Summary
Background
Supra-mesocolic surgery (SMS) is complicated in patients with portal vein cavernoma (PC) and portal decompression is recommended. The aim of this study was to report a large single centre of SMS in patients with PC without portal decompression.
Methods
Between 2006 and 2013, all patients who met inclusion criteria were analyzed retrospectively. The primary endpoint was the feasibility rate, surgical and postoperative outcome. The secondary endpoints were the long-term outcome of patients who underwent biliary bypass for cholangitis. Risk factors for complications were studied.
Results
Thirty patients underwent 51 procedures. Pancreatitis was the main etiology of PC (19/30) and biliary obstruction was mainly related to the underlying disease and not to portal cholangiopathy (12/14). All planned procedures were successfully completed. Fourteen patients underwent biliary bypass. Median blood loss (250 ml), transfusion (n = 7), mortality (n = 0), overall morbidity (n = 12) and the median hospital stay (10 days). Good long-term control of cholangitis was achieved in the 9 patients alive with available follow-up. Significant risk factors for complications were a previous abdominal wall scar, previous intra-abdominal surgical field and liver fibrosis.
Conclusion
SMS can be safely performed in patients with PC. In patients with risk factors for complications, portal decompression should be discussed.
Abbreviations: SMS, supra-mesocolic surgery; PC, portal vein cavernoma
Introduction
Portal vein cavernoma (PC) or cavernous transformation of the portal vein is defined by the presence of chronic obstruction of the main portal vein and the development of collateral venous circulation in the hepatic pedicle.1 This collateral circulation develops within the porta hepatis including the wall of the bile duct and gallbladder to maintain hepatofugal portal flow to the liver. Associated thrombosis of the superior mesenteric or splenic veins may occur resulting in segmental portal hypertension in the splenic and mesenteric systems. PC may be due to a procoagulant condition2, 3, 4 or secondary to a local inflammatory or neoplastic disease.3 Generally PC is asymptomatic, but patients can present with portal cholangiopathy characterized by cholangitis, jaundice or biliary colic3, 5, 6, 7 secondary to mechanical biliary obstruction from the venous collaterals or ischemia.8, 9, 10, 11, 12, 13 Patients with PC may undergo surgery for complications that are not directly related to PC but to the underlying etiology of the PC. Examples include distal biliary obstruction from chronic pancreatitis. PC is usually considered to be a contraindication to surgery due to the high risk of bleeding and mortality14 such that certain authors have suggested portal decompression before surgery.6, 14, 15, 16 In fact, the real risk in patients with PC and the added value of portal decompression are not well known. The aim of this study was to retrospectively evaluate all patients with PC who underwent supra-mesocolic surgery (SMS) without portal decompression since 2006.
Methods
Between February 2006 and September 2013, all patients who presented with PC and required SMS without prior portal decompression were reviewed. Demographic, clinical, past surgical history, radiological and surgical data as well as the postoperative outcome were prospectively recorded. Mortality was defined as any in-hospital death or death within 90 days after surgery and morbidity was defined according to the Clavien–Dindo classification.17 The postoperative outcome was evaluated in relation to the presence of previous abdominal wall scar, previous intra-abdominal surgical field, pedicular or extra-pedicular surgery. Previous intra-abdominal surgical field was defined as any redo surgery in a previously dissected surgical field, like biliary bypass in a patient who had already attempt of pancreaticoduodenectomy. Pedicular surgery was defined as any surgery on the hepatic pedicle (biliary bypass) or cholecystectomy. The primary endpoint was the feasibility rate, operative (transfusion, blood loss) and postoperative (complications, length of the hospital stay) outcomes. The secondary endpoints were the long-term outcome in patients who underwent biliary bypass for cholangitis. A good outcome was considered in patients who experienced <3 episodes of cholangitis/year treated simply by antibiotics without the need of any endoscopic or percutaneous treatment.
