Abstract
Background
Post-operative pancreatic fistula (POPF) is a common and potentially life-threatening complication following pancreaticoduodectomy. The aim of this study was to assess the predictive value of intra-operative amylase concentration (IOAC) in peri-pancreatic fluid after resection for the diagnosis of POPF.
Methods
Consecutive patients who underwent a pancreaticoduodectomy between September 2014 and October 2015 were included in the analysis. IOAC was measured intraoperatively followed by drain fluid analysis for amylase on post-operative days (POD) 1, 3 and 5. Receiver operator characteristic (ROC) analysis was performed to evaluate the discriminative capacity of IOAC as a predictor of POPF.
Results
IOAC was measured after pancreaticoduodectomy in 62 patients. The IOAC correlated significantly with i) POD 1 and 3 drain amylase (p < 0.01), ii) the development of POPF (p < 0.01), iii) the development of clinically relevant fistula (Type B, C) (p < 0.01), iv) delayed gastric emptying (p < 0.01), and v) grade of complication as per the Clavien-Dindo definition (p = 0.02). ROC curve analysis confirmed the predictive relationship of IOAC and POPF as a good test with an area under the curve of 0.93, 95% CI 0.87–0.99, p < 0.01. In patients with IOAC of 200 U/L or higher the POPF rate was 80% (OR = 50.1, p < 0.0001).
Discussion
Measurement of IOAC allows early and accurate categorization of patients at risk for POPF.
Introduction
Despite a multitude of technical studies by pancreatic surgeons, post-operative pancreatic fistula (POPF) continues to be a common and potentially life-threatening complication following pancreatic surgery.1, 2, 3 The rate of POPF is variably reported between 20 and 50%, and clinically relevant POPF can be associated with severe short and long term morbidity and mortality.4, 5
Recent studies have focused primarily on defining and predicting POPF. Controversy still remains regarding prophylactic drainage and its indications.5, 6 The International Study Group for Pancreatic Fistula (ISPGF) has recently standardized the definition of POPF.7 This definition is based on the concentration of amylase in drain fluid (or aspirated intra-abdominal fluid) on the third post-operative day. Theoretically, this would suggest that the POPF becomes established over the first three post-operative days. A more recent study by Fong et al. has shown that the amylase concentration in the drain on the first operative day can be used to predict the formation of a POPF.8 This finding suggests an earlier onset of the POPF. Other factors known to contribute to the development of a POPF are a soft pancreas, small (<3 mm) duct, pathology other than pancreatic adenocarcinoma and increased blood loss.9, 10, 11 Callery et al. established the Fistula Risk Score (FRS) based on the presence or absence of these very factors for the development of POPF.12
We hypothesized that a leak of pancreatic enzymes following a pancreaticoduodectomy (PD) is immediate, and therefore intra-operative amylase concentration (IOAC) in the peri-pancreatic fluid will be predictive of the formation of a POPF. The primary aim of this study was to assess the predictive value of IOAC after PD for the development of POPF. The secondary aims of this study were to analyse the correlation of IOAC with post-operative morbidity, to identify independent prognostic factors for POPF and to determine an IOAC threshold value for POPF prediction.
Methods
Data collection
Clinicopathologic data was prospectively collected from consecutive patients undergoing PD at the Royal North Shore Campus (Royal North Shore Hospital; North Shore Private Hospital; University of Sydney) between September 2014 and October 2015. All operations were performed by one of two surgeons involved in the study (JS and AM). This study was approved by the Northern Sydney Local Health District, Human Research Ethics Committee.
Surgical technique
PD was performed as previously described.13, 14 The pancreatic anastomosis was performed using either a ‘duct to mucosa’ or ‘dunking’ technique based on surgeon preference. Both of these anastomotic techniques have been previously described in the literature.15 Prior to closure of the abdomen two surgical drains were placed. One drain anterior to the pancreaticojejunostomy and one drain posterior to the hepaticojejunostomy. Immediately after the operation, the attending surgeon completed a short questionnaire documenting the type of type of anastomosis, consistency of the remnant pancreas (soft, normal or firm), and the size of the pancreatic duct. To identity patients with increased risk of POPF the FRS was also recorded for each patient.
