Abstract
Background
SMV/PV resection has become common practice in pancreatic surgery. The aim of this study was to evaluate the technical feasibility and surgical outcome of using cold-stored cadaveric venous allografts (AG) for superior mesenteric vein (SMV) and portal vein (PV) reconstruction during pancreatectomy.
Methods
Patients who underwent pancreatic resection with concomitant vascular resection and reconstruction with AG between January 2006 and December 2014 were identified from our institutional prospective database. Medical records and pre- and postoperative CT-images were reviewed.
Results
Forty-five patients underwent SMV/PV reconstruction with AG interposition (n = 37) or AG patch (n = 8). The median operative time and blood loss were 488 min (IQR: 450–551) and 900 ml (IQR: 600-2000), respectively. Major morbidity (Clavien ≥ III) occurred in 16 patients. Four patients were reoperated (thrombosis n = 2, graft kinking/low flow n = 2) and in-hospital mortality occurred in two patients. On last available CT scan, 3 patients had thrombosis, all of whom also had local recurrence. Estimated cumulative patency rate (reduction in SMV/PV luminal diameter <70% and no thrombosis) at 12 months was 52%.
Conclusion
Cold-stored cadaveric venous AG for SMV/PV reconstruction during pancreatic surgery is safe and associated with acceptable long-term patency.
Introduction
Venous resection during pancreatic surgery is often used to ensure radical removal of pancreatic and distal bile duct cancers and has become common practice.1 Surgery with venous resection for pancreatic cancers has been proven comparable to surgery without venous resection in terms of perioperative outcome and long-term survival.2, 3 However, the optimal method for venous reconstruction has not yet been established, and several different approaches are reported. Primary end-to-end anastomosis and venorrhaphy are reportedly used in 20–83% and 15–56% of patients, respectively.4, 5, 6, 7, 8, 9, 10, 11, 12 When primary anastomosis is difficult to achieve due to tension and the ensuing risk of stenosis, different types of grafts can be used. Autologous grafts from the internal jugular vein, saphenous vein, superficial femoral vein, left renal vein or gonadal vein have been reported, either as patch or interposition grafts.13, 14, 15, 16, 17, 18 The use of synthetic grafts, such as polytetrafluoroethylene (PTFE) grafts,19 has been described, and reconstruction with grafts made from bovine pericardium and parietal peritoneum,20, 21 or cryopreserved arterial homografts has also been reported.22 The use of cadaveric vein allografts (AG) for reconstruction during pancreatoduodenectomy (PD) has been described specifically in only two small series, while this technique has been included with small patient numbers in other reports.20, 23, 24, 25 Here, we report, to the best of our knowledge, the largest series of SMV/PV reconstruction with cold-stored cadaveric vein AG in patients undergoing pancreatic resection. The aim of the study was to assess the technical feasibility of using cold-stored cadaveric venous AG for SMV/PV reconstruction during pancreatectomy and to evaluate long-term patency at the reconstruction site.
Methods
We performed a retrospective review of all patients undergoing pancreatic surgery with vascular resection and reconstruction with AG at our hospital between January 2006 and December 2014. This study was approved as a quality assurance study by the hospital Data Protection Officer at our institution. Where applicable, the study was reported in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.26 Hospital records and pathology reports were reviewed. Preoperative workup included multidetector computed tomography (CT) with an optimized pancreatic protocol and a chest CT. Preoperative imaging was evaluated for tumor-vein circumferential interface (TVI) as described by Tran Cao et al.,27 and for the length of tumor-vein involvement (LTV). Intra -and postoperative data were evaluated. Postoperative complications were assessed according to the Clavien–Dindo (C–D) classification.28 Major complications were defined as C–D ≥ III. Length of stay was calculated from the day of surgery until discharge. In-hospital mortality was defined as death occurring after surgery and before patient discharge. Early patency was defined as adequate flow at the reconstruction site and the absence of thrombosis until postoperative day (POD) 30. Postoperative CT imaging was used to assess long-term patency. The change in SMV/PV diameter from preoperative to postoperative images was used to determine stenosis. The degree of stenosis was classified as grade A (0–49% reduction in diameter), grade B (50–69% reduction in diameter) or grade C (≥70% reduction in diameter). The presence of grade C stenosis (severe stenosis) and/or the presence of a thrombus were considered clinically relevant.29 Accordingly, grade C stenosis and/or the presence of thrombosis were considered not patent. Grade A and B stenosis was considered patent. Histologic diagnosis, tumor size, resection margins, the presence of positive lymph nodes and lymph node ratio were assessed. Resection margin status R1 was defined as tumor within 1 mm of the resection margin.