Preoperative assessment
The diagnosis of PC was based on CT with vascular reconstruction. Patients with only partial or tumoral portal vein obstruction with limited collateral veins were excluded from this study. The extension of venous obstruction to major venous axis and the distribution of the collateral circulation within the PC were evaluated on CT scan. Surgery was contraindicated if severe portal deprivation could not be avoided. A complete radiological assessment by endoscopic retrograde cholangiopancreaticogram and magnetic resonance imaging3 was performed in patients with biliary obstruction to assess the main cause and location of obstruction, either due to underlying etiology or portal cholangiopathy.
General surgical strategy
The gallbladder was systematically punctured during cholecystectomy to reduce tension, facilitate dissection and improve control of collateral veins. Dissection was retrograde (Fig. 1A). If the cystic duct was difficult to identify or hemorrhage was encountered, the pedicle was controlled and the cystic duct was sutured with the collateral veins. In general, there was some retraction of the infrahepatic area and inflammatory adhesions had to be released to obtain exposure. Mobilization of the right colonic angle was sometimes necessary for optimal control of the hepatic pedicle. In patients requiring a biliary bypass, the upper anterolateral main bile duct was exposed, which was generally free of collaterals (Fig. 1B). Identification of the bile duct can be difficult in patients with an inflammatory reaction due to PC, recurrent cholangitis or endoscopic treatment. In this situation the biliary stent was identified under ultrasound guidance, or using a small aspirating needle to obtain bile and identify the bile duct (Fig. 1C). The bile duct was carefully exposed and – transected by careful dissection but complete control before section is not recommended (Fig. 1D–F). There are two types of peribiliary venous collaterals. The paracholedochal veins of Petren 3,18 parallel to the duct wall, are relatively large and can be easily separated, ligated and divided. The epicholedochal plexus of Saint 4,19 on the surface of the bile duct, are smaller and more difficult to identify; ligations must be performed with an absorbable sutures. When the bile duct is completely transected, it is more easily freed from the surrounding tissue to facilitate anastomosis. In order to avoid the sump syndrome, the authors preferred complete transection of the bile duct with end to side anastomosis,20 however side to side anastomosis was considered if the dissection proved prohibitively difficult. With complete bile duct transection, significant portal deprivation is unlikely because only some collaterals veins on the right border of the hepatic pedicle are scarified. In some patients, more than one procedure was performed during the same surgical intervention.
Figure 1.
Anterograde cholecystectomy is performed (A). The upper right border of the bile duct (black arrow), relatively free of veins, is searched (B). The bile duct can be identified by puncture (C). Progressive transection of the bile duct (D). Stones extraction can be needed (E). The bile duct is ready for end anastomosis (G)
Statistics
Values are expressed as median and ranges, or percentages, as appropriate. The Chi-squared test was used to compare categorical variables. The independent t test and Mann–Whitney test were used to compare continuous variables. p < 0.05 was considered to be significant. All statistical analyses were performed using SPSS version 20.0.
Results
Surgery was contraindicated in 2/32 patients in the study. One patient was a 42-year-old woman with PC and symptomatic gallbladder stones. Cholecystectomy was not performed due to severe perivesicular collateral venous circulation with the risk of significant portal deprivation. The second patient was a 54-year-old man with PC related to chronic pancreatitis with diffuse venous thrombosis, complicated by diffuse portal cholangiopathy including the biliary confluence, was treated by metallic stents. The patient died 3 years later from septic complications.
Clinical and biological data are summarized in Table 1. PC was diagnosed a median 48 months (12–408) before surgery. Seven patients were receiving long-term therapeutic anticoagulation, one patient had history of variceal bleeding 2 years before surgery, one patient had a history of ascites and one underwent surgery with mild ascites. The indication for surgery was not directly related to PC in 12 patients. Liver biopsy was performed in the eight patients without biliary obstruction and showed normal or mild fibrosis (n = 5) and severe fibrosis (n = 3).
Table 1.
