Abstract
Introduction
Left-sided liver resection (LLR) for perihilar cholangiocarcinoma (PHC) may require right hepatic artery (RHA) resection and reconstruction because of its intimate relationship with the biliary confluence. Consequently right-sided resections (RLR) are preferred for Bismuth-Corlette IIIb tumours, and resections avoided in Bismuth-Corlette IV tumours with left lobar atrophy when the RHA is involved by tumour.
Methods
A retrospective analysis of patients with PHC who presented between December 2009 and June 2015.
Results
Thirty-six patients underwent resection for PHC (23 LLR, 13 RLR). The number of Bismuth-Corlette IV patients undergoing LLR was significantly greater than those undergoing RLR (8/23 vs 0/13, p = 0.032). The need for arterial reconstruction (AR) was significantly greater during LLR than RLR (10/23 vs 0/13, p = 0.006). Postoperative liver dysfunction was greater after RLR (5/13 vs 0/23, p = 0.003), and hospital stay was shorter after LLR (10 vs 15 days, p = 0.013).
Conclusions
Safe AR increases the ability to perform potentially curative LLR for PHC. This improves the resectability rate for PHC, particularly for Bismuth-Corlette Type IV tumours. The larger liver remnant after LLR results in less postoperative liver dysfunction and shorter hospital stay without increased operating time, blood loss or morbidity.
Introduction
Complete surgical resection provides the best chance of cure for perihilar cholangiocarcinoma (PHC).1, 2 Adequate surgery for this condition requires margin-free resection of the tumour, adjacent lymph nodes, the caudate lobe of liver and varying amounts of liver parenchyma.2 Although logic dictates that right-sided tumours (Bismuth-Corlette Stage IIIa) should be best treated by right-sided liver resections (RLR), and left-sided tumours (Bismuth-Corlette Stage IIIb) by left-sided liver resections (LLR), the anatomy of the liver hilum causes many surgeons to prefer right hepatectomy or right trisectionectomy even for left-sided PHC whenever possible. The long, extra-hepatic course of the left hepatic duct and the left portal vein make it easier to achieve tumour clearance on the hepatic duct and to perform portal vein resection respectively during right hepatectomy or right trisectionectomy. More importantly, the right hepatic artery, vital for preservation of the liver remnant after left-sided resection, is intimately related to the posterior surface of the biliary confluence and is often involved by tumour. Complete tumour clearance may require en-bloc resection and reconstruction of an involved artery, a procedure traditionally associated with high morbidity and mortality.3 This does not apply to right-sided resections because the left hepatic artery lies well away from the biliary confluence, enters the umbilical fissure at the extreme left of the hilum, and is rarely involved by tumour.
However RLR preserve a smaller liver remnant than corresponding LLR and is consequently associated with greater operative mortality in patients with cholestatic liver.4 Those undergoing these resections are more likely to require optimisation of the planned remnant with preoperative biliary drainage (PBD) and/or portal vein embolisation (PVE), and have to bear the additional time, expense and associated risk.4, 5 In the past few years, renewed efforts have been made to overcome the technical challenges of left hepatectomy and left trisectionectomy for PHC.6, 7, 8, 9 The success of these efforts has increased margin-free resectability rates of Bismuth-Corlette Stage IV tumours with left-sided vascular involvement, atrophy or tumour extension beyond the left margin of the umbilical fissure.
Over the past 6 years the authors have taken an aggressive approach towards arterial resection for PHC. In patients with Bismuth-Corlette Type IIIb (as an alternative to right trisectionectomy) and Type IV tumours the authors have considered LLR with resection and reconstruction of the RHA whenever it was involved by tumour. The aim of this study was to present the authors experience with this approach, focussing on the difference between right-sided and left-sided hepatic resections for this disease.
Methods
The medical records of all patients with PHC operated between October 2009 to June 2015 were evaluated.
All patients were evaluated by triphasic multislice CT scan. MRI was performed selectively whenever greater clarity on biliary anatomy was necessary. Endosonography, PET scan and preoperative biopsy were not performed.
