels (DRLs) in Japan
| Procedure and radiopharmaceutical | Average dosage in % value of nationwide survey results (MBq) | DRLs (MBq)a | ||
|---|---|---|---|---|
| 75 % | 80 % | 90 % | ||
| Bone: 99mTc-MDP | 932 | 962 | 1045 | 950 |
| Bone: 99mTc-HMDP | 937 | 963 | 1045 | 950 |
| Bone marrow: 111I n-Cl | 125 | 125 | 125 | 120 |
| Cerebral blood flow: 99mTc-HM-PAO (rest or stress) | 763 | 800 | 932 | 800 |
| Cerebral blood flow: 99mTc-HM-PAO (rest and stress) | 1155 | 1280 | 1464 | 1200 |
| Cerebral blood flow: 99mTc-ECD (rest or stress) | 775 | 800 | 848 | 800 |
| Cerebral blood flow: 99mTc-ECD (rest and stress) | 1007 | 1068 | 1130 | 1100 |
| Cerebral blood flow: 123I-IMP (rest or stress) | 200 | 211 | 236 | 200 |
| Cerebral blood flow: 123I-IMP (rest and stress) | 287 | 310 | 340 | 300 |
| Cerebral blood flow: Iomazenil (123I) | 193 | 195 | 244 | 200 |
| Dopamine transporter: Ioflupane (123I) | 186 | 189 | 195 | 190 |
| Cisternography: 111I n-DTPA | 63 | 63 | 72 | 70 |
| Thyroid imaging: 123I-NaI | 9 | 9 | 13 | 10 |
| Thyroid imaging: 99mTc-pertechnetate | 261 | 370 | 370 | 300 |
| Parathyroid: 201Tl-Cl | 120 | 120 | 175 | 120 |
| Parathyroid: 99mTc-pertechnetate | 300 | 370 | 391 | 300 |
| Parathyroid: 99mTc-MIBI | 784 | 824 | 848 | 800 |
| Lung ventilation: 81mKgas | 185 | 185 | 288 | 200 |
| Lung ventilation: 133Xe gas | 468 | 480 | 489 | 480 |
| Lung perfusion: 99mTc-MAA | 260 | 261 | 370 | 260 |
| Venography: 99mTc-MAA | 459 | 555 | 740 | 500 |
| Liver and spleen: 99mTc-phytate | 185 | 197 | 228 | 200 |
| Liver function: 99mTc-GSA | 251 | 260 | 261 | 260 |
| Hepatobiliary: 99mTc-PMT | 252 | 260 | 261 | 260 |
| Liver and spleen: 99mTc-Sn colloid | 157 | 185 | 185 | 180 |
| Myocardial perfusion: 201Tl-Cl | 180 | 180 | 196 | 180 |
| Myocardial perfusion: 99mTc-tetrofosmin (rest or stress) | 831 | 880 | 951 | 900 |
| Myocardial perfusion: 99mTc-tetrofosmin (rest and stress) | 1110 | 1130 | 1247 | 1200 |
| Myocardial perfusion: 99mTc-MIBI (rest or stress) | 818 | 848 | 900 | 900 |
| Myocardial perfusion: 99mTc-MIBI (rest and stress) | 1110 | 1125 | 1221 | 1200 |
| Myocardial fatty acid metabolism: 123I-BMIPP | 130 | 130 | 159 | 130 |
| Cardiac sympathetic nerve imaging: 123I-MIBG | 129 | 130 | 130 | 130 |
| Cardiac blood pool: 99mTc-HSA | 931 | 932 | 1045 | 1000 |
| Cardiac blood pool: 99mTc-HSA-D | 944 | 997 | 1045 | 1000 |
| Myocardial infarction: 99mTc-PYP | 750 | 925 | 1001 | 800 |
| Salivary gland: 99mTc-pertechnetate | 370 | 370 | 466 | 370 |
| Meckel’s diverticulum: 99mTc-pertechnetate | 466 | 523 | 740 | 500 |
| Gastrointestinal bleeding: 99mTc-HSA-D | 1036 | 1045 | 1046 | 1040 |