Surgical technique
The procedures for pancreatic surgery consisted of pancreatoduodenectomy with standard lymphadenectomy, or subtotal, total or distal pancreatectomy as deemed appropriate. A classic Whipple's procedure was the standard approach between 2006 and 2011, but from 2012 onward, this procedure was used only in patients with tumor involvement of the proximal duodenum or pylorus, while a pylorus-preserving procedure became the standard operation. The type of venous resection and reconstruction depended on the site and extent of tumor invasion of the vein. The length of the resected vein was not routinely measured. The decision on the reconstruction technique was based on intraoperative findings and the surgeon's preference, however, Cattell-Braasch mobilization was not routinely used. In general, the vein on either side of the tumor-involved segment was dissected free. In this way, inflow and outflow of the involved vein was secured, reducing potential bleeding and vascular clamp time. Splenic vein re-implantation or splenic vein preservation through an oblique transection line in the portal end of the resected vein was preferred. The artery-first approach was not routinely used, except for cases with SMV/PV TVI >180°, with occlusion, or with abutment of the superior mesenteric artery. Clamping of the superior mesenteric artery to reduce bowel ischemia was not used routinely. Perioperative use of heparin was administered on a routine basis. Iliac veins removed during multi-organ harvesting procedures by the transplantation unit were used as grafts. Immediately after harvesting, grafts were stored in University of Wisconsin solution at 4° C and matched to recipients according to the AB0-system. All anastomoses were performed free of tension with running 6-0 polypropylene sutures, and, in order to avoid any anastomotic stenosis, the anastomosis was expanded before complete revascularization by releasing the distal clamp first.
Postoperative management and surveillance
Patients remained in the postoperative ward for a minimum of one day. Doppler ultrasound of the reconstructed vein was performed routinely on POD 1. Patients were discharged to the local hospital or home as soon as the postoperative course was without suspicion of adverse events. Anticoagulation therapy with low-molecular heparin (LMWH) for a period of 1–3 months after surgery was recommended for all patients who had undergone reconstruction with an AG. The recommended LMWH dosage was 200 IE/kg for the first month and 100 IE/kg for the following two months. Lifelong aspirin at 75 mg daily was prescribed at the surgeon's discretion. Due to the retrospective nature of the study and the variety of pathology diagnoses, follow-up schedules varied. Local recurrence was defined as radiological evidence of intra-abdominal soft tissue in the resection area or along adjacent cardinal visceral vessels that (i) increased in size over time or (ii) had concomitant raised CA 19-9.30 Biopsy to confirm recurrence was not routinely performed.
Statistical analysis
Graft patency and overall survival were estimated using the Kaplan–Meier method. Graft patency was calculated from the time of surgery to the last available CT. Survival was defined as the time from surgery to death of any cause or the end of follow-up through October 31, 2015, which ever came first. Continuous variables were expressed as median or mean with interquartile range (IQR) or standard deviation (SD). All analyses were performed using the SPSS version 22, for Microsoft Windows.
Results
During the study period, a total of 734 patients underwent open pancreatic surgery, of whom 142 had vascular resection and reconstruction. Six patients undergoing only arterial resection and reconstruction, and one patient with resection and reconstruction of the inferior caval vein, were excluded from the analysis. Further excluded were patients in whom another type of reconstruction was used: primary end-to-end anastomosis or transverse suture (n = 76), patch (n = 3) or interposition (n = 3) grafts from autologous vein, synthetic interposition grafts (n = 5) or patch (n = 1), and graft from bovine pericardium (n = 2). Forty-five patients who had undergone reconstruction with AG were identified. Patient demographics, clinical features and postoperative data are summarized in Table 1. Median operative time and blood loss were 488 min (IQR; 450–551) and 900 ml (IQR; 600–2000), respectively. Median length of stay, defined as the time between surgery and discharge to a local hospital or home, was 13 days (IQR; 8–19). Segmental venous resection and reconstruction with an interposition graft was performed in 37 patients, while in eight patients a patch had been used. Sixteen patients had one or more C–D complication of grade ≥ III. The majority of patients (37/45) received low-molecular heparin during a period of 1–3 months after surgery, and 21 of 45 were prescribed lifelong aspirin. Splenic vein ligation was performed in three patients. Splenic vein re-implantation was performed in 10 patients. In 20 patients the splenic vein was preserved, following either resection of the SMV only or oblique transection through the portal vein distal to the splenic vein-SMV confluence. In 12 patients undergoing total or distal pancreatectomy, the splenic vein was resected en-bloc with the specimen. Pancreatic ductal adenocarcinoma (PDAC) and adenocarcinoma of the common bile duct (CBDCa) were the most common histological diagnoses (26/45 and 10/45, respectively, Table 2). Two patients were resected for suspected malignancy, but final histology revealed chronic pancreatitis. One patient was resected for a large serous cystadenoma. R0 resection was achieved in 15 patients. Preoperative CT revealed TVI in 44 of 45 patients, and in most patients TVI was <180° (27/44) (Table 3). Only three patients had occlusion of the SMV/PV on preoperative imaging.