Fluid collection
Intraoperative peri-pancreatic fluid was collected in a standardized manner. The standardized procedure was as follows: after the pancreaticojejunostomy and hepaticojejunostomy were completed, the peri-pancreatic space was irrigated with 200 ml of saline, and then this fluid was suctioned and disposed of. Three millilitres of newly developed intraoperative peri-pancreatic fluid was collected from superior, inferior and posterior aspects of the pancreatic anastomosis after construction of the gastroenterostomy. This fluid was then sent for biochemical analysis. Drain fluid amylase was measured on post operative days 1, 3 and 5 (POD 1,3,5). Each surgical drain was removed post-operative as soon as amylase level in the drain fluid was less than 500 U/L and drain production was less than 200 ml per day.
Definition and grading of complications
POPF was recorded and classified according to the ISGPF definition.7 Grade B and C POPF were classified as clinically relevant POPF. Delayed gastric emptying and post-operative haemorrhage were also recorded and classified according to the ISGPS definition.16, 17 Postoperative complications within 30 days were graded according to the Clavien-Dindo classification.18 Length of ICU stay and overall hospital stay were also recorded. Individual FRS scores were derived through the summation of each of the 4 weighted risk factors. Calculated scores are then discredited and assigned to 1 of 4 risk zones: negligible risk, 0 points; low risk, 1 to 2 points; moderate risk, 3 to 6 points; or high risk, 7 to 10 points.12
Statistical analysis
Data from patient characteristics, operative characteristics, characteristics of the pancreas are represented in numbers and percentages. Means with standard deviation or median values with minimum and maximum values were presented, as appropriate. χ2 test or logistic regression were used to analyse categorical variables. Continuous variables were compared using the Student's t-test for data with normal distribution, and Mann–Whitney U test for data that were not. Correlation between IOAC and postoperative outcome was determined with a Pearson correlation coefficient analysis.
The predicative capacity of IOAC for POPF development was determined using receiver operating characteristic (ROC) analysis as represented by the area under the curve (AUC). AUC ranged from 0.5 to 1, with 0.5 representing no predictive ability for the state variable, POPF positive. AUC greater than 0.7 represents good predictability, appearing as a curvilinear plot on the graph. Based on the ROC curve coordinates the most optimal cut-off point for IOAC predicting POPF was determined. Predictive factors for POPF were further analysed using univariate statistics. Multivariable binary logistic regression analysis was performed for variables selected by the univariate analysis.
P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS Version 21.0 (Statistical Package for the Social Sciences, Chicago, Illinois).
Results
Patient characteristics and operative outcome
Sixty-two patients underwent a pancreatic resection between September 2014 and October 2015. There were 31 men (50%) and the median age of the cohort was 64 years (±SD 14) (Table 1). The majority of patients (n = 41, 66.1%) underwent surgery for pancreatic adenocarcinoma. Other diagnoses were intraductal papillary mucinous neoplasms (n = 6, 9.6%), ampullary tumour (n = 4, 6.5%), mucinous cystic neoplasm (n = 2), tubulovillous adenoma (n = 2), cholangiocarcinoma (n = 2), pancreatic neuroendocrine tumour (n = 2), mass forming autoimmune pancreatitis (n = 2) and trauma (n = 1).
Table 1.
Patient characteristics
| Total N = 62 | % | |
|---|---|---|
| Gender | ||
| Male | 31 | 50.0% |
| Age, in years, Mean, ±SD | 64 (14.1) | |
| ASA classification | ||
| 1,2 | 44 | 71.0% |
| 3 | 18 | 29.0% |
| Diabetic, yes | 8 | 12.9% |
| Current smoker, yes | 4 | 6.5% |
| Diagnosis | ||
| Pancreatic adenocarcinoma | 41 | 66.1% |
| Neoadjuvant therapy | 22/41 | |
| IPMN | 6 | 9.7% |
| Ampullary | 4 | 6.5% |
| Other | 12 | 17.7% |
Operative characteristics are summarized in Table 2. The consistency of the gland was recorded as soft in 24 patients (38.7%), normal in 18 patients (29%) or firm in 20 patients (32.3%). The median diameter of the pancreatic duct was 2 mm with a range from 1 to 6 mm.
