Abstract
Introduction
Levels of Alzheimer's disease (AD)-related proteins in plasma neuronal derived exosomes (NDEs) were quantified to identify biomarkers for prediction and staging of mild cognitive impairment (MCI) and AD.
Methods
Plasma exosomes were extracted, precipitated, and enriched for neuronal source by anti-L1CAM antibody absorption. NDEs were characterized by size (Nanosight) and shape (TEM) and extracted NDE protein biomarkers were quantified by ELISAs. Plasma NDE cargo was injected into normal mice, and results were characterized by immunohistochemistry to determine pathogenic potential.
Results
Plasma NDE levels of P-T181-tau, P-S396-tau, and Aβ1–42 were significantly higher, whereas those of neurogranin (NRGN) and the repressor element 1-silencing transcription factor (REST) were significantly lower in AD and MCI converting to AD (ADC) patients compared to cognitively normal controls (CNC) subjects and stable MCI patients. Mice injected with plasma NDEs from ADC patients displayed increased P-tau (PHF-1 antibody)–positive cells in the CA1 region of the hippocampus compared to plasma NDEs from CNC and stable MCI patients.
Conclusions
Abnormal plasma NDE levels of P-tau, Aβ1–42, NRGN, and REST accurately predict conversion of MCI to AD dementia. Plasma NDEs from demented patients seeded tau aggregation and induced AD-like neuropathology in normal mouse CNS.
Keywords: Neuron, Exosomes, Mild cognitive impairment, Alzheimer's disease, Biomarker, Phospho-tau, Beta amyloid
1. Introduction
The number of individuals suffering from Alzheimer's disease (AD) is expected to triple by 2050 if no preventative measures are developed [1]. AD is a neurodegenerative process that results in cognitive impairment far worse than what would be expected as a result of normal aging. Mild cognitive impairment (MCI) often is a transitional stage between normal aging and dementia and is distinguishable clinically from AD and normal aging [2]. The prevalence of dementia in the United States is approximately 1% to 2%, whereas the prevalence of MCI is approximately 5% to 15% [2]. Although MCI is not associated with significant functional impairment, it carries an increased risk of developing dementia. MCI patients will progress to AD at a rate as high as 10% to 15% per year [3]. Initiating treatment at the stage of MCI has been the focus of much research activity because clinical trials of treatments for established AD have failed.
Given that MCI is a heterogeneous syndrome with many underlying etiologies, there is great interest in identifying biomarkers that predict conversion from MCI to AD dementia. Moreover, this is likely to delineate a population that is more responsive to preventative treatments. The most consistent AD biomarkers have been identified in cerebrospinal fluid (CSF) [4], [5], [6], [7]. CSF biomarkers in combination with recently improved neuroimaging have increased the rate of early diagnosis of preclinical AD patients, but CSF studies require invasive sample collection [8], [9], [10], [11], [12], [13], [14]. Studies have shown overlap in CSF biomarkers among MCI patients who later manifest AD, those who develop non-AD forms of dementia, and those who never exhibit signs of dementia [8], [9], [10], [11], [12], [13], [14]. The presence of nonspecific binding of imaging radiotracers in AD cases, especially those for phosphorylated tau (P-tau), may limit their utility as accurate and predictive biomarker tools for diagnosis of preclinical AD [15]. Prognostic uncertainty combined with the morbidity and the high expense of repeated CSF sampling and neuroimaging methods emphasizes the importance of seeking blood-based tests that have high diagnostic and prognostic ability for AD.
The pathologic hallmarks of AD include amyloid-beta (Aβ)—containing plaques and neurofibrillary deposits of P-tau protein. A recent study determined that elevated levels of the pathogenic proteins P-T181-tau, P-S396-tau, and Aβ1–42 contained within neuron-derived exosomes (NDEs) isolated from plasma accurately predicted the development of AD up to 10 years before clinical onset [16]. Several biomarkers measurable in NDEs may predict the transition of MCI to AD dementia [16], [17], [18], [19].
Exosomes represent a subtype of secreted membrane vesicles (40–100 nm in diameter) of endosomal origin that are released from most types of cells including neurons [20], [21]. Exosomes function in removal of damaged or excess proteins and cellular substituents, and also shuttle cargo, including proteins and RNAs between cells [22], [23]. Exosomes are reported to contain amyloid-β precursor protein and its metabolites (C-terminal fragments, amyloid intracellular domain, and Aβ) [22], [24]. Tau aggregates also may be packaged inside exosomes, some of which enter CSF or may even transit from the brain into blood. Although the neuro-pathogenic potential of NDEs has yet to be investigated, their role as biomarkers is being clarified.
Synapse loss and dysfunction is another pathologic hallmark of AD. Although synapse loss is an early event in AD pathogenesis, it is evident in all stages of AD [25], [26], [27]. Clinical pathological studies have shown that synapse loss correlates more closely with the degree of memory impairment than deposition of Aβ and P-tau in the brain [28], [29]. Neurogranin (NRGN) is a post-synaptic protein that is expressed exclusively in the brain, particularly in dendritic spines, and facilitates synaptic transmission [30]. Recently, elevated NRGN levels have been detected as a potential AD biomarker. CSF levels of NRGN were elevated in both AD [26], [27] and stable MCI patients [31] compared to those of cognitively normal controls (CNC).
Repressor element 1-silencing transcription factor (REST) has also been identified as a potential AD biomarker. REST represses expression of neural genes in non-neuronal cells and enhances neuronal survival [32], [33]. Central nervous system (CNS) levels of REST rise gradually in normal aging but are significantly reduced in brains of stable MCI patients and virtually absent in AD brains [34]. Changes in REST expression in response to neurodegeneration have yet to be measured in the CSF, but levels of REST in plasma NDEs are significantly lower in AD than in matched cognitively normal controls [18].