Table 1.
Demographic, clinical and intraoperative characteristics of the study population (n = 45)
| Number or Value | |
|---|---|
| Gender (male/female) | 27/18 |
| Age, years (median (IQR)) | 62 (54–73) |
| ASA score | |
| 1 | 2 |
| 2 | 21 |
| 3 | 22 |
| 4 | 0 |
| Neoadjuvant chemotherapy (yes/no) | 9/36 |
| Operating room time, min (median (IQR)) | 488 (450–551) |
| Estimated blood loss, ml (median (IQR)) | 900 (600–2000) |
| Length of stay, days (median (IQR)) | 13 (8–19) |
| Types of procedurea | |
| PPPD | 18 |
| cWP | 15 |
| TP | 7 |
| DP | 5 |
| Type of venous reconstruction | |
| Interponate | 37 |
| Patch | 8 |
| Concomitant arterial resection (yes/no) | 6/39 |
| Clavien–Dindo ≥ III complication | 16 |
| Anticoagulation therapy | |
| LMWH 1–3 months after surgery (yes/no) | 37/8 |
| Lifelong aspirin (yes/no) | 21/24 |
| Lifelong coumadin for other indications | 4 |
| <30 day thrombosis/no or low flow at reconstruction site | |
| Thrombosis | 2 |
| No/low flow | 2 |
| In-hospital mortality | 2 |
PPPD, pylorus-preserving pancreatoduodenectomy; cWP, classic Whipple; TP, total pancreatectomy; DP, distal pancreatectomy.
Table 2.
Histopathological findings and disease recurrence
| Number or Value | |
|---|---|
| Diagnoses | |
| Pancreatic ductal adenocarcinoma | 26 |
| Common bile duct adenocarcinoma | 10 |
| Intraductal papillary mucinous neoplasia | 1 |
| Serous cystic neoplasia | 1 |
| Pancreatic neuroendocrine tumor | 5 |
| Chronic pancreatitis | 2 |
| Tumor stage (n = 41)a | |
| 1 | 0 |
| 2 | 2 |
| 3 | 39 |
| 4 | 0 |
| Lymph node status (positive/negative) n = 41 | 30/11 |
| Lymph node ratio (<0,2/≥0,2) n = 41 | 23/18 |
| Tumor size, mm; mean (±SD) | 45 ± 37 |
| R-status (R0/R1), n = 41 | 15/26 |
| Recurrence (yes/no), n = 41 | 30/11 |
| Site of recurrence | |
| Local only | 13 |
| Distant only | 8 |
| Local and distant | 9 |
| Survival, PDAC, months; median (IQR) | 17 (13–31) |
| Survival, CBDCa, months; median (IQR) | 17 (5–27) |
| Disease-free survival, PDAC, months; median (IQR) | 12 (8–17.5) |
| Disease-free survival, CBDCa, months; median (IQR) | 9 (4–21) |
| Follow-up, entire study population, months; median (IQR) | 13 (7–30) |
| Time to last CT, months; median (IQR) | 8 (4.5–16.5) |
UICC, TNM classification of malignant tumors. 7th. Ed. Oxford:Wiley-Blackwell, 2009.
Table 3.
Pre- and postoperative radiology
| Preoperative imaging | |
| TVIa (yes/no) | 44/1 |
| TVI<180° (yes/no) | 27/18 |
| TVI > 180° (yes/no) | 14/31 |
| PV/SMV occlusion (yes/no) | 3/42 |
| Length of tumor-vein involvement, mm; median (IQR) | 19 (14–30) |
| Postoperative imaging (n = 43) | |
| Grade A stenosis, 0–49% | 11 |
| Grade B stenosis, 50–69% | 5 |
| Grade C severe stenosis, ≥70% or occlusion/thrombus | 27 |
The change in SMV/PV diameter was used to calculate stenosis. A reduction in the postoperative luminal diameter of 70% compared with the preoperative diameter, and/or the presence of thrombosis was considered not patent. Grade A and B stenosis was considered patent.
TVI, tumor-vein interface.
