Abstract
Background
Bone-targeted agents (BTAs) used for the prevention of skeletal-related events (SREs) associated with metastatic bone disease possess different attributes that factor into treatment decisions.
Objective
The aim of this study was to evaluate preferences of patients, caregivers, and nurses for features of BTAs used to prevent SREs in patients with a self-reported physician diagnosis of bone metastasis from solid tumors.
Methods
Patients (n = 187), primary caregivers (n = 197), or nurses (n = 196) completed a web-enabled discrete-choice experiment (10-question survey) in which they chose between pairs of hypothetical profiles of BTAs. Each profile was defined by six key treatment attributes, including efficacy and safety (two each) and route/frequency of administration and cost (one each). The relative importance of treatment attributes and levels was estimated.
Results
The most important treatment attribute for patients and nurses was out-of-pocket cost, and for caregivers, treatment-related risk of renal impairment. Risk of renal impairment was the second most important attribute for patients and nurses, while time until first SRE was the third most important attribute for all respondents. For nurses, risk of osteonecrosis of the jaw was least important, and for patients and caregivers, mode of administration was least important.
Limitations
Respondents considered hypothetical medications; therefore, their decisions may not have the same consequences as actual decisions.
Conclusions
The perspectives of patients, caregivers, and nurses are integral when making treatment decisions about BTAs to prevent SREs associated with solid tumors. Identifying the relative importance of attributes of BTAs will aid in the proper selection of therapy in this setting, which may improve patient outcomes.
Electronic supplementary material
The online version of this article (doi:10.1007/s40271-015-0158-4) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Little is known about preferences and trade-offs among important stakeholders (ie, patients, nurses, and caregivers) for treatment attributes for bone-targeted agents used to manage metastatic bone disease. |
| A web-enabled discrete-choice experiment identified that the most important treatment attributes for patients and nurses were, in order of importance, out-of-pocket cost to patients, risk of renal impairment, and time until first skeletal-related event; for caregivers, the order of importance was risk of renal impairment, out-of-pocket cost to patients, and time until skeletal-related event. |
| Understanding the perspectives of patients, caregivers, and nurses would help optimize treatment selection of bone-targeted agents for metastatic bone disease and ultimately improve patient outcomes. |
Introduction
Metastatic bone disease is a chronic condition commonly associated with skeletal complications (e.g. spinal cord compression, radiation to the bone, surgery to bone, and pathologic fractures), collectively referred to as skeletal-related events (SREs) [1–3]. Approximately 70 % of patients with advanced breast or prostate cancer show evidence of bone metastasis, making bone the most common site of metastases for these cancers [1, 4–7]. In one study of women with metastatic breast cancer, >80 % of those with bone-limited metastases developed SREs [3]. SREs affect patients’ ability to perform basic daily tasks necessary for normal functioning, cause pain, and impair overall quality of life while increasing mortality [4, 8, 9]. Often patients experience depression and anxiety associated with SREs, especially those who receive radiation to the bone [8]. The economic burden placed on the healthcare system by SREs is also substantial, with the mean SRE-related cost (in US dollars) per patient estimated to range from approximately $12,000 to $14,000, and the costs per episode ranging from approximately $4000 to $64,000 [10–13].
Current treatment options for SREs include bisphosphonates, such as zoledronic acid [14, 15] and, more recently, the monoclonal antibody denosumab [16]. Although denosumab was shown to have superior efficacy versus zoledronic acid in delaying the onset of SREs [17–19], other factors such as type/frequency of treatment-emergent adverse events, mode of administration, and out-of-pocket costs may also factor into treatment decisions [20, 21]. The influence of these various attributes on treatment preference may differ between patients and those responsible for their care. Indeed, in the current era of shared decision making in the oncology setting, clinicians should ensure treatments deliver the best quality of life possible for their patients, with minimal post-treatment decisional regrets [22]. Evidence suggests patient preferences and beliefs about treatments for cancer are not necessarily in agreement with those of their physicians [23–27]. However, nurses may be better positioned to help patients and their caregivers with treatment decisions and improve communication with physicians and other members of a patient’s healthcare team [28]. Furthermore, oncology nurses work in a range of roles and settings, such as nurse-run clinics, chemotherapy prescreening, and management of symptoms such as pain [29]. Therefore it is also important to understand their treatment preferences. The discrete-choice experiment (DCE) is commonly used to assess preferences for medical interventions [30]. DCE data have previously been gathered to determine physicians’ perspectives [31], but complementary information from patients, caregivers, and nurses is lacking. The main objective of this study was to quantify preferences, using DCE methodology, of patients, caregivers, and nurses for attributes of treatment options for bone metastasis from solid tumors.
Methods
Objectives
The primary objective of this study was to quantify the preferences of patients, primary caregivers, and nurses in the US for attributes associated with treatments for the prevention of SREs in patients with bone metastases from solid tumors. A secondary objective was to estimate the proportion of participants who would choose given treatment profiles with characteristics similar to those of denosumab or zoledronic acid, the two main treatment options for SRE prevention in the US.