LLR was performed in patients with left lobe atrophy and in those with Bismuth-Corlette Type IV tumours extending beyond the segment 4 hepatic duct. AR was preferred over PBD (±PVE) and right trisectionectomy in patients with Bismuth-Corlette Type IIIb tumours whenever possible. PBD was performed through the planned future liver remnant in all patients with cholangitis, serum bilirubin above 250umol/L (15 mg%) or those requiring prolonged preoperative optimisation. It was avoided if the estimated remnant volume was greater than 40%.7 Some patients presented after PBD had already been performed elsewhere. Once PBD had been instituted, surgery was performed after the serum bilirubin fell below 5 mg%. PVE was not performed in any of the patients in this series. When PBD was necessary for the above stated reasons in patients with Bismuth-Corlette Type IIIb tumours, right trisectionectomy was preferred if the artery was involved.
All patients underwent laparoscopy prior to laparotomy. Laparotomy was not performed in the presence of cirrhosis, peritoneal or liver metastases. Hilar lymph node metastases or direct infiltration of adjacent bowel were not considered contraindications to resection if complete resection was deemed feasible. For LLR the right lobe of liver was not mobilised. The caudate lobe was mobilised and resected entirely from the left side. Exploration was begun in Rouviere's sulcus to the right of the porta hepatis to identify tumour-free hepatic artery, portal vein and hepatic duct. The right posterior sectoral hepatic artery was followed towards the tumour to determine the optimal tumour-free right hepatic artery for potential arterial reconstruction (Fig. 1). With a reconstructable artery confirmed, the remaining operation was carried out as per established procedure. Left trisectionectomy was preferred over left hepatectomy when it was felt that left hepatectomy would not clear tumour extension into the right posterior sectoral duct, when there was right anterior sector atrophy, or when there was vascular involvement preventing preservation of the right anterior sectoral artery.
Figure 1.
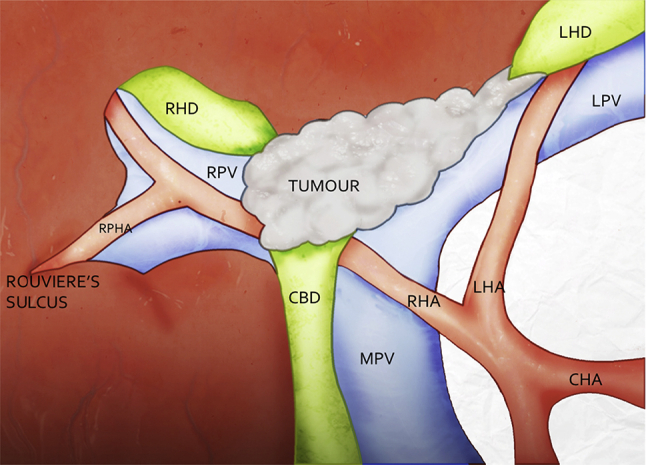
Identification of a reconstructable right posterior sectoral artery in Rouviere's sulcus to the right of the tumour is a prerequisite to arterial reconstruction for tumours involving the right hepatic artery. (CHA: Common Hepatic Artery, RHA: Right Hepatic Artery, LHA: Left Hepatic Artery, RPHA: Right Posterior Sectoral Hepatic Artery, MPV: Main Portal Vein, LPV: Left Portal Vein, RPV: Right Portal Vein, RHD: Right Hepatic Artery, LHD: Left Hepatic Artery, CBD: Common Bile Duct)
In LLR, vascular reconstructions were performed at the completion of parenchymal transection to avoid traction on the anastomosis during transection. Reconstruction of the hepatic artery (AR) when necessary was carried out by direct end-to-end anastomosis between healthy proximal right hepatic artery or proper hepatic artery to healthy distal right hepatic artery or right posterior sectoral artery using interrupted 8/0 prolene (Fig. 2). The gastroduodenal artery was divided and the common hepatic artery mobilised to its origin so as to approximate the two ends of the artery for reconstruction. In rare instances when direct anastomosis was still not feasible, an interposition vein graft using the inferior mesenteric vein or gonadal vein was used.