Early graft patency and in-hospital mortality
Forty-one patients had adequate flow and no signs of thrombosis at the reconstruction site within the first 30 days of surgery. Two patients with thrombosis on POD 11 and 22, respectively, underwent reoperation with thrombectomy, with no further complications at the reconstruction site. One patient was reoperated on POD 1 for low flow at the reconstruction site, which was due to kinking of the SMV/PV and resolved by a new reconstruction with an interposition graft. One patient died in ICU on POD 11 due to hepatic and renal failure. This patient had undergone concomitant reconstruction of the superior mesenteric artery and SMV/PV due to tumor adherence. In view of the long clamping time on both the arterial and venous segment, reconstruction of the biliary tract had been postponed. Thrombosis of the hepatic artery occurred on POD 4, which was treated with a new interposition graft between the proper hepatic artery and infrarenal aorta. During the same operation, low flow in the reconstructed SMV/PV was treated with a new interposition graft. A further patient died in hospital following total pancreatectomy with concomitant resection of the right hemicolon. After extubation, left-sided hemiparesis was identified and brain MR showed bilateral infarction. The patient was reoperated on POD 5 due to leakage of the ileocolic anastomosis. Subsequent abdominal CT showed thrombosis of the hepatic artery and partial liver necrosis. The patient developed multi-organ failure and died on POD 57.
Late graft patency, recurrence and survival
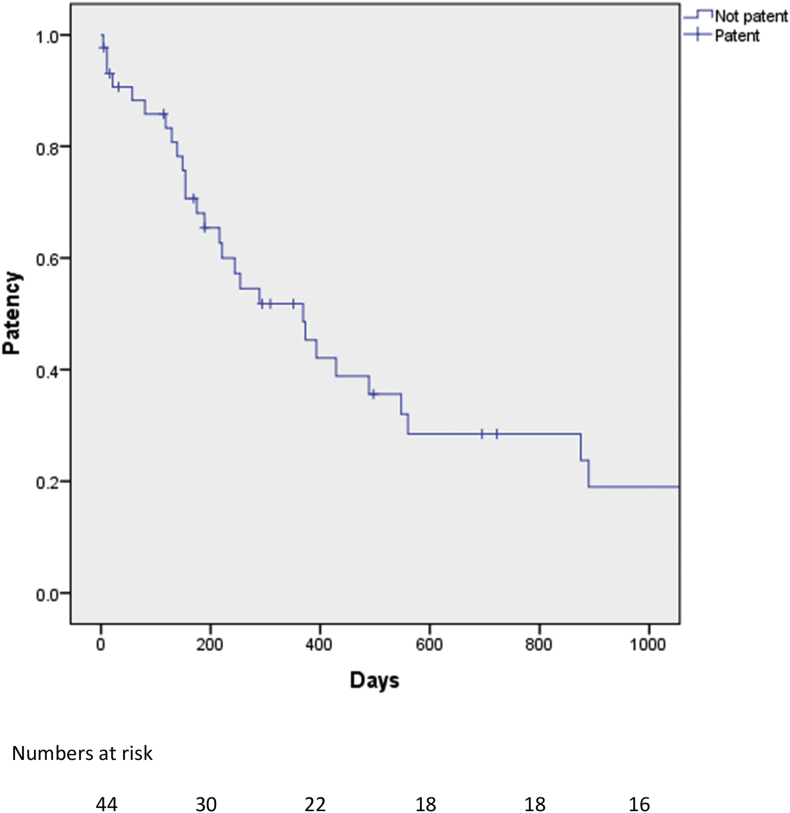
Postoperative CT imaging more than 30 days after surgery was available for 43 patients, while radiological follow-up was missing for one patient with serous cystadenoma and a further patient who died on POD 11. Median follow-up for the entire study population was 13 months (IQR; 7–30). The median time to last CT scan was eight months (IQR 4.5–16.5). Estimated cumulative patency rate (i.e. patients with grade A and B stenosis or no early and late thrombosis) at 12 months was 52% (Fig. 1). Eleven and 5 patients had grade A or B stenosis on the last available CT scan, respectively (Table 3), and 27 had severe stenosis. Review of the last available CT revealed local recurrence as the cause of SMV/PV stenosis in 24 of 27 patients with severe stenosis. SMV/PV thrombosis occurred in 3 of 27 patients and these patients also had local recurrence. Postoperative changes causing stenosis without evidence of tumor recurrence were found in three patients. Radiology revealed varicose veins around the hepaticojejunostomy (14/27) and at the gastric fundus (13/27). Ascites developed in 10 of 27 patients and venous shunts from the splenic vein, inferior mesenteric vein and veins in the small bowel mesentery were detected in 12 of the 27 patients with grade C stenosis. Median disease-free survival for PDAC and CBDCa was 12 months (IQR; 8–17.5) and 9 months (IQR; 4–21), respectively. Median overall survival for PDAC and CBDCa was 17 months (IQR; 13–31 and 5–27, respectively). Overall, recurrence was found in 30 of 41 patients with malignant histology.