Table 2.
Operative characteristics
| Total N=62 | % | |
|---|---|---|
| Blood loss [ml; mean ± SD] | 478 ± 416 | |
| Consistency gland | ||
| Soft | 24 | 38.7% |
| Normal | 18 | 29.0% |
| Firm | 20 | 32.3% |
| Pancreatic duct size [ mm; median, range] | 2 (1–6) | |
| Type of anastomosis | ||
| Duct to mucosa | 45 | 72.6% |
| Dunking | 17 | 27.4% |
| Fistula risk score [ median, range]∗ | 4 (0–8) | |
∗Fistula risk score, based on Callery MP et al.12
Based on the parameters of blood loss, gland texture, pathology and the diameter of the pancreatic duct the overall FRS in the present series was moderate with a median score of 4 (range 0–8).
Short term morbidity and mortality
Overall complications occurred in 33 patients (53.2%) (Table 3). The majority of complications (n = 18, 29.0%) were Clavien-Dindo grade 1 or 2. Severe complications (grade ≥3) occurred in 15 patients (24.2%). Postoperative haemorrhage occurred in 7 patients (11.3%), delayed gastric emptying in 8 patients (12.9%) and other complications such as bile leak, cardiopulmonary complications, wound infection and re-admission in 18 patients (29%). In hospital mortality occurred in one patient secondary to a medical complication (myocardial infarction).
Table 3.
Morbidity and mortality
| Total N = 62 | % | |
|---|---|---|
| Any complication | 26 | 51.0% |
| Delayed gastric emptying | ||
| Grade A | 5 | 8.1% |
| Grade B | 3 | 4.8% |
| Postoperative haemorrhage | ||
| Grade B | 5 | 8.1% |
| Grade C | 2 | 3.2% |
| POPF | ||
| Grade A | 22 | 35.5% |
| Grade B | 6 | 9.7% |
| Grade C | 1 | 1.6% |
| Grade of complications | ||
| <3 | 18 | 29.0% |
| ≥3 | 15 | 24.2% |
| In hospital mortality | 1 | 1.6% |
| LOS days (median, range) | 15 (4–106) | |
LOS, Length of hospitalization.
Postoperative pancreatic fistula
The overall grade A pancreatic fistula rate was 35.3% (n = 22). The grade B pancreatic fistula rate was 9.7% (n = 6) and one patient (1.6%) sustained a grade C fistula.
Intraoperative and postoperative amylase levels
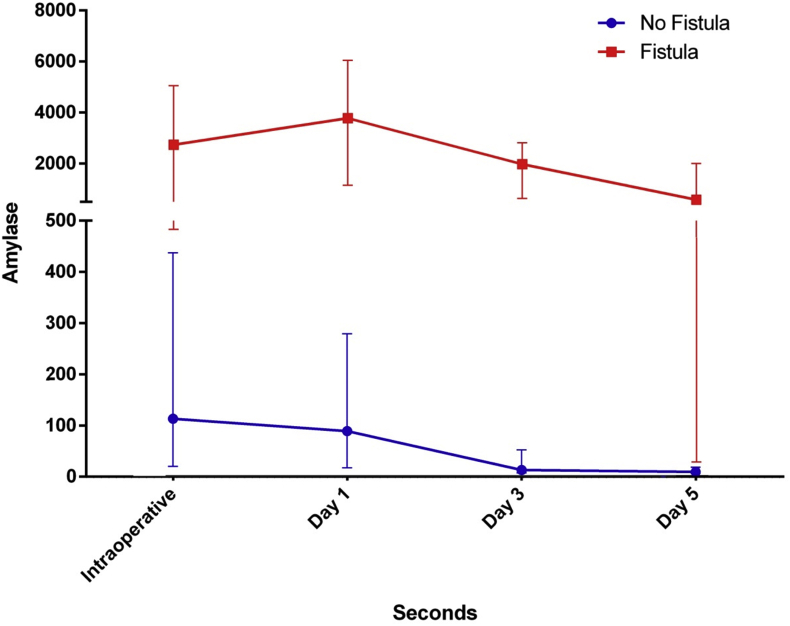
The median IOAC was 265 U/L (range 6 U/L–73,992 U/L). In patients without POPF, the median IOAC was significantly lower than in patients with POPF (34 U/L vs 618 U/L, p < 0.01) (Fig. 1).