In this study, we harvested exosomes from human plasma and enriched the content of those originating from neurons, termed NDEs, by absorption with anti-L1CAM antibody. Plasma NDE preparations were characterized by size (Nanosight) and shape (transmission electron microscopy, TEM), and levels of proteins of interest were quantified by ELISA. We analyzed the predictive value of levels of established and novel AD-related proteins extracted from plasma NDEs for the clinical progression of MCI to dementia, in the setting of biosamples collected in a randomized clinical trial. Additionally, we investigated the role that NDEs play in AD pathogenesis. Human NDEs isolated from the plasma of CNC subjects, stable MCI, and ADC patients were injected into the brains of wild-type, C57/Bl6 mice. We report evidence that P-tau species contained within plasma NDEs can propagate tau aggregation and have high pathogenic potential.
2. Methods
2.1. Study design, subject characterization, and tissue and blood collection
Sixty plasma samples were acquired through the Alzheimer's Disease Cooperative Study Biomarker Core at University of California, San Diego [3]. Four clinical cohorts were identified: cognitively normal controls (CNC, n = 10); patients with an established diagnosis of mild to moderate AD (AD, n = 10), patients with stable mild cognitive impairment (MCI; n = 20; mean age, 68.7 ± 7.76, Supplementary Table 1), and patients who transitioned within 36 months from MCI to AD (ADC, n = 20; mean age 75.35 ± 6.82, Supplementary Table 1). CNC, MCI, and AD groups were initially defined based on CSF Aβ concentrations and cognitive status as indicated by a mini-mental state examination score (MMSE): CNC, CSF Aβ1–42 level >190 pg/mL, and normal cognition (MMSE 27-30); MCI, memory complaint, cutoff score on the Logical Memory test, MMSE >24, intact ADL [3]; AD, CSF Aβ1–42 <190 pg/mL and MMSE <20. Patients in the ADC group were identified based on progression to a clinical diagnosis of AD during the clinical trial, and the MMSE scores 36 months after initial examination are reported (mean MMSE at month 36, 17.67 ± 0.70 ADC versus 28.82 ± 0.33 MCI, Supplementary Table 2).
2.2. Isolation and characterization of L1CAM-positive neuronal derived exosomes (NDEs) from plasma
Aliquots of 250 μL of plasma were incubated with thromboplastin-D (Fisher Scientific, Inc., Hanover Park, IL) followed by addition of calcium-free and magnesium-free Dulbecco's balanced salt solution (DBS−2) with protease inhibitor cocktail (Roche Applied Sciences, Inc., Indianapolis, IN) and phosphatase inhibitor cocktail (Pierce Halt, Thermo Scientific, Inc., Rockford, IL) [17], [18]. After centrifugation, supernatants were incubated with ExoQuick exosome precipitation solution (EXOQ; System Biosciences, Inc., Mountain view, CA), and resultant suspensions centrifuged at 1500× g for 30 minutes at 4°C. Each pellet was re-suspended in 300 μL of distilled water with inhibitor cocktails followed by immunochemical enrichment of exosomes from neural sources [19].
Total exosome suspensions were incubated with 2 μg of mouse anti-human CD171 (L1CAM, neural adhesion protein) biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) in 50 μL of 3% BSA for 60 min at 20°C followed by addition of 10 μL of Streptavidin-Plus UltraLink resin (Pierce-Thermo Scientific, Inc.) in 40 μL of 3% BSA and further incubation for 60 minutes [19]. After centrifugation at 400 × g for 5 minutes at 4°C, pellets were resuspended in 100 μL of 0.05-M glycine-HCl (pH 3.0), incubated at 4°C for 10 minutes, and re-centrifuged at 4000 × g for 10 minutes at 4°C. Each supernatant was transferred to a new Eppendorf tube containing 10 μL of 1-M Tris-HCl (pH 8.0) and 40 μL of 3% BSA, mixed and received 350 μL of M-PER mammalian protein extraction reagent (Thermo Scientific, Inc.) containing protease and phosphatase inhibitors, mixed and stored at −80°C.
L1CAM-positive NDE cargo proteins were quantified by human-specific ELISAs for P-T181-tau (Fujirebio US, Inc., Alpharetta, GA), Aβ1–42, and P-S396-tau (Life Technologies/Invitrogen, Camarillo, CA), REST (Cusabio, American Research Products, Inc., Waltham, MA), Neurogranin (Cloud-Clone Corp., American Research Products, Inc.), and tetraspanning exosome marker CD81 (Cusabio- American Research Products, Inc.) with verification of the CD81 antigen standard curve using purified human recombinant CD81 antigen (Origene Technologies, Inc., Rockville, MD), according to suppliers' directions. The mean value for all determinations of CD81 in each assay group was set at 1.00, and the relative values for each sample were used to normalize their recovery. Evidence for enrichment of exosomes from neural sources in plasma has been demonstrated previously [17], [19].
2.3. Characterization of L1CAM-positive plasma NDEs based on shape and size
L1CAM-positive plasma NDEs were characterized based on size and shape using TEM as previously described [35]. NTA was used to characterize L1CAM-positive plasma NDEs based on size distribution. NDEs from CNC (n = 4) and ADC (n = 4) patients were pooled in 100 μL of Phosphate buffer saline (PBS), diluted 1:2000, and visualized with a NanoSight LM10 instrument as described [36].
2.4. Injection of L1CAM-positive NDEs from plasma into normal mouse CNS and characterization by IHC
For all in vivo experiments, 2 μL of plasma NDEs from either control, stable MCI or ADC patients were injected into the right hippocampus of wild-type, C57/BL6 mice (n = 6/group, 8–10 month old) at the level of (−2.0, +1.5, −1.3) (from Bregma, lateral, into) and analyzed 1 month after injection using immunohistochemistry (IHC). Brain slices containing hippocampus were probed using the anti-P-tau (PHF-1) antibody as described [37].