Figure 2.
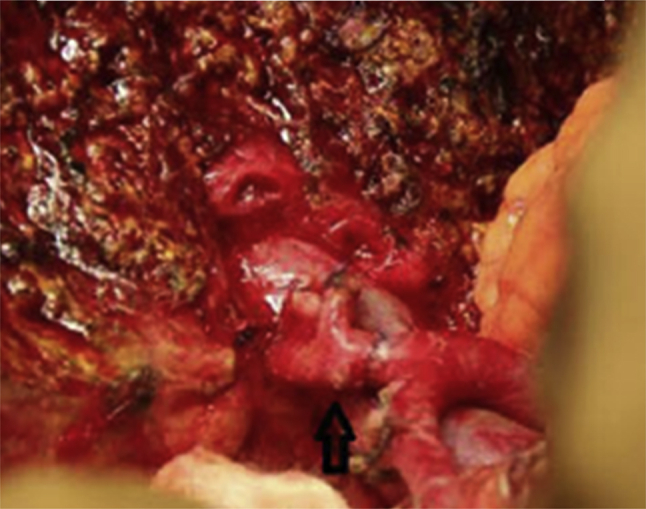
Completed arterial reconstruction after left hepatectomy
Portal Vein reconstruction (PVR) was carried out by end-to-end anastomosis using continuous 5/0 prolene. When performed during RLR the left-sided PVR was carried out prior to parenchymal transection.
The liver was never simultaneously deprived of both arterial and portal inflow at any time during vascular reconstruction. When both vessels had to be clamped PVR was performed first to reperfuse the liver quickly and reduce gut congestion. Intraoperative Doppler ultrasound was performed to document satisfactory arterial and portal flow after completion of vascular reconstruction. It was repeated on Day 1 after surgery, and then only if clinically indicated.
All proximal and the distal bile duct margins were confirmed to be clear on frozen section biopsy. When the margin was reported to be involved on frozen section, additional duct was resected whenever possible.10, 11
Patients who underwent vascular resections did not receive postoperative anticoagulation apart from routine prophylaxis against deep vein thrombosis. The only patient in whom an interposition graft was used was emperically placed on Aspirin 150 mg once daily.
All patients who had an arterial resection underwent a triphasic CT scan prior to discharge to document a patent vascular anastomosis with no pseudoaneurysm.
IBM SPSS Statistics v.20 for Windows (IBM, Armonk NY) was used for data entry and analysis. Continuous data was evaluated using medians, ranges and the Mann–Whitney U test. Categorical data was evaluated by Fisher's exact test. A p-value <0.5 was considered statistically significant for all tests of comparison. Survival was estimated using Kaplan Meier method and compared using the Log rank test.
Results
A total of 40 patients (26 Men, Median (range) Age 58 (20–74) years) with PHC met the inclusion criteria. Of these 13 were Bismuth-Corlette Type IIIa, 17 Bismuth-Corlette Type IIIb and 10 Bismuth-Corlette Type IV. PBD was performed in 14 patients.
Thirty-six of the 40 patients underwent resection with curative intent. Surgery was abandoned after laparoscopy in 3 patients (2 with peritoneal metastases and 1 with cirrhosis) and in 1 patient after laparotomy because there was no reconstructable vessel in Rouviere's sulcus. Of the remaining 36 patients, 23 underwent LLR and 13 RLR (Table 1). Nineteen patients underwent PVR and 10 underwent AR.
Table 1.
Operative procedures performed on our patients
| Operative procedure | N | PVR | AR |
|---|---|---|---|
| Right hepatectomy | 10 | 2 | 0 |
| Right trisectionectomy | 3 | 2 | 0 |
| Left hepatectomy | 16 | 11 | 8 |
| Left trisectionectomy | 7 | 4 | 2 |
Twenty-nine complications ocurred in 21 patients (Table 2). There were no complications related to PVR. Two patients experienced complications related to AR. The only patient in whom an interposition graft was used developed a small abscess in segment 8 secondary to a graft thrombosis 5 months after surgery. The liver was however well perfused through collaterals and the abscess was easily treated. Another patient developed a pseudoaneurysm, incidentally detected on routine CT prior to discharge, that was effectively treated with an intra-arterial stent.