| Renal imaging (static): 99mTc-DMSA | 210 | 230 | 261 | 210 |
| Renal imaging (dynamic): 99mTc-MAG3 | 390 | 400 | 424 | 400 |
| Renal imaging (dynamic): 99mTc-DTPA | 380 | 400 | 502 | 400 |
| Adrenal cortex: 131I-Adosterol | 44 | 44 | 44 | 44 |
| Adrenal medulla: 131I-MIBG | 40 | 40 | 48 | 45 |
| Adrenal medulla: 123I-MIBG | 130 | 130 | 170 | 130 |
| Tumor: 201Tl-Cl | 178 | 180 | 180 | 180 |
| Tumor and inflammation: 67Ga-citrate | 174 | 174 | 208 | 200 |
| Lymphatic system: 99mTc-HSA-D (not covered with health insurance) | 928 | 932 | 1045 | 950 |
| Sentinel lymph node: 99mTc colloid | 111 | 111 | 156 | 120 |
| Sentinel lymph node: 99mTc-phytate | 93 | 105 | 127 | 120 |
| RI angiography: 99mTc-HSA-D | 943 | 987 | 1046 | 1000 |
| Tumor: 18F-FDG (in-house-produced) | 235 | 240 | 260 | 240 |
| Tumor: 18F-FDG (delivery) | 252 | 260 | 280 | 240 |
| Brain: 18F-FDG (in-house-produced) | 227 | 233 | 248 | 240 |
| Brain: 18F-FDG (delivery) | 255 | 259 | 295 | 240 |
| 15O-CO2 gas: 2D | 7500 | 7700 | 8100 | 8000 |
| 15O-O2 gas: 2D | 4500 | 5400 | 8360 | 6000 |
| 15O-CO gas: 2D | 3000 | 3000 | 3800 | 3000 |
| 15O-CO2 gas: 3D | 2888 | 2910 | 2955 | 2900 |
| 15O-O2 gas: 3D | 6600 | 7300 | 7400 | 7000 |
| 15O-CO gas: 3D | 7125 | 7500 | 7750 | 7500 |
| Myocardial metabolism: 18F-FDG (in-house-produced) | 221 | 223 | 236 | 240 |
| Myocardial metabolism: 18F-FDG (delivery) | 251 | 258 | 287 | 240 |
| Myocardial perfusion: 13N-NH3 | 718 | – | 740 | 720 |
aAdult dosage (MBq)
The 75th percentile of 99mTc-MDP, 99mTc-HMDP (bone scintigraphy), 99mTc-HM-PAO, 99mTc-ECD, 123I-IMP (cerebral blood flow scintigraphy), 99mTc-Tetrofosmin, 99mTc-MIBI, 201Tl-Cl (myocardial perfusion scintigraphy) and 18F-FDG for oncology (in-house-produced and delivery) administered radioactivity were 932, 937, 763, 775, 200, 831, 818, 180, 235 and 252, respectively.
Distribution of administered radioactivity in a representative diagnostic nuclear medicine examination
The administered radioactivity distributions of bone scintigraphy, cerebral blood flow scintigraphy, myocardial perfusion scintigraphy for single photon emission computed tomography (SPECT) and 18F-fluorodeoxyglucose (FDG) tumor scintigraphy in PET are shown in Figs. 1, 2, 3, 4, 5, 6 and 7. It should be noted that in Figs. 1, 2, 4 and 6 the numbers of different response facilities are adjusted.
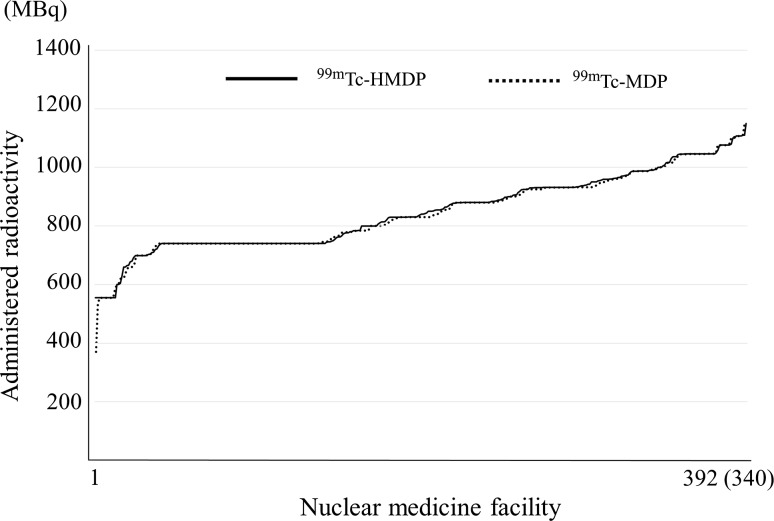
Fig. 1.
Bone: 99mTc-HMDP and 99mTc-MDP. Note: They are arranged in decreasing order to separate the nuclear medicine facilities with more from those with less radioactivity. Numbers responding for 99mTc-HMDP and 99mTc-MDP were 392 and 340, respectively
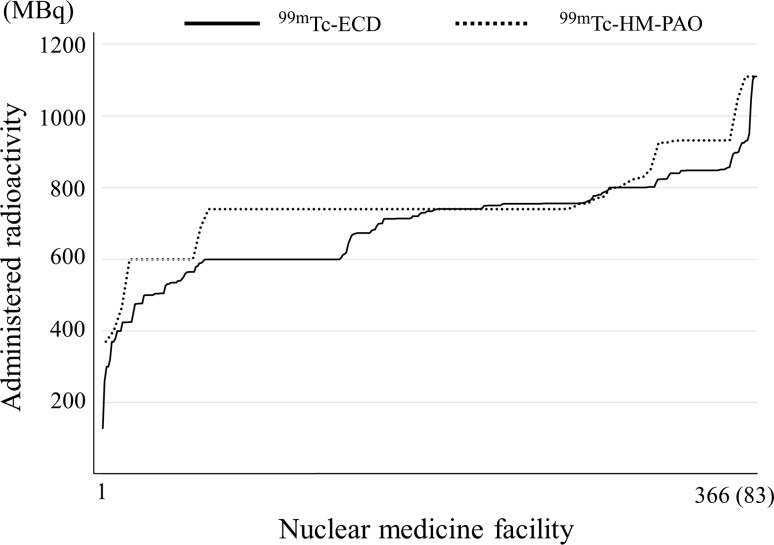
Fig. 2.