Frozen sections of 30 μM thickness were cut on a sliding microtome and stored at −20°C in cryoprotectant solution (20% glycerol and 30% ethylene glycol in 0.1-m phosphate buffer). PHF-1 was used to detect P-tau (1:500 dilution) in free-floating sections containing hippocampus using Mouse-on-Mouse Immunodetection Kit reagents (Vector Laboratories, Burlingame, CA) to avoid detection of endogenous mouse Ig. Endogenous peroxidase was quenched with 0.3% hydrogen peroxide to reduce free aldehydes. Reaction product was developed using a nickel-enhanced glucose oxidase method [38].
2.5. Statistical analyses
The statistical significance of differences between means for cross-sectional patient groups and between each patient group and their respective control group was determined with by one-way ANOVA with Newman–Keuls Multiple Comparison post hoc test (GraphPad Prism 6, La Jolla, CA). Discriminant classifier analyses were conducted by the Wilks' Lambda method to assess the performance of each NDE protein and the combined set in patient classification as described. Receiver operating characteristic (ROC) analyses were conducted under the non-parametric distribution assumption for standard error of area to determine the performance of classifier models (SPSS v21.0, IBM).
3. Results
3.1. Characterization of plasma L1CAM-positive plasma NDEs of stable MCI and ADC patients
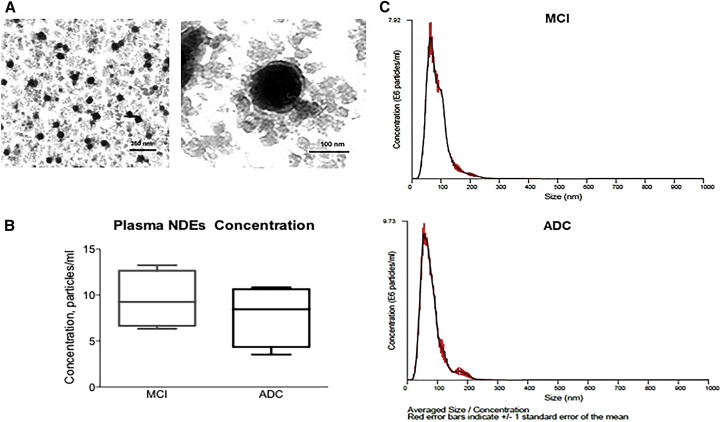
Plasma NDEs were analyzed for morphology and size distribution using TEM and a Nanosight system, respectively (Fig. 1). TEM revealed a homogenous population of morphologically distinctive particles of approximately 100-nm diameter for all patient populations (Fig. 1A; Plasma NDEs from stable MCI patients only; n = 4/group; scale bars, 350 nm and 100 nm). Nanoparticle tracking analysis detected a high concentration of plasma NDEs from both stable MCI (9.52 ± 1.93 × 1011 particle/mL) and ADC patients (7.39 ± 1.63 × 1011 particle/ml) with similar diameter distributions (Fig. 1B, 89.75 ± 2.15 nm vs. 94.5 ± 4.48 nm). Human plasma thus is a reliable source of authentic NDEs that are similar in appearance and size to those previously reported [20], [21], [39].
Fig. 1.
Plasma NDEs derived from stable MCI and ADC patients are similar in size and shape to previously reported exosome preparations. (A) Representative TEM image of plasma NDEs derived from a stable MCI patient (scale bars 350 nm; 100 nm). NTA detects high concentrations of plasma NDEs (B) with similar size distributions (C) that are not significantly different between the two patient populations. (C) Representative NTA plot of averaged size/concentration for plasma NDEs derived from a MCI and ADC patient (n = 4/group). Abbreviations: NDE, neuronal derived exosome; MCI, mild cognitive impairment; ADC, MCI converting to AD; TEM, transmission electron microscopy; NTA, nanoparticle tracking analysis.
3.2. Plasma NDE cargo contains P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST
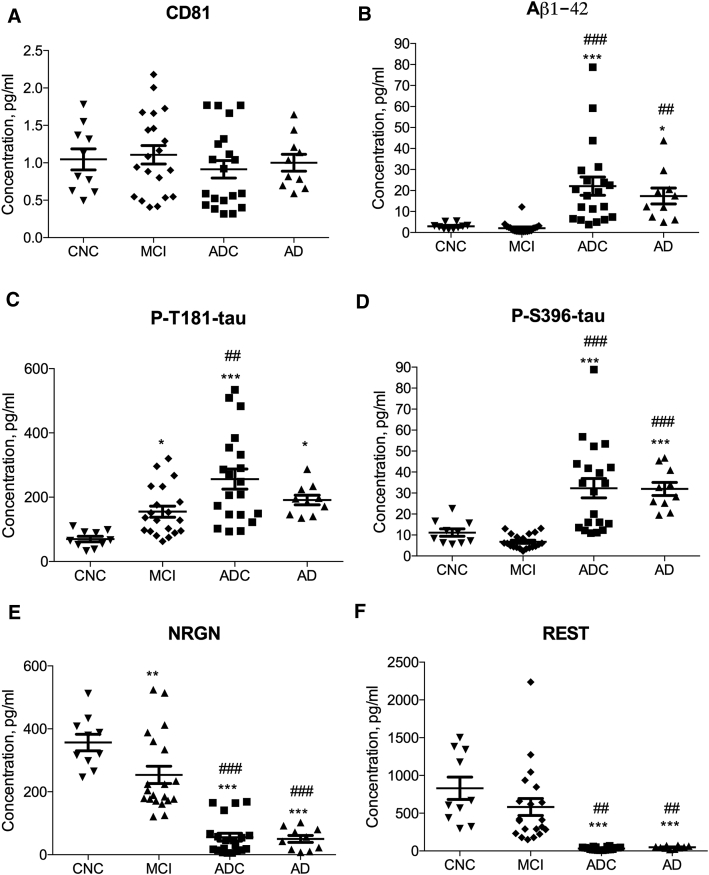
Plasma NDEs were isolated from the four clinical cohorts and their protein cargo were extracted and analyzed by enzyme-linked immunosorbent assays (ELISAs). Plasma NDEs from all patient groups and controls had indistinguishable levels of the exosome membrane marker protein CD81 (Fig. 2A). For ADC patients, CD81-normalized NDE concentrations of biomarkers were significantly higher than those of CNC subjects: Aβ1–42 (Fig. 2B, 22.07 ± 4.336 pg/mL vs. 2.979 ± 0.4485 pg/mL, P < .0001), P-T181-tau (Fig. 2C, 256.5 ± 31.15 pg/mL vs. 69.74 ± 8.319 pg/mL, P < .0001), and P-S396-tau (Fig. 2D, 32.32 ± 4.604 pg/mL vs. 11.15 ± 1.769 pg/mL, P < .0001). Similarly, for AD patients, CD81-normalized NDE concentrations of Aβ1-42 (17.38 ± 3.790 pg/ml, P < 0.05), P-T181-tau (191.3 ± 15.06 pg/ml, P < 0.05), and P - S396-tau (31.95 ± 3.100 pg/ml, P < 0.0001) and and were all significantly higher than those of CNC subjects. Interestingly, of the three core AD biomarker analytes quantified, only CD81-normalized NDE concentrations of P-T181-tau were significantly increased in stable MCI patients compared to those of CNC subjects (Fig. 2C, 155.1 ± 17.29 pg/mL, P < .05).