Study Sample
Patients included were ≥18 years of age and had a self-reported physician diagnosis of bone metastases from solid tumors. Caregivers were unpaid individuals taking care of patients with bone metastases from solid tumors. Nurses included in the study were involved in treating patients with bone metastases from solid tumors. Upon recruitment, all respondents provided online informed consent.
A survey research company (Harris Interactive, Inc., Rochester, NY, USA) recruited all of the respondents from existing online panels of patients who had previously been chosen and invited to participate in other health surveys. The survey research company coordinated the 25-min online survey between December 2012 and January 2013. The study materials were reviewed by the Office of Research Protection and Ethics at RTI International (Research Triangle Park, NC, USA) and were approved by their Institutional Review Board.
Discrete-Choice Experiments
DCEs compare and quantify preferences of various treatment characteristics/outcomes to the study population (patients, caregivers, and nurses in our study) [30, 32, 33]. There are a number of assumptions associated with DCEs [34]: (1) a medical treatment can be described by its characteristics or attributes (e.g. risk of adverse effects); (2) these attributes can be further specified by different levels of that characteristic (e.g. attribute levels for risk of adverse effects, 10 %, 20 %, 30 %, etc.); and (3) an individual’s preference for a treatment can be determined by the levels of those attributes (preferences for treatments were modeled as a function of the attribute levels). To evaluate the relative importance of treatment attributes and trade-offs in DCEs, respondents are presented with a series of questions and are asked to choose between two alternative hypothetical treatments with different combinations of attribute levels.
Survey Instrument
A web-enabled survey was used to elicit preferences and collect demographic information (e.g. age, sex, and employment status) and information about the respondents’ experience with bone metastases treatment. Two survey instrument versions were created that differed in the wording used to describe treatment attributes: a simplified version for patients and caregivers, and a version for nurses using standard medical terminology. The overall meaning of the attributes was the same for both versions. The study followed good practices for design and administration of DCEs [35]. The survey development process and survey questions are described in more detail in the appendix in the electronic supplementary material.
The six treatment attributes (Table 1) assessed in this survey were chosen after reviewing prescribing information and medical literature and consulting with clinical experts. The attributes included time until first SRE, time until 2-point increase in pain on the Brief Pain Inventory (BPI), risk of osteonecrosis of the jaw (ONJ) each year, risk of a 0.5-mg/dL increase in baseline creatinine each year (risk of renal impairment), mode of administration and frequency, and monthly out-of-pocket cost to the patient. Each of the attributes had three or four levels, and the levels were designed to encompass the range of outcomes observed in current clinical practices, as well as the range over which patients, caregivers, and nurses are willing to accept tradeoffs among attributes. Only attributes characterizing treatment risks (i.e. risk of ONJ and risk of increase in creatinine levels) were presented as probabilistic attributes because they are not expected to occur in all patients who start treatment. The choice scenarios in the DCE were prepared to address a specific preference-sensitive clinical decision, in which some degree of efficacy is obtained but a risk of adverse events is present, and stakeholders need to determine whether the possibility of facing adverse events is worth pursuing treatment. The choice scenario also excluded the possibility of opting out of treatments as a way to require that respondents provide information on the tradeoffs designed into the treatment profiles in each choice question. This approach maximizes the preference information collected with regard to attributes and attribute levels shown.
Table 1.
Attributes and levels for the choice questions
| Attributea | Levels |
|---|---|
| Time until first SRE (time until you/the patient have a complication from bone metastases), months | 28 |
| 18 | |
| 10 | |
| Time until a 2-point increase in pain on the BPI (time until your/the patient’s pain gets worse), months | 10 |
| 6 | |
| 3 | |
| Risk of ONJ each year (chance of a problem with your/the patient’s teeth and/or jawbone each year because of the medicine) | None |
| 1 of 100 (1 %) | |
| 5 of 100 (5 %) | |
| Risk of 0.5-mg/dL increase in baseline creatinine each year (chance of kidney problems because of the medicine each year) | None |
| 4 of 100 (4 %) | |
| 10 of 100 (10 %) | |
| Mode of administration and frequency (how you/the patient takes the medicine) | Injection every 4 weeks |
| 15-min infusion every 4 weeks | |
| 120-min infusion every 4 weeks | |
| Out-of-pocket cost to the patient each month (personal cost to you/the patient per month), $ | 25 |
| 75 | |
| 150 | |
| 330 |
BPI Brief Pain Inventory, ONJ osteonecrosis of the jaw, SRE skeletal-related event
aAttributes in parentheses represent those for patients and caregivers
The surveys were pretested to assess clarity and appropriateness of the descriptive information, as well as the relevance and thoroughness of the attributes and levels. The pretesting involved open-ended face-to-face or phone interviews with 15 patients, 11 caregivers, and 6 nurses in the US. The study team also included cheap-talk text to reduce the likelihood that respondents ignored the specific attribute levels presented to them in the choice questions [36, 37].