Cerebral blood flow: 99mTc-ECD and 99mTc-HM-PAO. Note: They are arranged in decreasing order to separate the nuclear medicine facilities with more from those with less radioactivity. Numbers responding for 99mTc-ECD and 99mTc-HM-PAO were 366 and 83, respectively
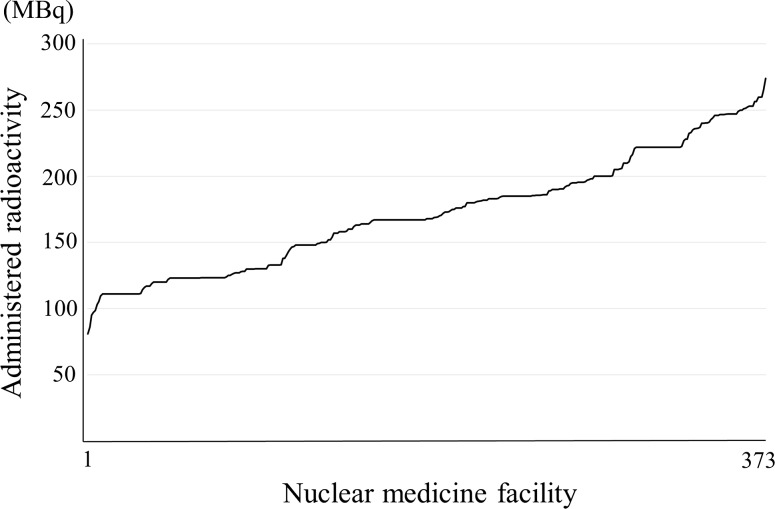
Fig. 3.
Cerebral blood flow: 123I-IMP. Note: They are arranged in decreasing order to separate the nuclear medicine facilities with more from those with less radioactivity. Number responding was 373
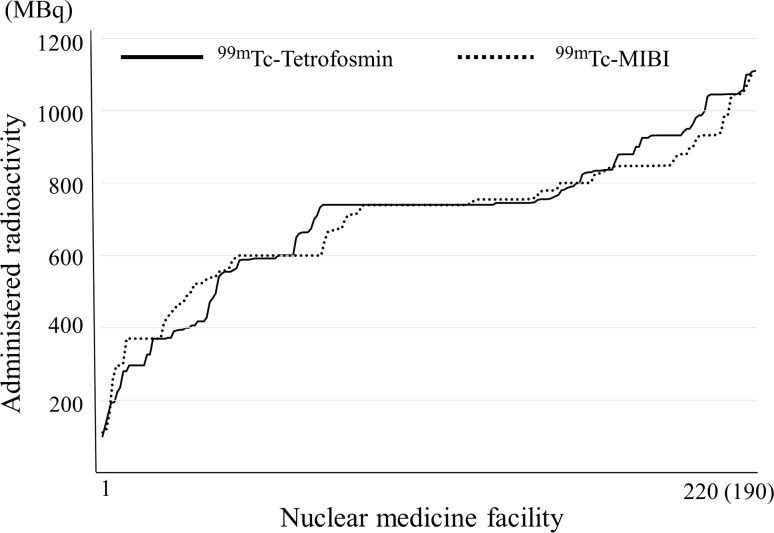
Fig. 4.
Myocardial perfusion: 99mTc-Tetrofosmin and 99mTc-MIBI. Note: They are arranged in decreasing order to separate the nuclear medicine facilities with more from those with less radioactivity. Numbers responding for 99mTc-Tetrofosmin and 99mTc-MIBI were 220 and 190, respectively
Fig. 5.
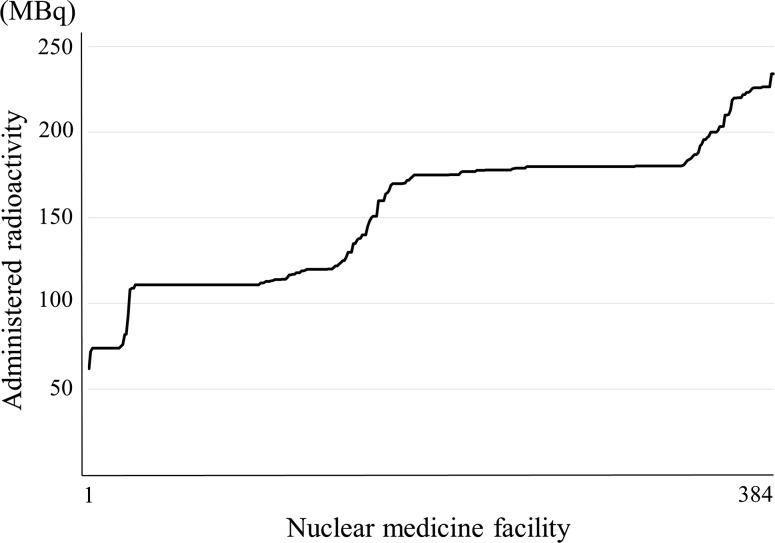
Myocardial perfusion: 201Tl-Cl. Note: They are arranged in decreasing order to separate the nuclear medicine facilities with more from those with less radioactivity. Number responding was 384
Fig. 6.