Fig. 2.
Human plasma NDE levels of P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST delineate stages of AD. (A) Plasma NDE levels of CD81 were not statistically different among the four clinical cohorts as measured by ELISA. Significantly different levels of (B) Aβ1–42, (C) P-T181-tau, (D) P-S396-tau, (E) NRGN, and (F) REST were detected by ELISAs in the plasmas of CNC (n = 10), stable MCI (n = 20), ADC (n = 20), and AD (n = 10) patients. Plasma NDE levels of P-T181-tau, P-S396-tau, and Aβ1–42 were significantly higher, whereas those of NRGN and REST were significantly lower for ADC and AD patients than CNC subjects and stable MCI patients. The horizontal line in each cluster depicts the mean for that set. * = P < .05, ** = P < .01, *** = P < .0001 versus CNC; ## = P < .01, ### = P < .0001 versus MCI. Abbreviations: NDE, neuronal derived exosome; CNC, cognitively normal controls; MCI, mild cognitive impairment; ADC, MCI converting to AD.
For inter-patient group analyses, CD81-normalized NDE concentrations of P-T181-tau (P < .001), P-S396-tau (P < .001), and Aβ1–42 (P < .01) all were significantly higher in ADC patients than in stable MCI patients. CD81-normalized NDE concentrations of P-S396-tau (P < .001) and Aβ1–42 (P < .01), but not P-T181-tau, were significantly higher in AD patients than in stable MCI patients.
CD81-normalized NDE concentrations of neurogranin (NRGN) were significantly lower in stable MCI patients (Fig. 2E, 253.7 ± 27.58 pg/mL vs. 356.5 ± 26.07 pg/mL, P < .01), ADC patients (Fig. 2E, 55.74 ± 12.70 pg/mL P < .0001) and AD patients (Fig. 2E, 50.29 ± 11.16 pg/mL, P < .001) than in CNC subjects. Furthermore, CD81-normalized NDE concentrations of NRGN were significantly lower in ADC patients (P < .0001) and AD (P < .0001) than in stable MCI patients. Similarly, CD81-normalized NDE concentrations of REST were significantly lower in ADC patients (Fig. 2F, 32.97 ± 4.161 pg/mL) and in AD patients (Fig. 2F, 48.17 ± 5.661 pg/mL) than in stable MCI patients (582.0 ± 112.5 pg/mL) and CNC subjects (829.9 ± 148.4 pg/mL; P < .0001 for all differences). We found no significant difference between CD81-normalized NDE concentrations of REST in stable MCI patients as contrasted with CNC subjects.
3.3. ROC analyses for patient characterization
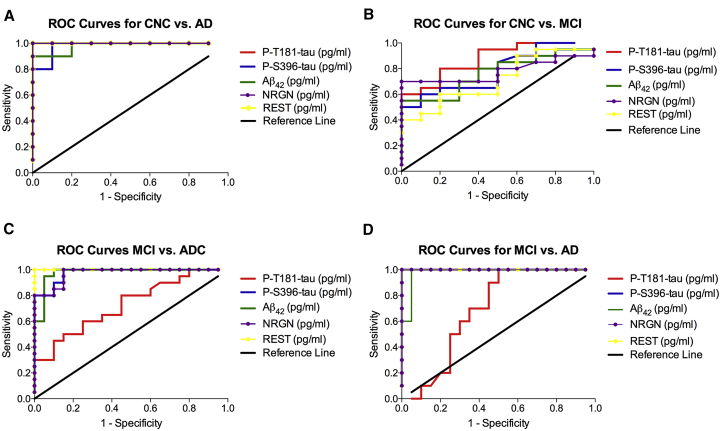
ROC analysis was conducted in order to determine if the diagnostic sensitivity of the five biomarkers individually or collectively increased their predictive ability in distinguishing two patient populations. (Fig. 3, see Supplementary Tables 3–6). When all the plasma NDE cargo proteins were considered individually, the sensitivity for distinguishing CNC subjects from AD patients was 100% for P-T181-tau, NRGN, and REST (confidence interval [CI]: 100%–100%) and 98% for P-S396-tau and Aβ1–42 (CI: 93%–100%; Fig. 3A). The sensitivity for distinguishing CNC subjects from stable MCI patients was 87.5± 0.06351% for P-T181-tau (CI: 75%–99%); 78.3± 0.08445% for P-S396-tau (CI: 62%–95%); 76.5± 0.08617% for Aβ1–42 (CI: 60%–93%), 77.8± 0.08546% for NGRN (61%–95%); and 71.5 ± 0.09499 for REST (CI: 53%–90%; Fig. 3B). The sensitivity for distinguishing stable MCI patients from ADC patients was 72.8± 0.07906% for P-T181-tau (CI: 57%–88%); 97.5± 0.01923% for P-S396-tau (CI: 97%–100%); 97.8± 0.02140% for Aβ1–42 (CI:93.6%-100%); 97.2 ± 0.02056% for NRGN (CI: 93.2 %–100%); and 100% for REST (CI: 100%–100%) (Fig. 3C). The sensitivity for distinguishing stable MCI patients from AD patients was 100% for P-S396-tau, NRGN, and REST (CI: 100%–100%); 69.3± 0.9475% for P-T181-tau (CI: 51%–88%); and 98± 0.02229% for P-S396-tau (CI: 94%–100%) (Fig. 3D).