Table 2.
Differences between right and left-sided liver resection
| RT resection (N = 13) | LT resection (N = 23) | P | |
|---|---|---|---|
| Preoperative biliary drainage | 6 | 8 | 0.723 |
| Median durn of PBD (days) | 23 (5–61) | 25 (16–56) | 0.188 |
| Bismuth-Corlette Type IV | 0 | 8 | 0.032 |
| Median operation time (minutes) | 540 (390–780) | 550 (240–840) | 0.724 |
| Median blood loss (CC) | 1200 (500–2000) | 750 (100–2300) | 0.452 |
| Portal vein resection | 4 | 15 | 0.082 |
| Arterial resection | 0 | 10 | 0.006 |
| Biliary complications | 1 | 5 | 0.385 |
| Postoperative liver dysfunction | 5 | 0 | 0.003 |
| Clavien-Dindo above 3A | 9 | 10 | 0.177 |
| Positive bile duct margin | 1 | 4 | 0.634 |
| Stage T3/T4 | 4 | 9 | 0.812 |
| Stage N1 | 5 | 12 | 0.502 |
| Mortality | 2 | 1 | 0.539 |
| Median hospital stay (days) | 15(11–28) | 10(5–40) | 0.013 |
| Median survival (months) | 20 | 22 | 0.893 |
| 2-Year survival | 44% | 39% | 0.934 |
Figures in brackets denote the range.
The bold type indicates the parameters that have a P value below 0.5 and are statistically significant.
Nine of 19 patients who underwent PVR and 4 of 10 who underwent AR had histologically demonstrable tumour infiltration of the vessel.
There were 3 postoperative deaths within 30 days of surgery – 2 after LLR and 1 after RLR. The postoperative death after RLR was related to postoperative liver insufficiency and sepsis. One death after LLR was due to sepsis in a patient who had prolonged PTBD prior to surgery and repeated episodes of cholangitis preoperatively. The second death after LLR was a sudden death 5 days after surgery and was probably due to a pulmonary embolus. The first two patients had not undergone AR during resection.
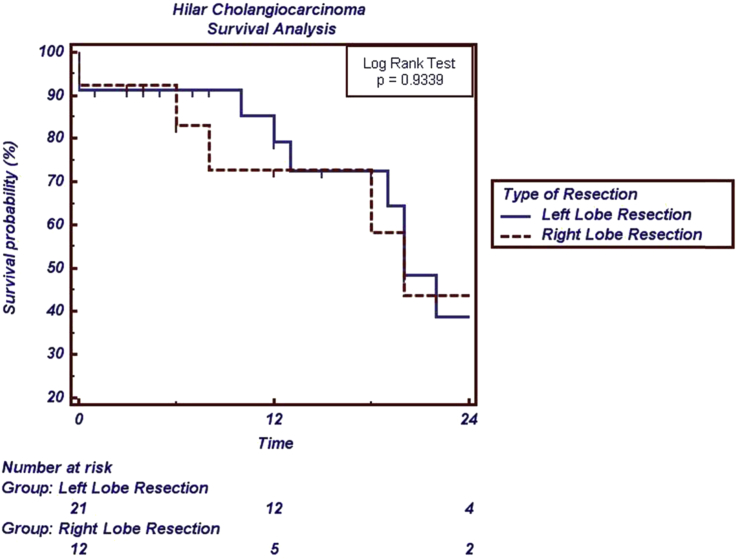
Long-term (Median (range) 14 (3–64) months) follow-up was available in 30 of the remaining 33 patients who underwent resection. Nineteen of these 30 patients were alive as of 1st August 2015. The median survival after LLR was 22 months, and after RLR was 20 months (Fig. 3). Neither arterial nor portal resection and reconstruction significantly affected the outcome. Median (95%CI) survival in those who did (N = 19) and did not (N = 17) have PVR was 22 (13–31) months and 19 (11–27) months respectively (p = 0.2675). Median survival (95%CI) for those who did (N = 10) and did not (N = 26) have arterial resection was 22 (10–31) and 20 (9–28) months respectively (p = 0.944).