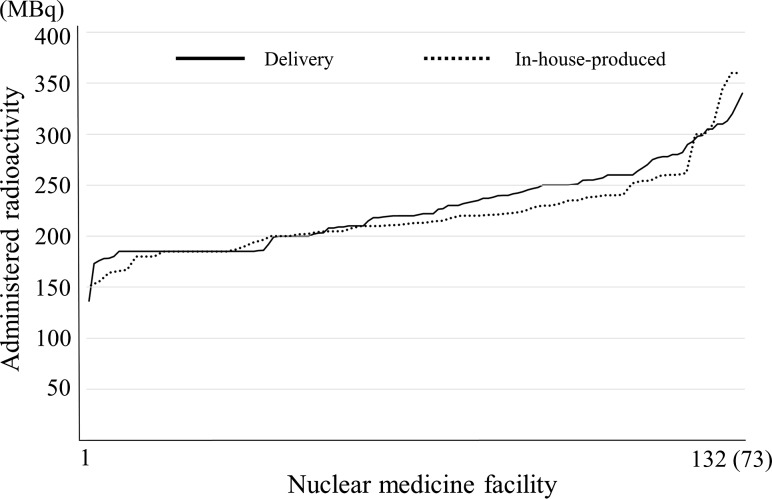
Tumor: 18F-FDG. Note: They are arranged in decreasing order to separate the nuclear medicine facility with more from those with less radioactivity. Numbers of delivery and in-house-produced were 132 and 73, respectively
Fig. 7.
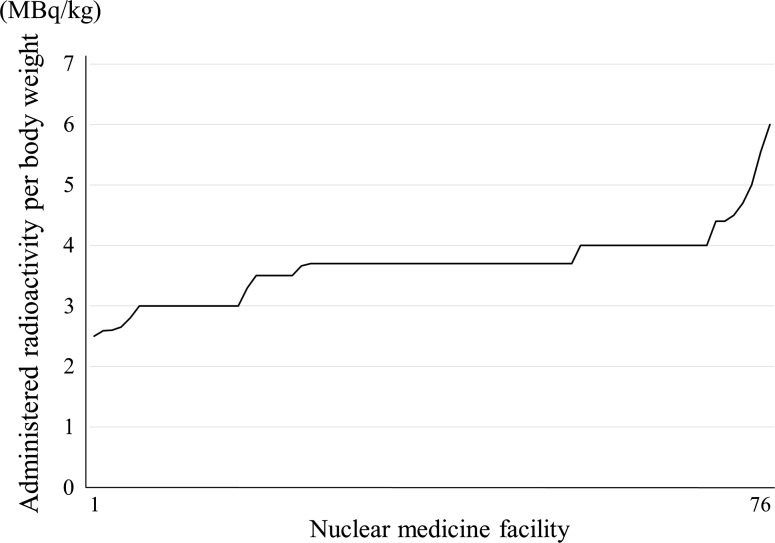
Tumor: 18F-FDG, administered activity per body weight. Note: They are arranged in decreasing order to separate the nuclear medicine facilities with more from those with less radioactivity. Number responding was 76
Figure 1 shows the administered radioactivity distribution of 99mTc-methylene diphosphonate (MDP) and 99mTc-Hydroxymethylene diphosphonate (HMDP). In Japan, 99mTc-MDP and 99mTc-HMDP have been used in bone scintigraphy as radiopharmaceuticals and there are two methods of on-site preparation of kits and ready-to-use radiopharmaceuticals. Two manufacturers have provided radiopharmaceuticals for bone scintigraphy. One has 555 and 740 MBq and the other has 370, 555, 740 and 925 MBq as assay radioactivity for one patient. For the present survey, information regarding whether kits were prepared on-site or ready-to-use radiopharmaceuticals was not obtained. The administered radioactivity distribution of 99mTc-MDP and 99mTc-HMDP was almost the same, and, the numbers of 740 MBq in both agents were the highest because administration of radioactivity of 555–740 MBq is recommended by the package insert, texts and guidelines as a standard administration activity in Japan. The percentages of response rates in the range of 740 MBq ± 5 % of 99mTc-MDP and 99mTc-HMDP were 30 and 31 %, respectively. The latter is around 930 MBq because the dose of 930 MBq corresponds to the case of administration of 740 MBq (assay activity at 12 am) at 10 am. Two radiopharmaceuticals for bone scintigraphy are recommended to be scanned from 2 to 3 h after administration. This may reflect the reality that many nuclear facilities are imaging at 1 pm after administration at 10 am using an assay radioactivity of 740 MBq (ready-to-use radiopharmaceuticals).