Clinical, biological and radiological data for the entire population
| Variables, n, median (range) | Total (n = 30) |
|---|---|
| Age | 48 (31–68) |
| Male gender | 18 |
| Causes of PC | |
| Chronic or acute pancreatitis | 17 and 2 |
| Hematologic diseases | 5 |
| Other | 6 |
| Abnormal liver function test | 29 |
| Liver failure | 0 |
| Vein obstruction | |
| Portal, splenic, mesenteric | 30, 18, 12 |
| All three veins | 10 |
| Collateral venous circulation territory | |
| Pedicular | 30 |
| Splenic, mesenteric, intrapancreatic | 23, 17, 13 |
Radiological findings are summarized in Table 1. In non cholecystectomized patients (26/30), radiological evaluation showed constant (26/26) perivesicular collateral venous circulation. All patients with biliary obstruction (n = 14) underwent a preoperative biliary evaluation by endoscopic retrograde cholangiopancreaticogram (n = 13) and magnetic resonance imaging (n = 14) and all except one had undergone endoscopic (n = 13) or percutaneous (n = 2) biliary stenting between 1 and 4 years before. In patients with chronic pancreatitis (n = 12), biliary obstruction was mainly related to the underlying disease and located in the intrapancreatic portion of the main bile duct and mildly affected by portal cholangiopathy (Fig. 2A–D). In patients with hematologic disease (n = 2), biliary obstruction was related to main bile duct stenosis (n = 2) with or without stones (1) with mild portal cholangiopathy at the biliary confluence (Fig. 2E–F).
Figure 2.
CT scan (A) of a patient with chronic pancreatitis and portal cavernoma and who presented with recurrent cholangitis. Endoscopic retrograde cholangiopancreaticogram (B) and lateral view of CT scan (C, D) showed distal bile duct obstruction related to chronic pancreatitis with no or mild portal cholangiopathy (black arrow). CT scan (E) in a patient with portal cavernoma related to hematological disease. Magnetic resonance imaging (F) showed stenosis on the main bile duct (white arrow) with associated stones
The surgical procedures are summarized in Table 2, all scheduled procedures (n = 51) were performed in all 30 patients. Surgery was performed by subcostal incision except in 3 patients who underwent isolated cholecystectomy by laparoscopic approach. An end to side anastomosis was performed in 13 of the 14 patients who underwent biliary bypass. In the eleven patients with previous abdominal wall scar, four underwent surgery in a previous intra-abdominal surgical field by a previous abdominal wall scar while the previous abdominal wall scar and/or previous intra-abdominal surgical field were avoided in the seven others. Among the 30 operated patients, only seven did not underwent any pedicular procedure.
Table 2.
All surgical procedures in 30 operated patients
| Main surgical procedures (n) | 51 Procedures 30 patients |
|---|---|
| Biliary bypass | 14 |
| Jejunal and duodenal | 6 and 8 |
| Cholecystectomy | 20 |
| Combined with other procedures | 15 |
| Isolated | 5 |
| Neoplasia | 8 |
| Left pancreatic resection | 5 |
| Liver resection | 2 |
| Gastric | 1 |
| Complications of chronic pancreatitis | 6 |
| Pseudocyst bypass | 3 |
| Gastroenterostomy | 3 |
| Other procedures | 3 |
Surgical and postoperative outcome data are summarized in Table 3: surgery lasted a median 125 (45–420) minutes, median blood loss was 250 ml (20–1800) and blood transfusion was needed in 7. There were no postoperative deaths. The main complications were ascites (n = 3), sepsis (n = 2), wound infections (n = 2), pancreatic fistula (2), pulmonary complications (2), fluid collections (4) requiring percutaneous drainage in one, colonic fistula (1), biliary fistula (1), confusion (2) and re-hospitalization (1). The surgical and postoperative outcomes were significantly more complicated in patients with previous abdominal wall scar but the outcome of pedicular and extra-pedicular surgeries was similar.
Table 3.