Figure 1.
Kaplan-Meyer analysis of patency rate on the study population. Patients with early and late graft failure are included in the analysis. For definitions of patency, see text
Complications of SMV/PV stenosis
Two patients resected for PDAC had severe stenosis and refractory ascites prior to the last follow-up CT. Both patients were treated with a stent in the reconstructed SMV/PV. The first patient developed a stenosis at the SMV/PV junction 24 months after surgery without signs of local or distant recurrence and was treated with a stent insertion via transhepatic access. Local recurrence was diagnosed 54 months after surgery, but imaging showed a patent stent. The second patient was stented for a recurrent stenosis at 8 months after surgery without evidence of local recurrence. Twelve months following surgery, local recurrence was detected, but the SMV/PV stent remained patent. Of the 27 patients with grade C stenosis on the last available CT scan, seven underwent gastroscopy. Four of these patients presented with gastrointestinal bleeding, due to portal hypertensive gastropathy (n = 1), varices at the gastric fundus (n = 1), varices around the gastroenterostomy (n = 1) and bleeding of unknown cause (n = 1).
Discussion
Pancreatic surgery with resection and reconstruction of the SMV/PV in patients with pancreatic or periampullary tumors is considered standard of care.2 However, the optimal vascular reconstruction technique has not yet been identified and validated. This study analyzes 45 patients undergoing pancreatic surgery with resection and reconstruction of the SMV/PV using cold-stored cadaveric venous AG. The results of this study show that reconstruction with AG is possible with acceptable morbidity and mortality.
Venous reconstruction following pancreatic surgery with SMV/PV resection is usually performed with either primary repair or autologous or prosthetic grafts.20, 31 Venous allografts have been used for other indications, e.g. hemodialysis access, infrainguinal bypass or portal vein reconstruction as part of the surgical treatment of hilar cholangiocarcinoma.32, 33, 34, 35, 36, 37 The use of AG for reconstruction during pancreatic resection has only been described specifically in two small case series.24, 25 When a tension-free primary end-to-end anastomosis cannot be achieved, the use of an interposition graft can contribute to the completion of the resection. In our hospital, the availability of allografts and their preferential use for venous reconstruction during pancreatic surgery is the result of close cooperation with the transplantation surgeons. The potential benefits of AG compared to other types of interposition grafts are multiple. Since there is no need to harvest a vein, local edema distal to the harvested vein is avoided. Furthermore, the possibility of infection to a second surgical site is eliminated. Making use of AGs from organ harvesting procedures and the availability of an AG bank is likely to save operative time compared to reconstruction with an autograft.
The use of interposition grafts undeniably prolongs clamping time with subsequent prolonged liver ischemia and bowel congestion. However, few studies have compared interposition grafts with primary end-to-end anastomosis with respect to early and late graft patency. Even though a recent study showed that long operative time and the use of prosthetic grafts were risk factors for portal vein reconstruction thrombosis, a further study comparing synthetic interposition grafts with primary end-to-end anastomosis did not observe a difference in postoperative morbidity, mortality and late graft patency.38, 39 Hence, the clinical significance of the potential risks related to the use of interposition grafts remains unclear.
There is a lack of consensus on the definition and reporting of SMV/PV patency after pancreatic surgery with SMV/PV resection and reconstruction. Based on two recent studies from Korea and Japan,29, 40 the current study uses a strict definition for severe stenosis at the reconstruction site, i.e. ≥70% reduction in the luminal diameter of the SMV/PV. This could explain why in our study the estimated patency rate of 52% at 12 months was inferior to that reported by others.15, 19, 39, 41 Fujii et al. correlated the degree of stenosis with complications associated with anastomotic stenosis of the portal venous system, including refractory ascites, hepatic encephalopathy and gastrointestinal bleeding, and found that these complications only occurred in patients with stenosis ≥70%.29 Furthermore, six out of 18 patients were reported to have complications without complete occlusion. This highlights the possibility that patency rates in previous reports may have been overestimated when using only occlusion/thrombosis as a definition for failed late graft patency. Regular follow-up with ultrasound Doppler examination of the portal vein including measurement of blood flow velocities could also be of value in order to assess the physiological significance of a reduced luminal diameter. In our study, two patients required SMV/PV stenting due to refractory ascites, and four patients underwent gastroscopy due to bleeding, of which only one had SMV/PV thrombosis. By evaluating patency after SMV/PV reconstruction, the present study confirms that adverse events related to the reconstruction site can occur even in the absence of thrombosis.