Figure 2 shows the distribution of 99mTc-hexamethylpropylene amine oxime (HM-PAO) and 99mTc-ethyl cysteinate dimer (ECD). In Japan, 99mTc-HM-PAO and 99mTc-ECD are used for cerebral blood flow scintigraphy of 99mTc agents as a radiopharmaceutical. 99mTc-HM-PAO is used only by on-site preparation of kits and 99mTc-ECD either by on-site preparation of kits or ready-to-use radiopharmaceuticals. The assay radioactivities of 99mTc-ECD are 400 and 600 MBq for one patient. Concerning 99mTc-HM-PAO, the number of the responses of 740 MBq was the highest because administration radioactivity of 370–740 MBq is recommended by the package insert, texts and guidelines as a standard administration radioactivity in Japan. For 99mTc-ECD, the number of responses of around 740 MBq was the most because an administration dose of around 370–740 MBq is highest by the package insert, texts and guidelines as a standard administration dose as well as 99mTc-ECD. The percentages of response rates in the range of 740 MBq ± 5 % of 99mTc-HM-PAO and 99mTc-ECD were 61 and 33 %, respectively.
Figure 3 shows the results of the distribution of N-isopropyl-p-[123I]iodoamphetamine (IMP). In Japan, for cerebral blood flow scintigraphy 123I-IMP is provided by only delivery. 123I-IMP has more kinds of assay radioactivities than other radiopharmaceuticals. One manufacturer has 111, 148, 167, 185 and 222 MBq and another has 111, 167 and 222 MBq as assay radioactivity for one patient. In addition, for 123I-IMP, a wide range of administration doses of 111–222 MBq is recommended by the package insert, texts and guidelines as a standard administration dose. In particular, a wide range of administration radioactivity (37–222 MBq) is recommended in the package insert. Thus, the distribution of radioactivity of 123I-IMP is scattered, which may reflect the various uses to which it is put at individual facilities. The response rate in the range of 111 MBq ± 5 % of 123I-IMP was 7 %.
Figure 4 shows the distribution of 99mTc-Tetrofosmin and 99mTc-hexakis-2-methoxyisobutylisonitrile (MIBI). In Japan, 99mTc-Tetrofosmin and 99mTc-MIBI have been used for myocardial perfusion scintigraphy as 99mTc agents, and, both agents have two methods of on-site preparation of kits and ready-to-use radiopharmaceuticals. 99mTc-Tetrofosmin has 296, 592 and 740 MBq and 99mTc-MIBI has 370, 600 and 740 MBq for one patient as the assay radioactivity. The response of around 740 MBq was the most common because an administration dose of 370–740 MBq is recommended by the package insert, texts and guidelines as a standard administration dose. The response rates in the range of 740 MBq ± 5 % of 99mTc-Tetrofosmin and 99mTc-MIBI were 37 and 30 %, respectively.
Figure 5 shows the distribution of 201Tl-Cl. In Japan, 201Tl-Cl for myocardial perfusion scintigraphy has been made available only by delivery. The assay radioactivities of 201Tl-Cl are 74, 11, 148 MBq for one patient provided by two manufacturers. The responses of 111 and 180 MBq were the most common. In Japan the manufacturers usually provide 201Tl-Cl to the nuclear medicine facility within 2 days before the assay date; therefore, 180 MBq corresponds to the radioactivity 2 days before the assay radioactivity 111 MBq. The administration radioactivity of around 74–111 MBq is recommended by the package insert, texts and guidelines as a standard administration activity for 201Tl-Cl. This study indicated that many nuclear medicine facilities administered the assay radioactivity 111 MBq for one patient in Japan (actual administered radioactivity is 180 MBq). The response rate in the range of 180 MBq ± 5 % of 201Tl-Cl was 43 %.
The distribution of administered radioactivity for 18F-FDG oncology PET is shown in Fig. 6 and the responses of 185 MBq were the greatest. In Japan, nuclear medicine facilities have two methods, in-housed-produced and delivery for 18F-FDG oncology PET. Provided manufacture of 18F-FDG is one and it has an assay radioactivity of only 185 MBq for one patient. For these reasons, it is presumed that the responses of 185 MBq were diverse. The response rate in the range of 185 MBq ± 5 % of 18F-FDG in-housed-produced and delivery were 19 and 27 %, respectively. In addition, the administered radioactivity per body weight (MBq/kg) was also investigated (Fig. 7). However, whether in-housed-produced or delivery was not distinguished by the survey items. An administered radioactivity per body weight of 2–5 MBq/kg (three dimensional collection) is recommended by the guidelines [29] and it was found that most of the facilities administered within the recommended radioactivity doses per body weight. Furthermore, the numbers of responses of 3.0, 3.7 and 4.0 MBq/kg were the highest, and the administered radioactivity dose per body weight was considered is be determined in accordance with the guidelines.
This survey reveals that many nuclear facilities determined the administered radioactivity dose according to the package insert, texts and guidelines.