Figure 1.
Median values of amylase. Vertical bars show 25th and 75th percentiles
The IOAC correlated significantly (Pearson correlation) with i) POD 1 and 3 drain amylase (p < 0.01), ii) the development of POPF (p = 0.05), iii) the development of clinically relevant fistula (Type B,C) (p < 0.01), iv) delayed gastric emptying (p < 0.01) and v) complication grade (as per the Clavien-Dindo definition; p = 0.02).
ROC-curve analysis for IOAC, FRS and optimal cut off for intraoperative amylase level
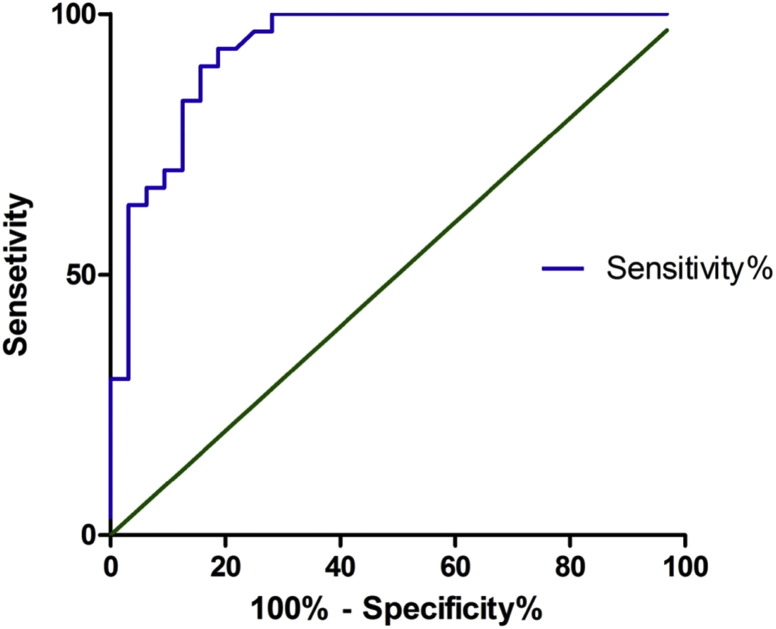
Fig. 2 illustrates the ROC-curve for IOAC and development of POPF. The ROC shows an AUC 0.93, 95% CI 0.87–0.99, p < 0.01. Based on the ROC, an IOAC of 200 U/L was determined to be an optimal cut-off value for predicting POPF. Twenty-seven patients (43.5%) had an IOAC of less than 200 U/L and POPF developed in only 2 (7.4%) cases, whereas in patients with IOAC of 200 U/L or higher (n = 35) the POPF rate was 80% (OR = 50.1, p < 0.0001). An IOAC <200 U/L has a negative predictive value for the development of POPF of 93% (sensitivity 93%, specificity 78%). ROC analysis for IOAC and clinically relevant POPF (type B, C) shows an AUC of 0.83 (95% CI 0.69–0.96, p < 0.01). The ROC analysis for the FRS and development of POPF shows an AUC of 0.71 (95% CI 0.58–0.84, p < 0.01). ROC analysis for the FRS and clinically relevant POPF (type B, C) shows a similar AUC of 0.71 (95% CI 0.54–0.86, p < 0.01).