Respondents answered 10 choice questions, choosing between pairs of hypothetical medication profiles (Fig. 1). Patients were asked to choose a hypothetical treatment profile for themselves, and caregivers were asked to choose a hypothetical treatment profile for the patient they were caring for. Because nurses see patients at different stages of the disease in their practice, two profiles—one of a typical breast cancer patient and one of a typical prostate cancer patient—were provided to nurses, and they were asked to make hypothetical treatment decisions based on these profiles (electronic supplementary Table S1); descriptions were provided to nurses to help illustrate typical patients. This practice was intended to reduce bias from the nurses’ own experiences. A main-effects D-efficient experimental design was prepared with a commonly used algorithm in SAS version 9.3 (SAS Institute, Cary, NC, USA) and in accordance with good research practices to optimize the statistical properties of the design, given the number of attribute levels and the complexity of the task layout [38], and included four sets of nine unique choice questions and one repeated choice question (10 questions in total for each survey version). The total balanced the number of questions needed for an identifiable model of preferences and the number of questions most of our pretest participants stated they could answer reliably before the instrument became overly burdensome. Questions avoided implausible or dominated treatment profiles (treatments that were unambiguously worse than the alternatives), and all levels varied across attributes. Each respondent was randomly assigned to one set.
Fig. 1.
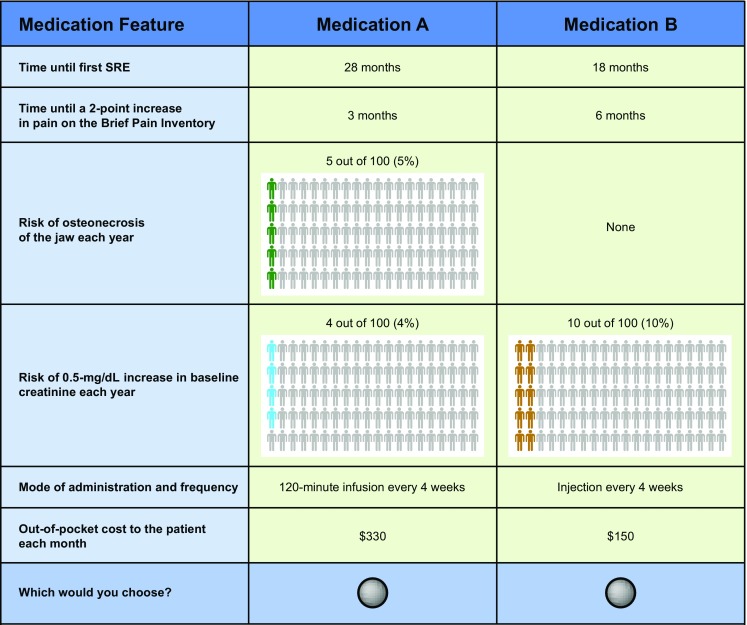
Hypothetical medication choice example. Comparison between two hypothetical pharmacologic therapies for SREs (medications A and B) presented to the nurses in the study. SRE skeletal-related event
Statistical Analysis
We reviewed time to completion of the survey and response variability as a way to gauge the validity of participants’ responses. Respondents who completed the survey in less than 6 min or those who always picked ‘medicine A’ or ‘medicine B’ in the choice questions were eliminated. We interpreted these responses as a strong indication that respondents were not paying attention to the information presented in the choice questions included in the survey.
Descriptive statistics, including respondents’ demographics and type of cancer (patients/caregivers), and clinical experience with bone metastasis (nurses) were reported. The respondents’ answers to the choice questions in the survey were modeled using a random parameters logit (RPL) model—a statistical model for examining discrete choices that provides estimates of mean preference weights (relative strength of preference or relative importance) for individual treatment attribute levels [39, 40].
Choice data from patients and caregivers were pooled to estimate a model that constrained preferences between the two groups to be the same for attributes for which no statistical differences were found between the two groups. Heterogeneity in the variability of responses between groups is known as scale heterogeneity and can limit the ability to pool data. We controlled for scale heterogeneity between patients and caregivers by estimating a group-specific random effect in the RPL model. Because logit models permit only the estimation of the variance of one group relative to that of another group, we normalized the variance of caregivers to 1 and estimated the variance of patients relative to the variance of caregivers. Choice data from nurses were analyzed in a separate model. All attribute levels were assumed to be normally distributed across respondents in each population. Other studies have used a similar modeling technique [32, 41].