Comparison with EU and North America
Basically DRLs are determined based on 75th percentile of the survey results. To compare the administered radioactivity between Japan and EU, a summary of Japanese and EU DRLs for diagnostic nuclear medicine is shown in Table 2 following a list of the EU DRLs [9]. Many DRL doses of radiopharmaceuticals: bone scintigraphy (99mTc-MDP and 99mTc-HMDP), cerebral blood flow scintigraphy (99mTc-HM-PAO) and myocardial perfusion scintigraphy (99mTc-Tetrofosmin and 99mTc-MIBI), etc. were within the range of the EU DRLs. Concerning 201Tl-Cl (myocardial perfusion scintigraphy), Japan DRL 180 MBq exceeds the range of the EU DRL (75–150 MBq). For 99mTc-pertechnetate (thyroid scintigraphy), Japan DRL 300 MBq exceeded the range of the EU DRLs (75–222 MBq). However, in the 18F-FDG for oncology PET and 123I-NaI for thyroid scintigraphy, Japan DRLs were at the lowest level in the range of the EU DRLs. These variations reflect the situation of each country, and so it is not considered that Japan DRLs are particularly high as compared with those of EU. Next, the results of this study were compared with SNMMI standard administered radioactivity in representative diagnostic nuclear medicine procedures: the upper limit of SNMMI standard administration radioactivity (bone scintigraphy: 1110 MBq [30], cerebral blood flow scintigraphy: 1110 MBq [31], myocardial perfusion 99mTc agents: 1110 MBq, 201Tl-Cl: 148 MBq [32], 18F-FDG oncology PET: 740 MBq [33]) following facilities were bone scintigraphy (99mTc-MDP: 99.4 %, 99mTc-HMDP: 99.7 %), cerebral blood flow scintigraphy (99mTc-HM-PAO: 100 %, 99mTc-ECD: 100 %), myocardial perfusion (99mTc-Tetrofosmin: 100 %, 99mTc-MIBI: 100 %, 201Tl-Cl: 41 %) and oncology PET (18F-FDG of both in-housed-produced and delivery: 100 %), respectively. Almost none of the administered radioactivity doses in Japan exceeded the upper limit of SNMMI standard administration radioactivity except for 201Tl-Cl for myocardial perfusion. In 18F-FDG for oncology PET, none of the doses at any of the facilities (100 %) exceeded the lower limit of SNMMI recommended administered radioactivity.
Table 2.
Comparisons of diagnostic reference levels (DRLs) between European Union (EU) and Japan
| Procedure and radiopharmaceutical | DRLs in EUa (MBq) | DRLs in Japan (MBq) | |
|---|---|---|---|
| Most common value | Range | ||
| Bone: 99mTc-MDP and HMDP | 600 | 500–1110 | 950 |
| Myocardial perfusion: 201Tl-Cl | 110 | 75–150 | 180 |
| Myocardial perfusion: 99mTc-tetrofosmin (rest or stress) | 1200 | 300–1500 | 900 |
| Myocardial perfusion: 99mTc-MIBI (rest or stress) | 1200 | 300–1480 | 900 |
| Tumor: 18F-FDG (in-housed-produced and delivery) | – | 200–400 | 240 |
| Thyroid: 99mTc-pertechnetate | 80 | 75–222 | 300 |
| Thyroid: 123I-NaI | 20 | 10–37 | 10 |
| Lung perfusion: 99mTc-MAA | 150 | 100–296 | 260 |
| Renal imaging (static): 99mTc-DMSA | – | 70–183 | 210 |
| Renal imaging (dynamic): 99mTc-MAG3 | 100 | 100–370 | 400 |
| Renal imaging (dynamic): 99mTc-DTPA | – | 150–540 | 400 |
| Parathyroid: 99mTc-MIBI | – | 400–900 | 800 |
| Cerebral blood flow: 99mTc-HM-PAO (rest or stress) | 500 | 500–1110 | 800 |
| Tumor and inflammation: 67Ga-citrate | – | 110–370 | 200 |
aEuropean Commission, 2010, DDM2 project report part 2: diagnostic reference levels (DRLs) in Europe
Convergence rate of the administered radioactivity, and the role of academic societies and experts
Table 3 shows the percentage of facilities that were within the range (25, 30 and 50 %) from the median of representative diagnostic nuclear medicine examinations in Japan. More than half of the facilities were within the range of 25 %. In addition, more than 90 % of the facilities were within the range of 50 %. In particular, the percentage of facilities was greater than 95 % in the range of 50 % in representative diagnostic nuclear medicine procedures except for the 99mTc-Tetrofosmin (myocardial perfusion scintigraphy) and 201Tl-Cl (myocardial perfusion scintigraphy). Our findings indicate that the administered radioactivity for diagnostic nuclear medicine has been in convergence zones in Japan.
Table 3.
Range from median value in this study results of representative administered radiopharmaceuticals
| Procedure and radiopharmaceutical |
|---|
| Bone: 99mTc-MDP |
| Bone: 99mTc-HMDP |
| Cerebral blood flow: 99mTc-HM-PAO |
| Cerebral blood flow: 99mTc-ECD |
| Cerebral blood flow: 123I-IMP |
| Myocardial perfusion: 99mTc-Tetrofosmin |
| Myocardial perfusion: 99mTc-MIBI |
| Myocardial perfusion: 201Tl-Cl |
| Tumor: 18F-FDG (in-house-produced) |
| Tumor: 18F-FDG (delivery) |
Essentially, optimization of the dose by DRL is performed at each facility, and is believed to lead to optimization in the whole country or region. However, nuclear medicine facilities have a strong tendency to adhere to the texts and guidelines in Japan. Therefore, in the optimization of radiopharmaceutical doses in Japan, a greater role of societies and organizations or experts is needed. As the finding of this study shows and the current state of Japan, to optimize radiopharmaceutical doses, Achievable Doses (ADs) [10, 12] might be useful, too.