In order to identify the optimal reconstruction technique, consensus must be reached on what is considered a sufficient luminal diameter after SMV/PV resection and reconstruction in the long term. We suggest that studies on graft patency after SMV/PV reconstruction during pancreatectomy report the number of patients with graft thrombosis and the number of patients with ≥70% reduction in luminal diameter. Furthermore, the cause of this reduction (thrombosis, local recurrence, stenosis without evidence of local recurrence) and the complications caused by the reduction (refractory ascites, gastrointestinal bleeding) should be reported. It could be argued that using the preoperative venous diameter for calculating the reduction in postoperative luminal diameter is unsatisfactory when reconstructing the SMV/PV with any kind of interponate, considering the unlikely event that the interponate has the exact dimension as the resected vein. However, we believe that reconstruction should aim at reestablishing the preoperative conditions and reinstate flow as before surgery. Importantly, the most frequent cause of severe stenosis at the reconstruction site was local recurrence. However, a small proportion of patients with severe stenosis had no signs of local recurrence, indicating that granulation tissue or fibrosis may cause SMV/PV stenosis in patients with potentially long-term survival. This fact also emphasizes the importance of systematic radiological follow-up in patients undergoing pancreatic surgery with vascular resection and reconstruction with AG.40
It is possible that an allogenic immune response directed against the graft tissue could play a role in late graft stenosis and reduced patency. The antigenicity of cold stored venous AG is not known, but it has been documented that their use leads to antibody formation even in the presence of immunosuppression.42, 43 Patients in the current series did not receive any immunosuppression due to the risk of accumulating complications, and donor -specific antibody formation following implantation was not routinely tested. This should be the subject of future studies. Furthermore, considering that a potential immune response may occur and that the predisposition of venous thrombosis is elevated in cancer patients, the use of anticoagulants in this study population was liberal, even though published data have failed to show any benefit for anticoagulation after PV resection during pancreatic surgery.44
An important limitation of this study lies in its retrospective design. There was heterogeneity in the study population with regard to follow-up. A control group with autologous vein or synthetic grafts is lacking, and future trials comparing AG, autologous grafts and synthetic grafts are needed to evaluate the optimal method of venous reconstruction after pancreatic surgery.
In conclusion, the use of cold-stored cadaveric venous allograft in SMV/PV reconstruction during pancreatic surgery is feasible and safe with acceptable morbidity, short- and long-term patency.
Conflict of interest
None declared.
Footnotes
The authors have received no funding for research or publication. This article is not based on previous communication to a society or meeting.
References
- 1.Liles J.S., Katz M.H. Pancreaticoduodenectomy with vascular resection for pancreatic head adenocarcinoma. Expert Rev Anticancer Ther. 2014;14:919–929. doi: 10.1586/14737140.2014.919860. [DOI] [PubMed] [Google Scholar]
- 2.Bockhorn M., Uzunoglu F.G., Adham M., Imrie C., Milicevic M., Sandberg A.A. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y., Zhang Z., Liu Y., Li B., Xu D. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J Surg. 2012;36:884–891. doi: 10.1007/s00268-012-1461-z. [DOI] [PubMed] [Google Scholar]
- 4.Carrere N., Sauvanet A., Goere D., Kianmanesh R., Vullierme M.P., Couvelard A. Pancreaticoduodenectomy with mesentericoportal vein resection for adenocarcinoma of the pancreatic head. World J Surg. 2006;30:1526–1535. doi: 10.1007/s00268-005-0784-4. [DOI] [PubMed] [Google Scholar]
- 5.Delpero J.R., Boher J.M., Sauvanet A., Le Treut Y.P., Sa-Cunha A., Mabrut J.Y. Pancreatic adenocarcinoma with venous involvement: is up-front synchronous portal-superior mesenteric vein resection still justified? A survey of the Association Francaise de Chirurgie. Ann Surg Oncol. 2015;22:1874–1883. doi: 10.1245/s10434-014-4304-3. [DOI] [PubMed] [Google Scholar]
- 6.Muller S.A., Hartel M., Mehrabi A., Welsch T., Martin D.J., Hinz U. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg. 2009;13:784–792. doi: 10.1007/s11605-008-0791-5. [DOI] [PubMed] [Google Scholar]