Estimated preference weights were used to calculate the predicted proportion of participants who would choose given treatment profiles. By applying the preference weights (relative importance) to the attribute levels included in each profile, we were able to predict the proportion of respondents who would select one of several drug profiles as a way to gauge respondents’ preferences for bundles of attribute levels, not just the pairwise comparisons of specific attribute-level preferences that are possible via comparison of preference weights. As a way to make this bundled evaluation of relative preferences more meaningful, we evaluated the likelihood of choice for treatment options that resembled currently available treatments. The profiles of attributes for denosumab and zoledronic acid used for this assessment were derived from prescribing information [15, 16] and clinical trial data [42].
Results
Response Rate and Sample Characteristics
Information on the recruitment and disposition of respondents is presented in electronic supplementary Fig. S1. The number of patients, caregivers, and nurses responding to the initial invitation to participate was 2340/97,500 (2.4 %), 461/17,800 (2.6 %), and 425/14,570 (2.9 %), respectively. Of those eligible to participate, 87.0 % (200/230) of patients, 80.0 % (200/250) of caregivers, and 77.5 % (200/258) of nurses completed the survey. A total of 13 patients, three caregivers, and four nurses were excluded from the final analysis because they always chose the same answer in the choice questions (indicating a lack of attention to the choice questions), leaving totals of 187, 197, and 196 respondents in the analysis for the respective groups. The cooperation rate was 81 % (187/230) for patients, 76 % (196/258) for nurses, and 79 % (197/250) for caregivers.
Electronic supplementary Table S2 shows the demographic characteristics of the eligible patients and caregivers included in the analysis. The mean age of the patients was 43 years, 58 % were men, 44 % were employed, 34 % had breast cancer, 45 % were diagnosed with bone metastases <1 year before the study, and 75 % were currently taking treatment to delay complications of bone metastases. The mean age of caregivers was 36 years, 61 % were men, 67 % were employed, and 30 % were caring for a patient with breast cancer. Approximately 73 % of patients and 74 % of caregivers noted a skeletal complication secondary to metastases. Electronic supplementary Table S3 shows the demographic characteristics of the nurses included in the analysis. Fifty-five percent were 46 years of age or older, 80 % were registered nurses, 76 % were oncology nurses, 55 % were hospital-based staff, and 47 % treated more than 10 patients with bone metastases from solid tumors each week.
Relative Importance Estimates
The mean preference weights for all attribute levels are shown in Fig. 2 and electronic supplementary Table S4. Across the three populations, the mean preference weights were consistent with the natural ordering of the levels of the represented attribute (i.e. better clinical outcomes were preferred to worse clinical outcomes, lower out-of-pocket cost was preferred to higher out-of-pocket cost, etc.). The lines connecting the data points (mean preference weights) in Fig. 2 indicate the relative strength of the preference for the most- and least-preferred attribute levels. The greater the vertical distance between data points for a particular attribute, the greater the importance of having a treatment with that attribute over the attribute ranges and levels included in the survey. These results form the basis for the data in Table 2, which lists the attributes’ relative importance in decreasing order by respondent group. The order of importance varied by respondent type. The three most important treatment attributes for patients and nurses were out-of-pocket cost to patients, treatment-related risk of renal impairment, and how long treatment delays time to first SRE. For caregivers, the risk of treatment-related renal impairment was a more important treatment attribute than out-of-pocket cost to patients.
Fig. 2.
a Patient and caregiver and b nurse preference weights for treatment attributes. Vertical bars surrounding mean preference weight point estimates denote the 95 % CI. Nonoverlapping 95 % CIs indicate statistically different mean estimates for an attribute. SRE skeletal-related event, CI confidence interval
Table 2.
Attribute importance by sample based on the attribute levels considered in the study
| Importance rank | Patients | Mean importancea | Caregivers | Mean importancea | Nurses | Mean importancea |
|---|---|---|---|---|---|---|
| 1 (most) | Out-of-pocket cost to the patient each month | 10.00 | Risk of 0.5-mg/dL increase in baseline creatinine each year | 13.87 | Out-of-pocket cost to the patient each month | 10.00 |
| 2 | Risk of 0.5-mg/dL increase in baseline creatinine each year | 6.01 | Out-of-pocket cost to the patient each month | 10.00 | Risk of 0.5-mg/dL increase in baseline creatinine each year | 6.20 |
| 3 | Time until first SRE | 2.65 | Time until first SRE | 4.86 | Time until first SRE | 4.68 |
| 4 | Time until a 2-point increase in pain on the BPI | 1.69 | Time until a 2-point increase in pain on the BPI | 3.10 | Time until a 2-point increase in pain on the BPI | 3.65 |
| 5 | Risk of ONJ each year | 1.16 | Risk of ONJ each year | 2.13 | Mode of administration | 3.32 |
| 6 (least) | Mode of administration | 1.13 | Mode of administration | 2.08 | Risk of ONJ each year | 1.70 |
BPI Brief Pain Inventory, ONJ osteonecrosis of the jaw, SRE skeletal-related event
aThe numbers in the ranking table had to be rescaled to reflect the importance of each attribute relative to the overall importance of out-of-pocket cost (where the importance of increasing treatment from $25 to $330 is set to be 10). Without this rescaling, the ranking numbers cannot be compared across populations
The relative importance of changes among the intermediate levels of an attribute is also reflected in the data lines in Fig. 2 (i.e. the less vertical distance between the data points or mean preference weights, the less important a change in level for that attribute is for the respondent). For example, patients and caregivers perceived relatively little difference between treatments administered via a 15-min infusion every 4 weeks and treatments administered via 120-min infusion every 4 weeks (Fig. 2a). Similarly, nurses perceived no difference between treatments when the risk of ONJ was reduced from 1 % to no risk because nurses perceived no difference between these two ONJ risk levels (Fig. 2b). Additional information on the RPL model can be found in electronic supplementary Table S5.