Development of Japan DRLs
Based on the results of this study, a draft of Japan DRLs was prepared by the JSNM radiological protection committee.
Subsequently, it was approved by the JSNM board of directors, board of directors of the societies and organizations that performed the collaboration investigation, J-RIME general meeting and J-RIME constituent bodies, respectively, and Japan DRLs were officially published on June 7, 2015 [34].
Limitation
Although the actual administered radioactivity doses to a standard body weight patient were obtained, the weight of the patients was not specified. It is necessary to pay attention to determine doses for DRLs when the standard body weight is different, because it is likely that the standard weight differs between Westerners and Asians.
Conclusions
For the first time a nationwide survey by nuclear medicine-related societies and organizations for the development of the Japanese DRLs of nuclear medicine was conducted in Japan. This study demonstrated that the administered radioactivity in diagnostic nuclear medicine in Japan has been in the convergence zone. Nuclear medicine facilities in Japan show a strong tendency to adhere to the package insert, texts and guidelines. Furthermore, the Japan administered radioactivities were within the range of variation of the EU and the SNMMI administration radioactivities. Whether nuclear facilities can optimize the dose, or whether this is required, depends on the role of the academic societies and experts.
Acknowledgments
This study was performed with financial support from the Japanese Society of Nuclear Medicine (JSNM). This study was carried out by JSNM, JSNMT, Japanese Society of Radiological Technology and JART. We would like to sincerely thank everyone in the nuclear medicine facilities who responded to and with this survey. Prof. Fumio Shishido, a committee member of the Japanese Radiological Society, supported the preparation of Japan DRLs in Nuclear Medicine. Part of this study was presented at JSNM/JSNMT Joint Symposium, the 55th Annual Meeting of the JSNM on September 14, 2015.
References
- 1.International Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37(2–4). [DOI] [PubMed]
- 2.International Commission on Radiological Protection (ICRP). Radiological protection in medicine. ICRP Publication 105. Ann ICRP. 2007;37(6). [DOI] [PubMed]
- 3.International Commission on Radiological Protection (ICRP). Radiological protection and safety in medicine. ICRP Publication 73. Ann ICRP. 1996;26(2). [PubMed]
- 4.International Atomic Energy Agency (IAEA). International basic safety standards for protection against ionizing radiation and for the safety of radiation sources, IAEA Safety Series No. 115. 1996.
- 5.Communication from the Commission concerning the implementation of Council Directive 96/29/Euratom of 13 May 1996 laying down basic safety standards for the protection of the health of the workers and the general public against the dangers arising from ionising radiation. http://eur-lex.europa.eu/legal-content/EN/NOT/?uri=CELEX:51998DC0087. Accessed 23 Dec 2015.
- 6.European Commission . Radiation protection 109. Guidance on diagnostic reference levels (DRLs) for medical exposures. Luxembourg: OOPEC; 1999. [Google Scholar]
- 7.Mettler FAJ, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 8.Mettler FAJ, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 2009;253:520–531. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 9.European Commission. DDM2 project report part 2: diagnostic reference levels (DRLs) in Europe. 2010.
- 10.National Council on Radiation Protection and Measurements. Reference levels and achievable doses in medical and dental imaging: recommendations for the United States. NCRP Report No. 172. Bethesda: National Council on Radiation Protection and Measurements. 2012.
- 11.Roch P, Aubert B. French diagnostic reference levels in diagnostic radiology, computed tomography and nuclear medicine: 2004–2008 review. Radiat Prot Dosim. 2013;154:52–75. doi: 10.1093/rpd/ncs152. [DOI] [PubMed] [Google Scholar]
- 12.Alessio AM, Farrell MB, Fahey FH. Role of reference levels in nuclear medicine: a report of the SNMMI Dose Optimization Task Force. J Nucl Med. 2015;56:1960–1964. doi: 10.2967/jnumed.115.160861. [DOI] [PubMed] [Google Scholar]
- 13.Committee for evaluation of proper use of radiopharmaceuticals, Japanese Society of Nuclear Medicine. Development of guideline for proper use of radiopharmaceuticals. Contract research of related academic society on trial project implementation for promotion of proper use of radiopharmaceuticals by Ministry of Health, Labour and Welfare 2001–2002. 2002. www.jsnm.org/files/pdf/guideline/iyakuhin_gaidorain.pdf. Accessed 22 Dec 2015.
- 14.A new text book of clinical nuclear medicine. 3rd ed. Kanehara & Co., Ltd; 1999.