Figure 2.
ROC curve for POPF. Area under the curve of 0.93, 95% CI 0.87–0.99, p < 0.001
Predictors for POPF in PD patients
Univariate and multivariable analyses for POPF in PD patients are summarized in Table 4. A soft pancreas (OR 2.6, 95% CI 0.9–7.3), a small duct (<3 mm) (OR 5.2, 95% CI 1.8–15.6), dunking anastomosis (OR 0.2, 95% CI 0.1–0.7), pathology other than pancreatic adenocarcinoma (OR 6.2, 95% CI 1.9–20.4), FRS of intermediate-high (OR 4.0, 95% CI 1.4–11.8) and an IOAC >200 U/L (OR 50.1, 95% CI 9.4–263.3) are associated with POPF. Older age, male gender, higher ASA classification, current smoking status, diabetes, neoadjuvant therapy and blood loss >300 ml were not associated with POPF. A multivariable analysis showed that an IOAC >200 U/L (OR 39.6, 95% CI 4.8–321) is the only independent predictor for POPF (p < 0.001) in the present series.
Table 4.
Univariate and Multivariable analysis for Fistula in Pancreaticoduodenectomy patients (n = 62)
| Factors | Fistula n (%) | Unadjusted odds ratio (95% CI) | P valuea | Adjusted odds ratio (95% CI) | P valueb |
|---|---|---|---|---|---|
| Pancreatic characteristics | |||||
| Consistency, normal/firm (n = 38) | 15 (39) | ||||
| Consistency, soft (n = 24) | 15 (62) | 2.6 (0.9–7.3) | 0.08 | 2.3 (0.2–21.6) | 0.45 |
| Diameter duct ≥3 mm (n = 29) | 8 (27) | ||||
| Diameter duct <3 mm (n = 33) | 22 (67) | 5.2 (1.8–15.6) | <0.01 | 2.0 (0.3–12.3) | 0.44 |
| Dunking anastomosis (n = 17) | 13 (76) | ||||
| DM anastomosis (n = 45) | 17 (38) | 0.2 (0.1–0.7) | 0.01 | 0.4 (0.04–2.8) | 0.32 |
| Histopathology | |||||
| Pancreatic adenocarcinoma (n = 41) | 14 (34) | – | |||
| Other (n = 21) | 16 (76) | 6.2 (1.9–20.4) | <0.01 | 3.2 (0.5–20.3) | 0.22 |
| Fistula risk score12 | |||||
| <4 (n = 27) | 8 (30) | ||||
| ≥4 (n = 35) | 22 (63) | 4.0 (1.4–11.8) | 0.01 | 1.2 (0.12–12.2) | 0.86 |
| IOAC cut-off, <200 U/l (n = 27) | 2 (7) | ||||
| IOAC cut-off, ≥200 U/l (n = 35) | 28 (80) | 50.1 (9.4–263.3) | <0.01 | 39.6 (4.8–321) | <0.01 |
Factors analysed in univariate analysis that were not significant included age, gender, ASA classification, current smoker, diabetic, neoadjuvant therapy, and blood loss >300 ml. DM = duct to mucosa. CI = confidence interval. IOAC = Intra operative amylase concentration. Fistula Risk Score, based on Callery MP et al.12
p value of binary logistic regression.
p value of remaining significant independent variables after multivariable logistic regression analysis.
Discussion
This is the first study to show that the immediate leak of pancreatic enzymes after pancreaticojejunostomy can predict the development of a pancreatic fistula. This finding represents a paradigm shift in our understanding of the underlying pathophysiology of POPF. The ability to identify patients intra-operatively who have a leak of pancreatic enzymes and are likely to develop POPF will potentially allow for individualized intra-operative and post-operative decision making; including the use of intra-abdominal drains and application of enhanced recovery protocols.