Preference estimates from the DCE survey results (not including cost) and the profiles of actual product attributes were used to calculate the predicted proportion of participants who would choose a given treatment profile (respondents were not directly asked about their preference for existing treatments). Based on these preference estimates, the model predicted 75 % of patients, 78 % of caregivers, and 96 % of nurses would prefer a drug therapy with attributes similar to those of denosumab over a drug therapy with attributes similar to those of zoledronic acid (Table 3). Given that participants were not allowed to opt out of treatment in the choice questions, respondents were not allowed to reveal whether they preferred no treatment over the treatment alternatives in each choice question. For this reason, it is important to note that the predicted proportion of respondents who would select a given drug profile is only a measure of preference (i.e. likelihood of choice) and cannot be considered a demand analysis for any of these treatments.
Table 3.
Predicted choice probabilities
| Attribute | Characteristics similar to denosumaba | Characteristics similar to zoledronic acida |
|---|---|---|
| Time until first SRE, months | 27.7 | 19.5 |
| Time until worsening of pain, months | 6.6 | 4.7 |
| Risk of ONJ, % | 1.8 | 1.3 |
| Risk of renal impairment, % | 0 | 9.3 |
| Mode of administration | Injection every 4 weeks | 15-min infusion every 4 weeks |
| Predicted choice probabilitiesb, % | ||
| Patients | 74.7 | 25.3 |
| Caregivers | 77.7 | 22.3 |
| Nurses | 95.8 | 4.2 |
ONJ osteonecrosis of the jaw, SRE skeletal-related event
aValues derived from prescribing information for denosumab [16] and zoledronic acid [15]
bValues represent the predicted probability that each alternative with its associated attributes would be chosen if these profiles represented the only available treatment options
Discussion
The results of our analysis show that out-of-pocket cost of bone-targeted agents (BTAs) for bone metastases was an important concern for all groups of respondents. For patients and nurses, this was the most important attribute, whereas caregivers considered it the second most important attribute. This result has implications for the use of older therapies versus newer therapies that may have higher drug acquisition costs [20, 21]. It also underscores the importance of assistance programs that facilitate patient access to lower-cost medications. Improved communication regarding available programs is important to facilitate patients’ access to therapies. Patients and caregivers should be proactive in inquiring about these programs; however, the existence of such programs is not always apparent to patients and their caregivers. Furthermore, older patients may lack the knowledge or resources to search for such programs online or elsewhere [28]. Nurses often have more information than physicians regarding drug assistance programs and may also be more attuned to patients’ financial concerns and, thus, would be expected to play an important role in this area, especially with regard to educating patients and caregivers regarding program availability. In addition, the results of this study provide nurses with information regarding patients’ and caregivers’ treatment preferences with respect to bone-targeting agents and, because patients’ and nurses’ preferences aligned well in our study, increase their ability to provide patient-driven treatment decisions.
For patients and nurses, the second most important attribute after out-of-pocket cost was risk of renal impairment, whereas for caregivers the risk of renal impairment was the most important attribute. Results from a recent DCE that evaluated patients’ preferences for bone metastases treatment in France, Germany, and the UK also showed that time until first SRE and risk of renal impairment were important attributes for BTAs (out-of-pocket costs were not evaluated) [43]. This is consistent with the results for patients in our study in that risk of renal impairment and time until first SRE were the most important attributes after out-of-pocket costs. Another recent DCE conducted with US physicians also reported out-of-pocket costs as the most important attribute for BTAs in this setting, followed by time until first SRE and risk of renal impairment [31].