- 7.Ravikumar R., Sabin C., Abu Hilal M., Bramhall S., White S., Wigmore S. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg. 2014;218:401–411. doi: 10.1016/j.jamcollsurg.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Sgroi M.D., Narayan R.R., Lane J.S., Demirjian A., Kabutey N.K., Fujitani R.M. Vascular reconstruction plays an important role in the treatment of pancreatic adenocarcinoma. J Vasc Surg. 2015;61:475–480. doi: 10.1016/j.jvs.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Smoot R.L., Christein J.D., Farnell M.B. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg. 2006;10:1371–1375. doi: 10.1016/j.gassur.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Tseng J.F., Raut C.P., Lee J.E., Pisters P.W., Vauthey J.N., Abdalla E.K. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. [discussion 949–950] [DOI] [PubMed] [Google Scholar]
- 11.Wang F., Arianayagam R., Gill A., Puttaswamy V., Neale M., Gananadha S. Grafts for mesenterico-portal vein resections can be avoided during pancreatoduodenectomy. J Am Coll Surg. 2012;215:569–579. doi: 10.1016/j.jamcollsurg.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Yekebas E.F., Bogoevski D., Cataldegirmen G., Kunze C., Marx A., Vashist Y.K. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg. 2008;247:300–309. doi: 10.1097/SLA.0b013e31815aab22. [DOI] [PubMed] [Google Scholar]
- 13.Fleming J.B., Barnett C.C., Clagett G.P. Superficial femoral vein as a conduit for portal vein reconstruction during pancreaticoduodenectomy. Arch Surg. 2005;140:698–701. doi: 10.1001/archsurg.140.7.698. [DOI] [PubMed] [Google Scholar]
- 14.Fuhrman G.M., Leach S.D., Staley C.A., Cusack J.C., Charnsangavej C., Cleary K.R. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154–162. doi: 10.1097/00000658-199602000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D.Y., Mitchell E.L., Jones M.A., Landry G.J., Liem T.K., Sheppard B.C. Techniques and results of portal vein/superior mesenteric vein reconstruction using femoral and saphenous vein during pancreaticoduodenectomy. J Vasc Surg. 2010;51:662–666. doi: 10.1016/j.jvs.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto Y., Yamamoto J., Saiura A., Koga R., Kokudo N., Kosuge T. Reconstruction of hepatic or portal veins by use of newly customized great saphenous vein grafts. Langenbecks Arch Surg. 2004;389:110–113. doi: 10.1007/s00423-003-0452-9. [DOI] [PubMed] [Google Scholar]
- 17.Smoot R.L., Christein J.D., Farnell M.B. An innovative option for venous reconstruction after pancreaticoduodenectomy: the left renal vein. J Gastrointest Surg. 2007;11:425–431. doi: 10.1007/s11605-007-0131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto Y., Sakamoto Y., Nara S., Ban D., Esaki M., Shimada K. Reconstruction of the portal and hepatic veins using venous grafts customized from the bilateral gonadal veins. Langenbecks Arch Surg. 2009;394:1115–1121. doi: 10.1007/s00423-009-0500-1. [DOI] [PubMed] [Google Scholar]
- 19.Chu C.K., Farnell M.B., Nguyen J.H., Stauffer J.A., Kooby D.A., Sclabas G.M. Prosthetic graft reconstruction after portal vein resection in pancreaticoduodenectomy: a multicenter analysis. J Am Coll Surg. 2010;211:316–324. doi: 10.1016/j.jamcollsurg.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Jara M., Malinowski M., Bahra M., Stockmannn M., Schulz A., Pratschke J. Bovine pericardium for portal vein reconstruction in abdominal surgery: a surgical guide and first experiences in a single center. Dig Surg. 2015;32:135–141. doi: 10.1159/000370008. [DOI] [PubMed] [Google Scholar]
- 21.Dokmak S., Aussilhou B., Sauvanet A., Nagarajan G., Farges O., Belghiti J. Parietal peritoneum as an autologous substitute for venous reconstruction in hepatopancreatobiliary surgery. Ann Surg. 2015;262:366–371. doi: 10.1097/SLA.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 22.Mascoli C., D'Ambra M., Casadei R., Ricci C., Taffurelli G., Ancetti S. Portal/superior mesenteric vein reconstruction during pancreatic resection using a cryopreserved arterial homograft. Dig Surg. 2015;32:284–290. doi: 10.1159/000381194. [DOI] [PubMed] [Google Scholar]
- 23.Boggi U., Del Chiaro M., Croce C., Vistoli F., Signori S., Moretto C. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery. 2009;146:869–881. doi: 10.1016/j.surg.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Meniconi R.L., Ettorre G.M., Vennarecci G., Lepiane P., Colasanti M., Laurenzi A. Use of cold-stored vein allografts for venous reconstruction during pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:1233–1239. doi: 10.1007/s11605-013-2201-x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q., Yan S., Wang W., Shen Y., Zhang M., Ding Y. Use of allograft for portomesenteric vein interposition in radical resection of pancreatic tumor. Surg Pract. 2013;17:22–27. [Google Scholar]