The current ISPGF definition of POPF focuses on drain fluid amylase levels on the third post-operative day.7 However, previously published reports have shown that both intra-operative factors and early analysis of drain fluid on the first operative day can predict the eventual development of a POPF.8, 19, 20 For example, Wada et al. reported the outcomes of their 266 consecutive patients in whom the pancreaticojejunostomy was performed with surgical loupes (2.5× magnification) or with a surgical microscope (12.5× magnification).20 The leak rate in the loupes group was 15% vs. 2.9% in the microscope group (p = 0.008). By using the microscope, they observed several technical errors such as crossed sutures, subsequent needle passage through the same area of the already-placed sutures, and inappropriate tension on tissues. The conclusion of this study was that enhanced vision provided by the surgical microscope allowed precise construction of the anastomosis resulting in a significant decrease in fistula rates. These observed technical errors suggest that immediate pancreatic leaks and a subsequent POPF is influenced by intra-operative factors.
The Verona group was the first to show the predictive value of day 1 drain amylase measurement in a large cohort of patients.19 The ROC analysis reported in that study showed a very similar outcome to the present study – an AUC of 0.92 (p < 0.01) compared with an AUC of 0.93, p < 0.01 in the current study. For further analysis in their 137 patients, the Verona group chose an arbitrary cut-off for amylase concentration on POD1 of 5000 U/L, based on the corresponding 70th percentiles of the amylase concentration. In the present study, the cut-off value of 200 U/L was based on the coordinates of the ROC curve which were analysed to determine an optimal negative predictive value of 93% (95% CI 0.76–0.99). Patients with an IOAC below 200 U/L are very low risk for the development of POPF. Predicting type B or C fistulae is of most clinical relevance, as these fistulae are associated with a high morbidity and are potentially life-threatening. The present analysis has shown that PD patients with an IOAC below 200 U/L are very low risk patients for developing a clinically relevant fistula.
Massachusetts General Hospital confirmed the findings from the Verona group in a validation study including only PD patients (n = 495).8 In this study the day 1 amylase value's predictability was proven with an AUC from the ROC of 0.91 in a training cohort (n = 126) and confirmed in the validation cohort (n = 369) with an AUC of 0.85. Based on this analysis the authors determined that an amylase level of 612 U/L or higher showed the best sensitivity (93%) and specificity (79%) for predicting POPF. In the subsequent multivariable analysis, it was shown that a POD 1 drain amylase level of lower than 600 U/L had an OR of 0.019 (p < 0.0001) and was a stronger predictor of the absence of POPF than pancreatic gland texture (OR = 0.193, p = 0.002) or duct diameter (OR = 0.861, p = 0.835). This supports the findings in the present analysis where on multivariable analysis, the only independent predictor of POPF was the IOAC.
It has recently been shown that the principles of enhanced recovery can be applied in pancreatic surgery.21 The benefits of enhanced recovery protocols in pancreatic surgery include reductions in delayed gastric emptying, postoperative stay and costs.22 This study shows that an IOAC >200 U/L correlates with increased rates of post-operative complications including POPF, delayed gastric emptying and other medical complications. Therefore, patients with an IOAC <200 U/L could be enrolled in an enhanced recovery program while those with an IOAC of >200 U/L perhaps should have a more traditional recovery program.
Several studies have reported the benefits of early drain removal.19, 23 Kawai et al. demonstrated a higher incidence of postoperative abscess in patients with drains removed on day 8 compared to those removed on day 4 (38% vs 8%, p < 0.01).24 A single centre study from Memorial Sloan Kettering showed no difference in complication rates between the groups (63% vs 57% in the no-drain group, p > 0.05).23, 25, 26 However, a multi-centre trial by Van Buren et al. randomized 137 patients to PD with (n = 68) and without (n = 69) intraperitoneal drainage.27 This study showed that PD without intraperitoneal drainage was associated with an increase in the number of complications per patient (p = 0.029) and with a higher average complication severity (p = 0.027). The authors concluded that elimination of intraperitoneal drainage in all cases of PD increases the frequency and severity of complications and therefore advocated selective drainage. The use of IOAC with a cut-off of <200 U/L may be used to identify patients who are unlikely to have a pancreatic leak and thus drains can be avoided. Similarly, an IOAC >200 U/L may indicate that placement of abdominal drains is indicated, especially where other risk factors for POPF (e.g. soft pancreas) are present.