It is plausible to speculate that differences in the order of important treatment attributes amongst our study populations reflect different priorities—patients are directly affected by the reduced quality of life that accompanies SREs, and caregivers and nurses may be more concerned with avoiding treatment toxicities that may reduce survival. Indeed, data support that patients may prefer quality of life to added survival [41, 44, 45]. Recent data from a DCE in patients with advanced prostate cancer from the UK and Sweden showed more patients would choose to receive BTAs to delay bone metastases as the number of months’ delay of bone metastases increased and the associated risk of ONJ decreased [46]. However, a majority of patients (52 % for the UK and 60 % in Sweden) in that study would still choose a treatment that minimally delayed bone metastasis (by 5 months, the lowest-choice question level), even at a relatively high risk for ONJ (9 %; the highest-choice question) [46]. Moreover, 74 % of patients from the UK and 85 % of patients from Sweden indicated they would forego at least 3 months’ survival time to avoid an SRE [46]. Therefore, when making treatment decisions, healthcare providers cannot assume patients value survival above other considerations, underscoring the importance of eliciting treatment and outcome preferences from patients. Similarly, a recent DCE study calculated the willingness to pay (WTP) for various end-of-life treatments. The patients’ WTP to extend their life by up to 1 year was not greater than their WTP to avoid severe pain. In addition, caregivers had a higher WTP to extend the patient’s life [45].
This study also indicated that most patients, caregivers, and nurses preferred a hypothetical drug therapy with attributes similar to those of denosumab over a hypothetical drug therapy with attributes similar to those of zoledronic acid (based on five clinical attributes included in the study design, excluding out-of-pocket cost), in keeping with the preference indicated by the recent DCE of US physicians [31]. This treatment preference was largely driven by improved efficacy and reduced risk of renal toxicity attributes. Out-of-pocket costs were not included in the predicted choice probabilities assessment because, in practice, out-of-pocket costs vary by insurance coverage and use of patient assistance programs. In the recent abovementioned patient DCE study, more than 90 % of patients from France, Germany, and the UK also strongly preferred an agent with characteristics similar to those of denosumab over agents with characteristics similar to those of the bisphosphonates zoledronic acid, clodronate, and pamidronate [43], consistent with results for US patients in our study.
A number of study limitations should be considered. Because respondents were asked to consider hypothetical medications, their decisions may not have the same clinical, financial, and emotional consequences of actual decisions. We have attempted to minimize this possible bias by striving to make the hypothetical choice mimic, as closely as possible, real-world tradeoffs by verifying the attributes included in the DCE survey through open-ended interviews during testing. In addition, Internet surveys represent a convenience sample, and therefore they may not represent the general population. Furthermore, low response rates suggest that caution should be taken when generalizing these results to the population of patients, caregivers, and nurses who would conceivably be involved in the decisions evaluated in this study. Because demographic data for individuals not included in the final analysis were not collected, it cannot be determined whether the recruitment procedure introduced selection bias. However, the total number of participants was in line with our prespecified criteria.
Another general limitation of preference-elicitation studies, and DCEs among them, is that no power calculation can be conducted to determine minimum sample size for hypothesis testing without prior information on respondents’ expected preferences. Most published choice experiments have a sample size from 100 to 300 respondents [30]; however, minimum sample size depends on a number of criteria, including the question format, complexity of the choice task, desired precision of the results, and need to conduct subgroup analyses [47]. Moreover, although we did consult with clinical experts regarding the survey, we did not perform external validation; consequently, it is unknown to what extent our results agree with real-world choices. The preference comparisons presented here were aimed at evaluating the relative importance of the attributes and attribute levels in the choice questions to contrast each group’s perspective on treatments and their features rather than formally testing for consistency of preferences across groups.
The results of this study underscore the importance of establishing patient (and/or caregiver) treatment preferences and cost considerations, information that can help nurses and other clinicians facilitate communication between patients and providers. Although physicians are primary sources of information regarding bone metastases and associated treatments to patients with solid tumors, there is a gap between physician assumptions about patient knowledge of bone health and patient perceptions [27]. Physicians tend to overestimate patient education and understanding of the diagnosis and treatment explanations, and these discordances are associated with patient dissatisfaction with their interactions with physicians [48]. Patients and caregivers value family involvement in cancer treatment decision making, although there is often disagreement on who should take the decisional leadership [49]. Oncology nurses often have more opportunities to spend time with patients and their families than physicians do (e.g. during chemotherapy infusions and radiation treatments), and therefore are well positioned to elicit a patient’s preferred level of participation in decisions about treatment and to encourage patients to share their preferences with their physicians [50].
Conclusions
Our study showed patients, caregivers, and nurses generally had clear preferences for drug characteristics of BTAs used to manage metastatic bone disease. Improved understanding of specific treatment preferences and overall disease comprehension of patients and caregivers will help to identify potential gaps among important stakeholders involved in the treatment decision making, improve the transparency and openness of treatment decisions, and ultimately empower patients to participate in the decisions about their care, thereby improving treatment adherence and optimizing outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Rick Davis, MS, of Complete Healthcare Communications, LLC, whose work was funded by Amgen Inc., and Holly Zoog, PhD, and Lori Smette, PhD, of Amgen Inc. for assistance in the preparation of this manuscript.