Figure 3.
Kaplan Meier survival curves between right-sided and left-sided liver resections for perihilar cholangiocarcinoma
The number of Bismuth-Corlette Type IV patients undergoing LLR was significantly greater than those undergoing RLR (8 of 23 vs 0 of 13, p = 0.032). The number of patients undergoing AR was significantly greater during LLR than RLR (10 of 23 vs 0 of 13, p = 0.006). The number of patients experiencing postoperative liver dysfunction (defined as encephalopathy, prolonged ascites) was significantly greater after RLR than LLR (5 of 13 vs 0 of 23, p = 0.003). The hospital stay after LLR was significantly shorter than after RLR (Median (range) 10 (5–40) days vs 15 (11–28) days, p = 0.013).
Oncological outcomes were similar between resection types (Table 2, Fig. 3).
Discussion
One of the reasons why RLR is preferred over LLR12 for PHC is the potential need for reconstruction of the RHA to achieve R0 resection status during the latter.2 Not until Nagino13 reported 50 patients who underwent combined arterial and portal vein resections for PHC with a 2% operative mortality and 30% 5-year survival, did LLR became more widely performed for Bismuth-Corlette Type IIIb and IV PHC.
The current series demonstrates that although the propensity for complications related to AR is high they can be minimised with the use of precautionary measures. Liver resection, including the caudate lobe resection is best performed without mobilisation of the right lobe from its attachments, permitting collaterals to form through them should the AR fail,14, 15 simultaneous occlusion of arterial and venous flow to the remnant is best avoided and the anastomosis should be performed by surgeons experienced in microvascular reconstruction. It is recommended that a contrast CT scan be performed prior to discharge from hospital to ensure the AR is healthy and functional.
The success of resection for PHC is dependent on the ability to achieve tumour-free proximal bile duct margins.11 Japanese authors have demonstrated6, 7 that left trisectionectomy for PHC in patients with a supraportal variant of right hepatic duct bifurcation (86% of the population) provides an additional length of 6–9 mm of potentially resectable duct margin than left hepatectomy, providing the best chance at achieving ductal clearance in Bismuth Corlette Type IV tumours. The current series also demonstrates a greater (though not statistically significant) incidence of positive margin status after left hepatectomy compared to left trisectionectomy (4 vs 0, p = 0.273). Significantly, 8 of 23 patients who underwent LLR in the current series had Bismuth-Corlette Type IV disease. Four had left lobe atrophy, and tumour extended beyond the left margin of the umbilical fissure in another. These patients would have been unresectable by RLR. Left Trisectionectomy therefore increased the resectability rate of PHC from 31/40 to 36/40. The remaining 3 patients had small remnants that would have required PBD and PVE prior to resection. LLR greatly simplified the treatment plan.
The remnant liver is considerably larger after LLR than after a corresponding RLR. Consequently postoperative liver dysfunction is less frequent after LLR, translating to a shorter hospital stay and a trend towards a lower operative mortality despite the greater need for AR. In view of the larger remnant, PBD ± PVE are less likely to be necessary for LLR, reducing the duration and cost of treatment as well as the risk of septic complications and tumour seeding. The current series was unable to demonstrate these benefits, in part because many patients presented after PBD had been performed elsewhere. Perhaps interventions prior to RLR were underutilised in the current series as suggested by the incidence of liver dysfunction after RLR.
The operation for PHC has to be designed based on the extent of vascular and biliary involvement, and associated liver atrophy. Whereas the need for LLR in the presence of left lobar atrophy or Bismuth-Corlette Type IV tumours with tumour extension to the umbilical fissure is well accepted, Type IIIb tumours can be treated by either LLR or RLR, although PBD and PVE may be necessary in the latter case. The surgeon must weigh the balance between the morbidity and expense of PBD, and the greater risk of a positive tumour margin on the left hepatic duct after right trisectionectomy against the risk of a potential AR. The authors suggest that AR can be performed safely when appropriate precautions are taken and should not be a reason to contraindicate resection.