Further research is required into the underlying pathophysiology and classification of POPF. The current study demonstrates that a leak of pancreatic enzymes post-resection is immediate. However, there may be another group of patients in whom late fistulas develop. Pratt et al. investigated latent pancreatic fistulas, defined as fistulas which lacked an amylase-rich effluent on the third post-operative day but ultimately exhibit the clinical findings indicative of fistula or radiographic evidence of peri-pancreatic fluid collections.28 In their series latent fistulas occurred in five per cent of all resections and were associated with more interventions, resulted in longer hospitalizations and incurred greater hospital costs. Therefore, it could be hypothesized that there are different types of POPF; the more common immediate pancreatic leak/fistula and the less common delayed or latent fistula. Patients with latent fistulae would not benefit from surgical drainage as their day three drain amylase is by definition low and surgical drains would have been removed per protocol.
Although the present study has several limitations, it is the first to show the benefit of measuring IOAC. Laboratory results of the IOAC test were available before closure of the abdomen, which would ideally facilitate IOAC being used to inform decision making. Although this study is limited by small patient numbers, the correlations are strong and outcomes are similar to previous larger studies. The present study needs future validation in a larger prospective cohort and preferably in another institution for external validation.
In conclusion, the dogma that a POPF develops over time is challenged by the current findings. Based on these results it could be hypothesized that the present definition for POPF is static and a sub-classification of immediate (high IOAC) and latent (suggested by Pratt et al.) could be added. Further research is now needed to ascertain if it is possible for the surgeon to individualize decision making regarding insertion of abdominal drains and enrolment in an enhanced recovery program based on the IOAC.
Funding sources
None.
Conflicts of interest
None to declare.
Footnotes
Presented at ANZHPBA Annual meeting October 2015 Cairns, Queensland.
References
- 1.Winter J.M., Cameron J.L., Campbell K.A., Arnold M.A., Chang D.C., Coleman J. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Gouma D.J., van Geenen R.C., van Gulik T.M., de Haan R.J., de Wit L.T., Busch O.R. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho C.K., Kleeff J., Friess H., Büchler M.W. Complications of pancreatic surgery. HPB. 2005;7:99–108. doi: 10.1080/13651820510028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron J.L., Riall T.S., Coleman J., Belcher K.A. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strobel O., Büchler M.W. Drainage after pancreaticoduodenectomy: controversy revitalized. Ann Surg. 2014 Apr;259:613–615. doi: 10.1097/SLA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 6.Fong Z.V., Ferrone C.R., Thayer S.P., Wargo J.A., Sahora K., Seefeld K.J. Understanding hospital readmissions after pancreaticoduodenectomy: can we prevent them? A 10-year contemporary experience with 1,173 patients at the Massachusetts General Hospital. J Gastrointest Surg. 2014;18:137–144. doi: 10.1007/s11605-013-2336-9. discussion 144–145. [DOI] [PubMed] [Google Scholar]
- 7.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Fong Z.V., Correa-Gallego C., Ferrone C.R., Veillette G.R., Warshaw A.L., Lillemoe K.D. Early drain removal-the middle ground between the drain versus no drain debate in patients undergoing pancreaticoduodenectomy: a prospective validation study. HPB. 2005;7:99–108. doi: 10.1097/SLA.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 9.Ansorge C., Nordin J.Z., Lundell L., Strömmer L., Rangelova E., Blomberg J. Diagnostic value of abdominal drainage in individual risk assessment of pancreatic fistula following pancreaticoduodenectomy. Br J Surg. 2014 Jan;101:100–108. doi: 10.1002/bjs.9362. [DOI] [PubMed] [Google Scholar]