Author contributions
Yi Qian, Guy Hechmati, Jorge Arellano, and A. Brett Hauber contributed to the concept and design of the study. Yi Qian, Jorge Arellano, A. Brett Hauber, Ateesha F. Mohamed, and Juan Marcos Gonzalez contributed to the collection and acquisition of patient data. Yi Qian, Guy Hechmati, Francesca Gatta, Jorge Arellano, A. Brett Hauber, Ateesha F. Mohamed, Juan Marcos Gonzalez, and Cynthia Campbell-Baird contributed to the analysis and interpretation of the data and were involved in drafting and revising the manuscript.
Compliance with Ethical Standards
Funding
Funding for this study was obtained from Amgen Inc., Thousand Oaks, CA, USA.
Conflict of interest
This study was conducted by RTI Health Solutions and was funded by Amgen Inc.
Brett Hauber and Juan Marcos Gonzalez are employees of RTI Health Solutions, an independent scientific research organization. Ateesha F. Mohamed was employed by RTI Health Solutions at the time of development of this manuscript, but is now currently employed by Bayer HealthCare Pharmaceutical Inc., Whippany, NJ, USA, and owns stock in Bayer AG. Cynthia Campbell-Baird has received speaking fees and reimbursement of travel and accommodation expenses from Amgen Inc. and Novartis. Yi Qian, Jorge Arellano, and Guy Hechmati are employees of Amgen Inc. and hold stock in Amgen Inc. Francesca Gatta is an employee of Amgen Inc. Stacey Harrelson reports no conflicts of interest.
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–1594. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 3.Domchek SM, Younger J, Finkelstein DM, Seiden MV. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer. 2000;89(2):363–368. doi: 10.1002/1097-0142(20000715)89:2<363::AID-CNCR22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64(24):9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 7.Chow E, Hoskin P, van der Linden Y, Bottomley A, Velikova G. Quality of life and symptom end points in palliative bone metastases trials. Clin Oncol (R Coll Radiol). 2006;18(1):67–69. doi: 10.1016/j.clon.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16(4):579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 9.Langer C, Hirsh V. Skeletal morbidity in lung cancer patients with bone metastases: demonstrating the need for early diagnosis and treatment with bisphosphonates. Lung Cancer. 2010;67(1):4–11. doi: 10.1016/j.lungcan.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67(5–6):390–396. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 11.Delea T, McKiernan J, Brandman J, Edelsberg J, Sung J, Raut M, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol. 2006;4(7):341–347. [PubMed] [Google Scholar]
- 12.Lage MJ, Barber BL, Harrison DJ, Jun S. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care. 2008;14(5):317–322. [PubMed] [Google Scholar]
- 13.Hagiwara M, Delea TE, Cong Z, Chung K. Utilization of intravenous bisphosphonates in patients with bone metastases secondary to breast, lung, or prostate cancer. Support Care Cancer. 2014;22(1):103–113. doi: 10.1007/s00520-013-1951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berenson JR. Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist. 2005;10(1):52–62. doi: 10.1634/theoncologist.10-1-52. [DOI] [PubMed] [Google Scholar]
- 15.Zometa®, zoledronic acid: prescribing information. East Hanover (NJ): Novartis Pharmaceutical Corp. 2014. Available at: https://www.pharma.us.novartis.com/product/pi/pdf/Zometa.pdf.
- 16.XGEVA®: denosumab: prescribing information. Thousand Oaks (CA): Amgen Inc. 2014. Available at: http://pi.amgen.com/united_states/xgeva/xgeva_pi.pdf.
- 17.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 20.Ford J, Cummins E, Sharma P, Elders A, Stewart F, Johnston R, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17(29):1–386. doi: 10.3310/hta17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo K, Lam K, Mittmann N, Konski A, Dennis K, Zeng L, et al. Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support Care Cancer. 2013;21(6):1785–1791. doi: 10.1007/s00520-013-1790-y. [DOI] [PubMed] [Google Scholar]
- 22.Aning JJ, Wassersug RJ, Goldenberg SL. Patient preference and the impact of decision-making aids on prostate cancer treatment choices and post-intervention regret. Curr Oncol. 2012;19(Suppl 3):S37–S44. doi: 10.3747/co.19.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emberton M. Medical treatment of benign prostatic hyperplasia: physician and patient preferences and satisfaction. Int J Clin Pract. 2010;64(10):1425–1435. doi: 10.1111/j.1742-1241.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 24.Muhlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–180. doi: 10.1007/s40258-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 25.Bruera E, Willey JS, Palmer JL, Rosales M. Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076–2080. doi: 10.1002/cncr.10393. [DOI] [PubMed] [Google Scholar]
- 26.Thiel FC, Schrauder MG, Fasching PA, Lohberg CR, Bani MR, Haberle L, et al. Shared decision-making in breast cancer: discrepancy between the treatment efficacy required by patients and by physicians. Breast Cancer Res Treat. 2012;135(3):811–820. doi: 10.1007/s10549-012-2218-y. [DOI] [PubMed] [Google Scholar]
- 27.Tripathy D, Durie BG, Mautner B, Ferenz KS, Moul JW. Awareness, concern, and communication between physicians and patients on bone health in cancer. Support Care Cancer. 2014;22(6):1601–1610. doi: 10.1007/s00520-014-2127-1. [DOI] [PubMed] [Google Scholar]
- 28.Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp KG. Physician, patient, and contextual factors affecting treatment decisions in older adults with cancer and models of decision making: a literature review. Oncol Nurs Forum. 2012;39(1):E70–E83. doi: 10.1188/12.ONF.E70-E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieger PT, Yarbro CH. Role of the oncology nurse. Holland-Frei Cancer Medicine. Hamilton: BC Decker; 2003.