Fig. 3.
ROC curve analyses assess diagnostic accuracy for plasma NDEs levels of P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST. ROC curves based on the plasma NDE levels of P-T181-tau, P-S396-tau, Aβ1–42, NRGN, and REST for (A) CNC subjects versus AD patients, (B) CNC subjects versus stable MCI patients, (C) stable MCI versus ADC patients, and (D) stable MCI versus AD patients. Abbreviations: ROC, receiver operating curve; NDE, neuronal derived exosome; CNC, cognitively normal control; MCI, mild cognitive impairment; ADC, MCI converting to AD.
When plasma NDE levels of all the proteins were considered together, the sensitivity of distinguishing CNC subjects from AD patients was 99.2%; the sensitivity of distinguishing CNC subjects from stable MCI patients was 78.3%; the sensitivity for distinguishing stable MCI patients from ADC patients was 93.1%; and the sensitivity for distinguishing stable MCI patients from AD patients was 93.2%.
3.4. Human plasma NDEs with P-tau seed tau aggregation in normal mice
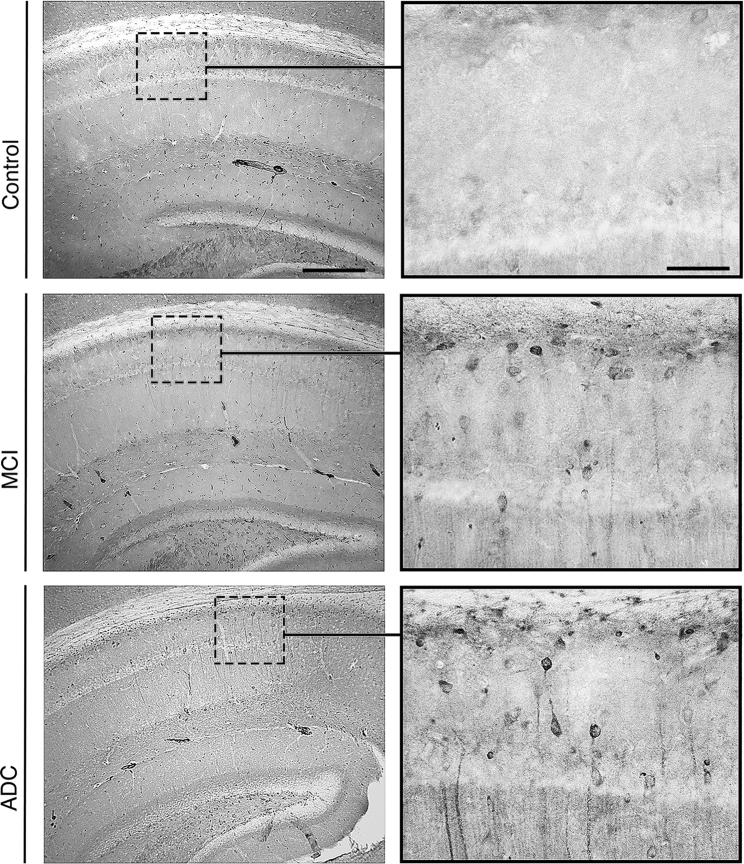
To determine the neuropathogenic potential of NDEs derived from human MCI and ADC plasma as compared to controls alone, these preparations were injected into the right hemisphere of 8–10 month old, C57/Bl6, female mice. One month after injection, all mice were sacrificed, and brain tissues were analyzed by IHC. We found that mice injected with plasma NDEs from stable MCI and ADC patients displayed PHF-1 immunoreactivity in the CA1 region of the hippocampus, whereas no PHF-1 immunoreactivity was observed in the hippocampus of mice injected with plasma NDEs from CNC subjects (Fig. 4, n = 6/group). PHF-1 immunoreactivity was rarely observed in mice injected with plasma NDEs from stable MCI patients, whereas a significant number of PHF-1 positive, pyramidal CA1 neurons were observed in mice that were injected with plasma NDEs from ADC patients. Moreover, diffuse staining of PHF-1 also extended into the dendritic processes of PHF-1 positive neurons observed in mice injected with plasma NDEs from ADC patients.
Fig. 4.
Plasma NDEs from stable MCI and AD patients seed tau aggregation in the brains of normal mice. Representative photomicrographs of hippocampal sections obtained from wild-type, C57/Bl6 mice that were injected with plasma NDEs from control, stable MCI, and ADC patients. At 1-month post-injection, mice injected with plasma NDEs from stable MCI patients display modest enhancement of PHF-1 immunoreactivity in the CA1 region of the hippocampus, whereas mice injected with plasma NDEs from ADC patients display a significant increase in PHF-1 immunoreactivity in the CA1. Extensive cellular staining as well as staining within the dendritic processes can be observed in these mice. Mice-injected NDEs from control plasma displayed no PHF-1 immunoreactivity (n = 6/group; scale bars 250 μm; 25 μm). Abbreviations: NDE, neuronal derived exosome; MCI, mild cognitive impairment; ADC, MCI converting to AD.