The benefits of vascular resection in LLR for PHC has been published in the Japanese literature.13 Although the current series is hampered by its retrospective nature, small numbers and the relatively short duration of follow-up, it is the only one outside of the Far East to present the potential benefits of LLR despite the need for AR.
The authors conclude that the ability to perform safe arterial resection increases the ability to perform potentially curative LLR resections for PHC. This in turn increases the resectability rate for PHC, particularly for Bismuth-Corlette Type IV tumours. LLR is associated with less postoperative liver dysfunction and shorter hospital stay without increased operating time or blood loss.
Conflicts of interest
None declared.
References
- 1.Furusawa N., Kobayashi A., Yokoyama T., Shimizu A., Motoyama H., Miyagawa S. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 2014;38:1164–1176. doi: 10.1007/s00268-013-2394-x. [DOI] [PubMed] [Google Scholar]
- 2.Govil S., Reddy M.S., Rela M. Surgical resection techniques for locally advanced hilar cholangiocarcinoma. Langenbecks Arch Surg. 2014;399:707–716. doi: 10.1007/s00423-014-1216-4. [DOI] [PubMed] [Google Scholar]
- 3.Abbas S., Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB. 2013;15:492–503. doi: 10.1111/j.1477-2574.2012.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratti F., Cipriani F., Piozzi G., Catena M., Paganelli M., Aldrighetti L. Comparative analysis of left- versus right-sided resection in Klatskin tumor surgery: can lesion side be considered a prognostic factor? J Gastrointest Surg. 2015;19:1324–1333. doi: 10.1007/s11605-015-2840-1. [DOI] [PubMed] [Google Scholar]
- 5.Nagino M., Kamiya J., Arai T., Nishio H., Ebata T., Nimura Y. “Anatomic” right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28–32. doi: 10.1097/01.sla.0000193604.72436.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu H., Kimura F., Yoshidome H., Ohtsuka M., Kato A., Yoshitomi H. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251:281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]
- 7.Natsume S., Ebata T., Yokoyama Y., Igami T., Sugawara G., Shimoyama Y. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg. 2012;255:754–762. doi: 10.1097/SLA.0b013e31824a8d82. [DOI] [PubMed] [Google Scholar]
- 8.Hosokawa I., Shimizu H., Yoshidome H., Ohtsuka M., Kato A., Yoshitomi H. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg. 2014;259:1178–1185. doi: 10.1097/SLA.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 9.Nimura Y. Radical surgery of left-sided Klatskin tumors. HPB. 2008;10:168–170. doi: 10.1080/13651820801992674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shingu Y., Ebata T., Nishio H., Igami T., Shimoyama Y., Nagino M. Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery. 2010;147:49–56. doi: 10.1016/j.surg.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Ribero D., Amisano M., Lo Tesoriere R., Rosso S., Ferrero A., Capussotti L. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011;254:776–781. doi: 10.1097/SLA.0b013e3182368f85. discussion 81–83. [DOI] [PubMed] [Google Scholar]
- 12.Ramos E. Principles of surgical resection in hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:139–146. doi: 10.4251/wjgo.v5.i7.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagino M., Nimura Y., Nishio H., Ebata T., Igami T., Matsushita M. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252:115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]
- 14.Miyake H., Tashiro S., Fujii M., Sasaki K., Takagi T. Efficacy of only left side approach in a case of unsuccessful reconstruction of the right hepatic artery after left hepatic lobectomy with caudal lobectomy. Hepato-gastroenterology. 2004;51:372–374. [PubMed] [Google Scholar]
- 15.Liang G., Wen T., Mi K., Li C., Wang C., Li K. Resection of hilar cholangiocarcinoma combined with left hepatectomy and common hepatic arteriectomy without reconstruction. Hepato-gastroenterology. 2012;59:364–365. doi: 10.5754/hge11554. [DOI] [PubMed] [Google Scholar]