- 26.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 27.Tran Cao H.S., Balachandran A., Wang H., Nogueras-Gonzalez G.M., Bailey C.E., Lee J.E. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg. 2014;18:269–278. doi: 10.1007/s11605-013-2374-3. [discussion 278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii T., Nakao A., Yamada S., Suenaga M., Hattori M., Takami H. Vein resections >3 cm during pancreatectomy are associated with poor 1-year patency rates. Surgery. 2015;157:708–715. doi: 10.1016/j.surg.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Heye T., Zausig N., Klauss M., Singer R., Werner J., Richter G.M. CT diagnosis of recurrence after pancreatic cancer: is there a pattern? World J Gastroenterol. 2011;17:1126–1134. doi: 10.3748/wjg.v17.i9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siriwardana H.P., Siriwardena A.K. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93:662–673. doi: 10.1002/bjs.5368. [DOI] [PubMed] [Google Scholar]
- 32.Benedetto B., Lipkowitz G., Madden R., Kurbanov A., Hull D., Miller M. Use of cryopreserved cadaveric vein allograft for hemodialysis access precludes kidney transplantation because of allosensitization. J Vasc Surg. 2001;34:139–142. doi: 10.1067/mva.2001.114206. [DOI] [PubMed] [Google Scholar]
- 33.Bosman P.J., Blankestijn P.J., van der Graaf Y., Heintjes R.J., Koomans H.A., Eikelboom B.C. A comparison between PTFE and denatured homologous vein grafts for haemodialysis access: a prospective randomised multicentre trial. The SMASH Study Group. Study of Graft Materials in Access for Haemodialysis. Eur J Vasc Endovasc Surg. 1998;16:126–132. doi: 10.1016/s1078-5884(98)80153-6. [DOI] [PubMed] [Google Scholar]
- 34.Heintjes R.J., Eikelboom B.C., Steijling J.J., van Reedt Dortland R.W., van der Heijden F.H., Bastini M. The results of denatured homologous vein grafts as conduits for secondary haemodialysis access surgery. Eur J Vasc Endovasc Surg. 1995;9:58–63. doi: 10.1016/s1078-5884(05)80226-6. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.G., Song G.W., Hwang S., Ha T.Y., Moon D.B., Jung D.H. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 36.Streinchenberger R., Barjoud H., Adeleine P., Larese A., Nemoz C., Chatelard P. Venous allografts preserved at 4 degrees C for infrainguinal bypass: long-term results from 170 procedures. Ann Vasc Surg. 2000;14:553–560. doi: 10.1007/s100169910103. [DOI] [PubMed] [Google Scholar]
- 37.Albers M., Romiti M., Pereira C.A., Antonini M., Wulkan M. Meta-analysis of allograft bypass grafting to infrapopliteal arteries. Eur J Vasc Endovasc Surg. 2004;28:462–472. doi: 10.1016/j.ejvs.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Glebova N.O., Hicks C.W., Piazza K.M., Abularrage C.J., Cameron A.M., Schulick R.D. Technical risk factors for portal vein reconstruction thrombosis in pancreatic resection. J Vasc Surg. 2015;62:424–433. doi: 10.1016/j.jvs.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 39.Liao K., Wang H., Chen Q., Wu Z., Zhang L. Prosthetic graft for superior mesenteric-portal vein reconstruction in pancreaticoduodenectomy: a retrospective, multicenter study. J Gastrointest Surg. 2014;18:1452–1461. doi: 10.1007/s11605-014-2549-6. [DOI] [PubMed] [Google Scholar]
- 40.Kang M.J., Jang J.Y., Chang Y.R., Jung W., Kim S.W. Portal vein patency after pancreatoduodenectomy for periampullary cancer. Br J Surg. 2015;102:77–84. doi: 10.1002/bjs.9682. [DOI] [PubMed] [Google Scholar]
- 41.Dua M.M., Tran T.B., Klausner J., Hwa K.J., Poultsides G.A., Norton J.A. Pancreatectomy with vein reconstruction: technique matters. HPB. 2015;17:824–831. doi: 10.1111/hpb.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balzer K.M., Luther B., Sandmann W., Wassmuth R. Donor-specific sensitization by cadaveric venous allografts used for arterial reconstruction in peripheral arterial occlusive vascular disease. Tissue Antigens. 2004;64:13–17. doi: 10.1111/j.0001-2815.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 43.Boulland L.M., Naper C., Skauby M.H. Presensitization revisited: pitfalls of vascular allografts in transplant candidates. Clin Kidney J. 2014;7:65–67. doi: 10.1093/ckj/sft145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandrasegaram M.D., Eslick G.D., Lee W., Brooke-Smith M.E., Padbury R., Worthley C.S. Anticoagulation policy after venous resection with a pancreatectomy: a systematic review. HPB. 2014;16:691–698. doi: 10.1111/hpb.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]