Together, these data suggest that human plasma NDEs carry pathogenic tau cargo capable of seeding the propagation of P-tau pathology in normal mice.
4. Discussion
The current procedures of AD diagnostics are invasive (CSF sampling), expensive (neuroimaging), and time consuming (neuropsychological testing) [40]. The consequent need for less invasive and more cost-effective tools to identify AD at an early and perhaps more treatable stage has fueled research into blood-based biomarkers. A recent study found that a combination of P-T181-tau, P-S396-tau, and Aβ1–42 contained within NDEs from blood predicted the development of AD up to 10 years before clinical onset [16]. Step-wise discriminant modeling of auto-lysosomal protein levels [19], transcription factors [18], and phosphorylated forms of the insulin receptor substrate [17] in plasma NDEs have also correctly distinguished 100% of patients with AD from normal controls. Our goal for characterizing plasma NDEs was to create a profile that included primary (P-tau and Aβ1–42) and amplifying (NRGN and REST) blood-based biomarkers that measure proteins related to pathologic events of brain changes described in AD. Additionally, we wanted to determine the pathogenic potential of AD-related proteins contained within plasma NDEs. In the present study, we confirmed that plasma NDE protein profiles can distinguish among different stages of AD and predict which patients with clinically defined MCI are most likely to progress to AD. Moreover, we demonstrated for the first time that the plasma NDEs from ADC (and MCI) patients may propagate tau pathology in the brains of normal mice similar to that seen in human AD brains.
The diagnostic accuracy of the five different biomarkers was assessed by ROC analysis. ROC analysis showed that individually, all five biomarkers were highly predicative (sensitivity, 98 to 100%) in distinguishing CNC subjects from AD patients and from MCI patients who progressed to AD. In contrast, the sensitivity to distinguish CNC subjects from stable MCI patients was relatively low and more variable among the five biomarkers that were tested.
When plasma NDE levels of all the markers were considered together, the sensitivity of distinguishing CNC subjects from stable MCI patients was 78.3%. When plasma NDE levels of P-T181-tau, P-S396-tau, and Aβ1–42 were considered together, the sensitivity of distinguishing CNC subjects from stable MCI patients slightly increased to 80.8%. Although NRGN and REST were highly predictive for distinguishing AD patients from CNC subjects and MCI patients, individually, their sensitivities were significantly less for distinguishing CNC subjects from MCI patients (77.8% and 71.6%, respectively). These data suggest that decreases in plasma NDE levels of NRGN and REST may occur later in AD progression. The most sensitive plasma NDE analyte distinguishing CNC subjects from stable MCI patients was P-T181-tau (sensitivity, 87.5 ± 0.06351%), although P-T181-tau was the least sensitive analyte for distinguishing stable MCI patients from AD patients (sensitivity, 69.3± 0.09475%).
In current clinical practice, the combination of P-tau and Aβ1–42 in CSF has provided the greatest predictive accuracy for the conversion of MCI to dementia [4], [5], [6], [7]. One study reported that a low Aβ1–42/P-tau ratio in CSF was the best predictor of cognitive decline and conversion to AD in MCI patients [5]. In contrast, we find that a high expression of P-tau and Aβ1–42 in plasma NDEs, that reflect CNS tissue levels of these biomarkers, was the best predictor of the conversion from MCI to AD. It is possible that NDEs reflect neuronal attempts to eliminate pathogenic proteins during neurodegeneration.
Studies show that higher baseline CSF levels of NRGN in MCI patients who progressed to AD correlated with a more rapid loss of cognition [27] and were highly predictive of the progression from MCI to AD [26]. Here, we found that levels of NRGN were significantly reduced in plasma NDEs derived from stable MCI, ADC, and AD patients compared to CNC subjects. It is possible that NRGN is released preferentially into CSF and not via exosomes as a result of synaptic damage occurring in AD. The expression of REST in neurons increases with aging and is markedly decreased in the brain in AD [18] and Huntington's Disease [18]. Reduced REST expression has been reported in plasma NDEs from AD patients [18]. To date, no studies have been conducted analyzing REST in CSF. We found reduced protein levels of REST in plasma NDEs derived from ADC and AD patients, and a smaller reduction, with overlap with CNC subjects, in stable MCI patients. The trafficking of REST in neurons and how it may be released through exosomes remains to be defined.
The use of blood-based biomarkers for AD diagnostics could improve effectiveness and reliability of patient characterization. Well-established protocols have to be designed, validated, and implemented to demonstrate their clinical utility, accuracy, and effectiveness in distinguishing different patient populations. One study found the combination of 18 different proteins in plasma predicted the progression to AD in stable MCI patients with over 90% accuracy [41]; however, a subsequent study demonstrated that the diagnostic accuracy for most of these markers was not reproducible [42]. High P-tau levels in CSF have shown to be excellent biomarkers to detect AD. However, most clinical settings use serum or plasma for diagnostic testing where P-tau levels are normally very low in AD patients [43]. Given that recent in vitro evidence suggests that most tau protein that is present in blood is encapsulated in NDEs, validating the usefulness of plasma NDE proteins as biomarkers in AD with standardized assays could improve screening and stratifying patients for clinical trials.