- 10.Shyr Y.M., Su C.H., Wu C.W., Lui W.Y. Does drainage fluid amylase reflect pancreatic leakage after pancreaticoduodenectomy? World J Surg. 2003 May;27:606–610. doi: 10.1007/s00268-003-6841-y. [DOI] [PubMed] [Google Scholar]
- 11.Chen J.Y., Feng J., Wang X.Q., Cai S., Dong J.H., Chen Y.L. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2015 May 21;21:5926–5933. doi: 10.3748/wjg.v21.i19.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callery M.P., Pratt W.B., Kent T.S., Chaikof E.L., Vollmer C.M., Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013 Jan;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Gundara J.S., Wang F., Alvarado-Bachmann R., Williams N., Choi J., Gananadha S. The clinical impact of early complete pancreatic head devascularisation during pancreatoduodenectomy. Am J Surg. 2013 Oct;206:518–525. doi: 10.1016/j.amjsurg.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 14.De Reuver P.R., Mittal A., Neale M., Gill A.J., Samra J.S. Extended pancreatoduodenectomy as defined by the International Study Group for Pancreatic Surgery is associated with worse survival but not with increased morbidity. Surgery. 2015 Jul;158:183–190. doi: 10.1016/j.surg.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Shukla P.J., Barreto S.G., Fingerhut A., Bassi C., Büchler M.W., Dervenis C. Toward improving uniformity and standardization in the reporting of pancreatic anastomoses: a new classification system by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2010 Jan;147:144–153. doi: 10.1016/j.surg.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Wente M.N., Bassi C., Dervenis C., Fingerhut A., Gouma D.J., Izbicki J.R. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Wente M.N., Veit J.A., Bassi C., Dervenis C., Fingerhut A., Gouma D.J. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007 Jul;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molinari E., Bassi C., Salvia R., Butturini G., Crippa S., Talamini G. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007 Aug;246:281–287. doi: 10.1097/SLA.0b013e3180caa42f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada K., Traverso L.W. Pancreatic anastomotic leak after the Whipple procedure is reduced using the surgical microscope. Surgery. 2006 Jun;139:735–742. doi: 10.1016/j.surg.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Balzano G., Zerbi A., Braga M., Rocchetti S., Beneduce A.A., Di Carlo V. Fast-track recovery programme after pancreatico- duodenectomy reduces delayed gastric emptying. Br J Surg. 2008 Nov;95:1387–1393. doi: 10.1002/bjs.6324. [DOI] [PubMed] [Google Scholar]
- 22.Williamsson C., Karlsson N., Sturesson C., Lindell G., Andersson R., Tingstedt B. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg. 2015 Aug;102:1133–1141. doi: 10.1002/bjs.9856. [DOI] [PubMed] [Google Scholar]
- 23.Correa-Gallego C., Brennan M.F., D'Angelica M., Fong Y., Dematteo R.P., Kingham T.P. Operative drainage following pancreatic resection: analysis of 1122 patients resected over 5 years at a single institution. Ann Surg. 2013;258:1051–1058. doi: 10.1097/SLA.0b013e3182813806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai M., Tani M., Terasawa H., Ina S., Hirono S., Nishioka R. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006 Jul;244:1–7. doi: 10.1097/01.sla.0000218077.14035.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heslin M.J., Harrison L.E., Brooks A.D., Hochwald S.N., Coit D.G., Brennan M.F. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg. 1998;2:373–378. doi: 10.1016/s1091-255x(98)80077-2. [DOI] [PubMed] [Google Scholar]
- 26.Conlon K.C., Labow D., Leung D., Smith A., Jarnagin W., Coit D.G. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487–493. doi: 10.1097/00000658-200110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Buren G., 2nd, Bloomston M., Hughes S.J., Winter J., Behrman S.W., Zyromski N.J. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014 Apr;259:605–612. doi: 10.1097/SLA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 28.Pratt W.B., Callery M.P., Vollmer C.M., Jr. The latent presentation of pancreatic fistulas. Br J Surg. 2009 Jun;96:641–649. doi: 10.1002/bjs.6614. [DOI] [PubMed] [Google Scholar]