- 30.Marshall D, Bridges JF, Hauber B, Cameron R, Donnalley L, Fyie K, et al. Conjoint analysis applications in health: how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–256. doi: 10.2165/11539650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Arellano J, Hauber AB, Mohamed AF, Gonzalez JM, Collins H, Hechmati G, et al. Physicians’ preferences for bone metastases drug therapy in the United States. Value Health. 2015;18(1):78–83. doi: 10.1016/j.jval.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Hauber AB, Arden NK, Mohamed AF, Johnson FR, Peloso PM, Watson DJ, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage. 2013;21(2):289–297. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed AF, Hauber AB, Neary MP. Patient benefit-risk preferences for targeted agents in the treatment of renal cell carcinoma. Pharmacoeconomics. 2011;29(11):977–988. doi: 10.2165/11593370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Ryan M. Discrete choice experiments in health care. BMJ. 2004;328(7436):360–361. doi: 10.1136/bmj.328.7436.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Ozdemir S, Johnson FR, Hauber AB. Hypothetical bias, cheap talk, and stated willingness to pay for health care. J Health Econ. 2009;28(4):894–901. doi: 10.1016/j.jhealeco.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Johnson FR, Mohamed AF, Ozdemir S, Marshall DA, Phillips KA. How does cost matter in health-care discrete-choice experiments? Health Econ. 2011;20(3):323–330. doi: 10.1002/hec.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Muhlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. [DOI] [PubMed]
- 39.Train K. Discrete choice methods with simulation. 1. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 40.Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Applications of simulation methods in environmental and resource economics. Dordrecht: Springer; 2005. pp. 117–134. [Google Scholar]
- 41.Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224–231. doi: 10.1016/j.lungcan.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 42.von Moos R, Body JJ, Egerdie B, Stopeck A, Brown JE, Damyanov D, et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer. 2013;21(12):3497–3507. doi: 10.1007/s00520-013-1932-2. [DOI] [PubMed] [Google Scholar]
- 43.Hechmati G, Hauber AB, Arellano J, Mohamed AF, Qian Y, Gatta F, et al. Patients’ preferences for bone metastases treatments in France, Germany and the United Kingdom. Support Care Cancer. 2015;23(1):21–28. doi: 10.1007/s00520-014-2309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauber AB, Johnson FR, Fillit H, Mohamed AF, Leibman C, Arrighi HM, et al. Older Americans’ risk-benefit preferences for modifying the course of Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(1):23–32. doi: 10.1097/WAD.0b013e318181e4c7. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra C, Farooqui MA, Kanesvaran R, Bilger M, Finkelstein E. Comparison of preferences for end-of-life care among patients with advanced cancer and their caregivers: a discrete choice experiment. Palliat Med. 2015;29(9):842–850. doi: 10.1177/0269216315578803. [DOI] [PubMed] [Google Scholar]
- 46.Hauber AB, Arellano J, Qian Y, Gonzalez JM, Posner JD, Mohamed AF, et al. Patient preferences for treatments to delay bone metastases. Prostate. 2014;74(15):1488–1497. doi: 10.1002/pros.22865. [DOI] [PubMed] [Google Scholar]
- 47.Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and applications. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 48.Coran JJ, Koropeckyj-Cox T, Arnold CL. Are physicians and patients in agreement? Exploring dyadic concordance. Health Educ Behav. 2013;40(5):603–611. doi: 10.1177/1090198112473102. [DOI] [PubMed] [Google Scholar]
- 49.Shin DW, Cho J, Roter DL, Kim SY, Sohn SK, Yoon MS, et al. Preferences for and experiences of family involvement in cancer treatment decision-making: patient-caregiver dyads study. Psychooncology. 2013;22(11):2624–2631. doi: 10.1002/pon.3339. [DOI] [PubMed] [Google Scholar]
- 50.Tariman JD, Doorenbos A, Schepp KG, Singhal S, Berry DL. Older adults newly diagnosed with symptomatic myeloma and treatment decision making. Oncol Nurs Forum. 2014;41(4):411–419. doi: 10.1188/14.ONF.411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