Finally, we report for the first time that plasma NDE cargo has high pathogenic potential. Plasma NDEs from stable MCI and ADC patients induced tau pathology in the brains of normal mice (Fig. 4). Interestingly, we observed more extensive staining of CA1 pyramidal neurons and of their dendritic processes in the brains of mice injected with plasma NDEs from ADC patients. This could be due to plasma NDEs from ADC patients having a higher concentration of P-T181-tau and P-S396 as measured by ELISA. As disease severity increases, it is not clear if the accumulation of toxic proteins in the brain drives the activation of this alternative secretory pathway or if the presence of P-tau and Aβ1–42 is due to passive release by dead and/or dying neurons. Our observation of more PHF-1 positive cells in the brains of mice injected with plasma NDEs of ADC patients suggests that the tau species themselves may serve as the agents of spread. The secretion and release of tau aggregates or “seeds” from neurons has been reported with other aggregation-prone proteins such as Aβ1–42 [22], α-synuclein [44], [45], prion protein [46], and SOD1 [47]. We cannot exclude that other aggregation-prone proteins may be trafficked in NDEs from the CNS and potentially propagate pathology in the brain.
In conclusion, our findings suggest that plasma NDE cargo proteins traffic from the CNS to blood and allow accurate assignment of the stage of development of AD by predicting the conversion of MCI to AD. Creating a more detailed profile with additional NDE markers may significantly enhance their diagnostic utility for AD as well other neurodegenerative diseases. Recently, a preliminary study reported elevated levels of P-tau contained within plasma exosomes extracted from former National Football League players compared to a control group of noncontact sport athletes [48]. Although the authors do not report a method to ensure that their plasma exosomes were derived from a neural source, these data still suggest that tau-positive exosomes can act as potential biomarkers for another neurodegenerative disease, chronic traumatic encephalopathy. Our study is limited by the relatively small number of subjects in each category and the cross-sectional sampling of plasma. Further studies need to address how well a panel of NDE markers may help to stage AD and other neurodegenerative diseases, and how they change over time in longitudinal studies. Finally, we plan to characterize the neuropathologic and behavioral consequences of plasma NDEs derived from stable MCI and ADC patients injected into normal mice at longer time points after injection.
Research in context.
-
1.
Systematic review: Biomarkers of Alzheimer's disease (AD) can dramatically clinical trial design and analysis. Although much biomarker work has focused on tau phosphorylation, another important intermediate to consider is neuronally derived exosomes (NDE). Better understanding of the trafficking of NDE from CNS to CSF and blood could be important to clarify the pathogenic significance of tau containing exosomes in AD and might contribute to validate the detection of exosomes as biomarkers of AD; which in turn could improve clinical trial design and analysis, increasing the likelihood of successful drug development.
-
2.
Interpretation: We demonstrate that the protein content profile of NDE can accurately differentiate AD from control patients and accurately predict conversion of MCI to AD. In animal models, intrahippocampal injection of NDE from MCI and MCI-AD converters induced tau pathology in naïve mice.
-
3.
Future directions: We recommend advancement of the NDE program for validation in early-mid phase AD trials to determine the biomarker utility of NDE content in defining dementia groups (e.g., AD and MCI). More exploratory trial work could focus on how treatment regimen impacts NDE content. In terms of basic science work, our current studies aim to understand (1) the total number of exosomes in blood and how they traffic from CNS to periphery, (2) the portion of exosomes that are neuronally derived, (3) the profile of pathogenic proteins contained in NDE versus other exosome populations and (4) how this cargo changes with aging and disease.
Acknowledgments
This work was supported by grants to R.A.R from NIH AG047484, BX003040 and AG0051839, the Alzheimer's Association and the Alzheimer's Art Quilt Initiative.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.04.001.
Supplementary data
References
- 1.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward A., Arrighi H.M., Michels S., Cedarbaum J.M. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Grundman M., Petersen R.C., Ferris S.H., Thomas R.G., Aisen P.S., Bennett D.A. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 4.van Rossum I.A., Vos S., Handels R., Visser P.J. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J Alzheimers Dis. 2010;20:881–891. doi: 10.3233/JAD-2010-091606. [DOI] [PubMed] [Google Scholar]
- 5.Parnetti L., Farotti L., Eusebi P., Chiasserini D., De Carlo C., Giannandrea D. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson's Disease. Front Aging Neurosci. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanari A., Parnetti L. Cerebrospinal fluid biomarkers and prediction of conversion in patients with mild cognitive impairment: 4-year follow-up in a routine clinical setting. ScientificWorldJournal. 2009;9:961–966. doi: 10.1100/tsw.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blennow K., Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R., Tripathi C.B. Diagnostic Utility of CSF Tau and Abeta(42) in Dementia: A Meta-Analysis. Int J Alzheimers Dis. 2011;2011:503293. doi: 10.4061/2011/503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreasson U., Lautner R., Schott J.M., Mattsson N., Hansson O., Herukka S.K. CSF biomarkers for Alzheimer's pathology and the effect size of APOE varepsilon4. Mol Psychiatry. 2014;19:148–149. doi: 10.1038/mp.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zetterberg H., Lautner R., Skillback T., Rosen C., Shahim P., Mattsson N. CSF in Alzheimer's disease. Adv Clin Chem. 2014;65:143–172. doi: 10.1016/b978-0-12-800141-7.00005-x. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson N., Insel P.S., Landau S., Jagust W., Donohue M., Shaw L.M. Diagnostic accuracy of CSF Ab42 and florbetapir PET for Alzheimer's disease. Ann Clin Transl Neurol. 2014;1:534–543. doi: 10.1002/acn3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen C., Hansson O., Blennow K., Zetterberg H. Fluid biomarkers in Alzheimer's disease - current concepts. Mol Neurodegener. 2013;8:20. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen C., Rosen H., Andreasson U., Bremell D., Bremler R., Hagberg L. Cerebrospinal fluid biomarkers in cardiac arrest survivors. Resuscitation. 2014;85:227–232. doi: 10.1016/j.resuscitation.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Rosen C., Zetterberg H. Cerebrospinal fluid biomarkers for pathological processes in Alzheimer's disease. Curr Opin Psychiatry. 2013;26:276–282. doi: 10.1097/YCO.0b013e32835f6747. [DOI] [PubMed] [Google Scholar]