- 15.The Japanese Society of Radiological Technology and the Japan Association of Radiological Technologists. Medical exposure guideline committee Medical exposure guideline (reduction target dose) for patients. J JART. 2000;47:1694–1750. [Google Scholar]
- 16.Watanabe H. Medical exposure guideline 2006 (6; nuclear medicine) (reduction target dose in radiation practice) J JART. 2007;54:29–42. [Google Scholar]
- 17.Takahashi Y, Ono K, Kikuchi K, Saito K, Takase T, Morozumi K. Radiopharmaceutical administered doses for nuclear medicine. J JART. 2013;60:359–367. [Google Scholar]
- 18.Piepsz A, Hahn K, Roca I, Ciofetta G, Toth G, Gordon I, et al. A radiopharmaceuticals schedule for imaging in paediatrics. Paediatric Task Group European Association Nuclear Medicine. Eur J Nucl Med. 1990;17(3–4):127–129. doi: 10.1007/BF00811439. [DOI] [PubMed] [Google Scholar]
- 19.Smith T, Gordon I. An update of radiopharmaceutical schedules in children. Nucl Med Commun. 1998;19:1023–1036. doi: 10.1097/00006231-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs F, Thierens H, Piepsz A, Bacher K, Van de Wiele C, Ham H, et al. Optimised tracer-dependent dosage cards to obtain weight-independent effective doses. Eur J Nucl Med Mol Imaging. 2004;32:581–588. doi: 10.1007/s00259-004-1708-5. [DOI] [PubMed] [Google Scholar]
- 21.Lassmann M, Biassoni L, Monsieurs M, Franzius C, Jacobs F. The new EANM paediatric dosage card. Eur J Nucl Med Mol Imaging. 2007;34:769–798. doi: 10.1007/s00259-007-0370-0. [DOI] [PubMed] [Google Scholar]
- 22.Treves ST, Davis RT, Fahey FH. Administrated radiopharmaceutical doses in children: a survey of 13 pediatric hospitals in North America. J Nucl Med. 2008;49:1024–2027. doi: 10.2967/jnumed.107.049908. [DOI] [PubMed] [Google Scholar]
- 23.Gelfand MJ, Parisi MT, Treves ST. Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines. J Nucl Med. 2011;52:318–322. doi: 10.2967/jnumed.110.084327. [DOI] [PubMed] [Google Scholar]
- 24.Treves ST, Parisi MT, Gelfand MJ. Pediatric radiopharmaceutical doses: new guidelines. Radiology. 2011;261:347–349. doi: 10.1148/radiol.11110449. [DOI] [PubMed] [Google Scholar]
- 25.Fahey FH, Treves ST, Adelstein SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52:1240–1251. doi: 10.2967/jnumed.109.069609. [DOI] [PubMed] [Google Scholar]
- 26.Zanzonico P. Virtual reality for dose optimization in pediatric nuclear medicine: better than the real thing. J Nucl Med. 2011;52:1845–1847. doi: 10.2967/jnumed.111.097188. [DOI] [PubMed] [Google Scholar]
- 27.Koizumi K, Masaki H, Matsuda H, Uchiyama M, Okuno M, Oguma E, et al. Japanese consensus guidelines for pediatric nuclear medicine Part 1: pediatric radiopharmaceutical administered doses (JSNM pediatric dosage card). Part 2: Technical considerations for pediatric nuclear medicine imaging procedures. Ann Nucl Med. 2014;28:498–503. doi: 10.1007/s12149-014-0826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Japan Network for Research and Information on Medical Exposures (J-RIME). Newsletter No. 1. 2011. http://www.radher.jp/J-RIME/rimelite/rimeliteNo.1.pdf. Accessed 22 Dec 2015.
- 29.The Japanese Society of Nuclear Medicine. FDG-PET PET/CT clinical practice guideline. 2012. http://www.jsnm.org/files/pdf/guideline/2012/fdgpet_guideline2012_120912.pdf. Accessed 23 Dec 2015 (in Japanese).
- 30.Donohoe KJ, Brown ML, Collier BD, Carretta RF, Henkin RE, O’Mara RE, et al. Society of nuclear medicine procedure guideline for bone scintigraphy. 2003. http://snmmi.files.cms-plus.com/docs/pg_ch34_0403.pdf. Accessed 10 Jan 2016. [PubMed]
- 31.Donohoe KJ, Agrawal G, Frey KA, Gerbaudo VH, Mariani G, Nagel JS, et al. SNM practice guideline for brain death scintigraphy 2.0. 2012. http://snmmi.files.cms-plus.com/docs/Brain_Death_Scintigraphy_V2.0_Final_Draft_for_Approval.pdf. Accessed 10 Jan 2016. [DOI] [PubMed]
- 32.Strauss HW, Miller DD, Wittry MD, Cerqueira MD, Garcia EV, Iskandrian AS, et al. Procedure guideline for myocardial perfusion imaging 3.3. 2008. http://snmmi.files.cms-plus.com/docs/Myocardial%20Perfusion%20Imaging%203.3.pdf. Accessed 10 Jan 2016. [DOI] [PubMed]
- 33.Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0*. 2006. http://snmmi.files.cms-plus.com/docs/jnm30551_online.pdf. Accessed 10 Jan 2016. [PubMed]
- 34.Japan Network for Research and Information on Medical Exposures (J-RIME). Diagnostic reference levels based on latest surveys in Japan-Japan DRLs 2015. 2015. http://www.radher.jp/J-RIME/report/DRLhoukokusyoEng.pdf. Accessed 22 Dec 2015.