- 15.Okamura N., Harada R., Furumoto S., Arai H., Yanai K., Kudo Y. Tau PET imaging in Alzheimer's disease. Curr Neurol Neurosci Rep. 2014;14:500. doi: 10.1007/s11910-014-0500-6. [DOI] [PubMed] [Google Scholar]
- 16.Fiandaca M.S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J.B. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapogiannis D., Boxer A., Schwartz J.B., Abner E.L., Biragyn A., Masharani U. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetzl E.J., Boxer A., Schwartz J.B., Abner E.L., Petersen R.C., Miller B.L. Low neural exosomal levels of cellular survival factors in Alzheimer's disease. Ann Clin Transl Neurol. 2015;2:769–773. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzl E.J., Boxer A., Schwartz J.B., Abner E.L., Petersen R.C., Miller B.L. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons M., Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 22.Rajendran L., Honsho M., Zahn T.R., Keller P., Geiger K.D., Verkade P. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budnik V., Ruiz-Cañada C., Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vingtdeux V., Hamdane M., Loyens A., Gele P., Drobeck H., Begard S. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem. 2007;282:18197–18205. doi: 10.1074/jbc.M609475200. [DOI] [PubMed] [Google Scholar]
- 25.Davidsson P., Blennow K. Neurochemical dissection of synaptic pathology in Alzheimer's disease. Int Psychogeriatr. 1998;10:11–23. doi: 10.1017/s1041610298005110. [DOI] [PubMed] [Google Scholar]
- 26.Kester M.I., Teunissen C.E., Crimmins D.L., Herries E.M., Ladenson J.H., Scheltens P. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA Neurol. 2015;72:1275–1280. doi: 10.1001/jamaneurol.2015.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portelius E., Zetterberg H., Skillbäck T., Törnqvist U., Andreasson U., Trojanowski J.Q. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain. 2015;138:3373–3385. doi: 10.1093/brain/awv267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingelsson M., Fukumoto H., Newell K.L., Growdon J.H., Hedley-Whyte E.T., Frosch M.P. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 29.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 30.Chang J.W., Schumacher E., Coulter P.M., Vinters H.V., Watson J.B. Dendritic translocation of RC3/neurogranin mRNA in normal aging, Alzheimer disease and fronto-temporal dementia. J Neuropathol Exp Neurol. 1997;56:1105–1118. doi: 10.1097/00005072-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Kvartsberg H., Duits F.H., Ingelsson M., Andreasen N., Öhrfelt A., Andersson K. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease. Alzheimers Dement. 2015;11:1180–1190. doi: 10.1016/j.jalz.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Chong J.A., Tapia-Ramírez J., Kim S., Toledo-Aral J.J., Zheng Y., Boutros M.C. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 33.Coulson J.M. Transcriptional regulation: cancer, neurons and the REST. Curr Biol. 2005;15:R665–R668. doi: 10.1016/j.cub.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Lu T., Aron L., Zullo J., Pan Y., Kim H., Chen Y. REST and stress resistance in ageing and Alzheimer's disease. Nature. 2014;507:448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burghardt R.C., Droleskey R. Transmission electron microscopy. Curr Protoc Microbiol. 2006;Chapter 2:Unit 2B.1. doi: 10.1002/9780471729259.mc02b01s03. [DOI] [PubMed] [Google Scholar]
- 36.Mitsuhashi M., Taub D.D., Kapogiannis D., Eitan E., Zukley L., Mattson M.P. Aging enhances release of exosomal cytokine mRNAs by Abeta1-42-stimulated macrophages. FASEB J. 2013;27:5141–5150. doi: 10.1096/fj.13-238980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell S.N., Zhang C., Monte L., Roe A.D., Rice K.C., Tache Y. Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing corticotropin-releasing factor. J Alzheimers Dis. 2015;43:967–976. doi: 10.3233/JAD-141281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu S.Y., Ju G., Fan L.Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 39.van der Pol E., Coumans F.A., Grootemaat A.E., Gardiner C., Sargent I.L., Harrison P. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12:1182–1192. doi: 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
- 40.Laske C., Sohrabi H.R., Frost S.M., López-de-Ipiña K., Garrard P., Buscema M. Innovative diagnostic tools for early detection of Alzheimer's disease. Alzheimers Dement. 2015;11:561–578. doi: 10.1016/j.jalz.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Ray S., Britschgi M., Herbert C., Takeda-Uchimura Y., Boxer A., Blennow K. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 42.Soares H.D., Chen Y., Sabbagh M., Roher A., Rohrer A., Schrijvers E. Identifying early markers of Alzheimer's disease using quantitative multiplex proteomic immunoassay panels. Ann N Y Acad Sci. 2009;1180:56–67. doi: 10.1111/j.1749-6632.2009.05066.x. [DOI] [PubMed] [Google Scholar]
- 43.Sparks D.L., Kryscio R.J., Sabbagh M.N., Ziolkowski C., Lin Y., Sparks L.M. Tau is reduced in AD plasma and validation of employed ELISA methods. Am J Neurodegener Dis. 2012;1:99–106. [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-Erviti L., Seow Y., Schapira A.H., Gardiner C., Sargent I.L., Wood M.J. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi M., Liu C., Cook T.J., Bullock K.M., Zhao Y., Ginghina C. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grad L.I., Pokrishevsky E., Silverman J.M., Cashman N.R. Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion. 2014;8:331–335. doi: 10.4161/19336896.2014.983398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern R.A., Tripodis Y., Baugh C.M., Fritts N.G., Martin B.M., Chaisson C. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J Alzheimers Dis. 2016;10:1099–1109. doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.