Operative data and complications for the all population and according to the presence or not of previous abdominal wall scar (PAWS) and pedicular or extrapedicular surgery
| Variables n, median (range) | All n = 30 | PAWS n = 11 | No PAWS n = 19 | p | Pedicular surgery n = 23 | Extrapedicular surgery n = 7 | p |
|---|---|---|---|---|---|---|---|
| Blood loss (ml) | 250 (20–1800) | 450 (100–1800) | 200 (20–750) | 0.048 | 200 (20–1800) | 465 (275–750) | 0.963 |
| Transfusion | 7 | 5 | 2 | 0.015 | 5 | 2 | 0.896 |
| Operative time (mn), | 125 (45–420) | 240 (120–450) | 115 (45–210) | <0.001 | 110 (45–450) | 210 (130–320) | 0.390 |
| Overall complications | 12 | 8 | 4 | 0.002 | 7 | 5 | 0.129 |
| Clavien–Dindo > III | 6 | 4 | 2 | 0.053 | 3 | 3 | 0.148 |
| Hospital stay (days) | 10 (1–77) | 17 (8–77) | 10 (1–26) | 0.013 | 9 (1–77) | 13 (8–27) | 0.460 |
In the eleven patients with previous abdominal wall scar and compared to those without (n = 19), the surgical and postoperative outcome was very complicated in patients who underwent surgery in a previous intra-abdominal surgical field (n = 4) with a median blood loss (1075 vs 250, p < 0.001), transfusion (4 vs 2, p < 0.001), overall morbidity (4 vs 4, p = 0.003), Clavien–Dindo III (3 vs 2, p = 0.004) and a prolonged long hospital stay (34 vs 8, p < 0.001). However outcomes were similar if the previous abdominal wall scar and/or previous intra-abdominal surgical field (n = 7) could be avoided.
The median overall follow-up was 36 months (9–96) and 4 patients were lost during the follow-up. One of the patients operated for malignancy (n = 5), died from recurrent pancreatic cancer 23 months after resection and the 4 others are still alive and recurrence free. Two of the patients operated for benign disease (n = 25) died, 1 due to acidocetotic coma 8 months after biliary bypass and the second from pancreatic cancer on a background of chronic pancreatitis 27 months after biliary bypass. Among the 14 patients with biliary bypass, good control of cholangitis was obtained in the 9 patients who are still alive with available follow-up.
Discussion
In the current study, chronic pancreatitis was the leading etiology of PC. At present anticoagulation therapy can prevent the extension of thrombosis to the mesenteric system and small bowel infarction21 and chronic pancreatitis can be effectively managed allowing more patients with PC to undergo surgery. Because PC has been considered a contraindication to surgery due to the risk of bleeding, most of these patients are not referred to surgeons for this indication. The current study of 30 patients demonstrated for a wide range of indications that SMS in patients with PC is safe with no mortality and an acceptable risk of complications even in the absence of portal decompression.
The current study demonstrated that biliary obstruction is the main presentation and indication for surgery in patients with PC. Due to the high surgical risk in patients with PC,3, 14 non-surgical options have been described and developed, including mainly endoscopic6, 22, 23 or percutaneous biliary stenting. Although the short-term results of biliary stenting have been good,24 long-term results have been poor with the risk of recurrence after stent removal.25 With this treatment, repeated stenting is necessary for effective long-term control, bleeding from epicholedochal plexus veins may occur during sphincterotomy,26 resistant poly-microbial germs may emerge and pedicular inflammation may develop which can complicate surgery and chronic obstruction can lead to secondary biliary cirrhosis. Whatever the results of this option, in the authors experience like with others, biliary stenting is the first line treatment6, 14, 22, 23, 27 and surgery has only been indicated after failure of endoscopic treatment. Biliary stenting also facilitates identification of the bile duct during surgery and can prevent bile duct stenosis from uncontrolled suturing when faced with inadvertent bleeding during dissection.
Previously surgical treatment of portal cholangiopathy has mainly focused on portal decompression as both the therapeutic and anti-haemorrhagic effects can be obtained and the results can be more effective than simple biliary bypass.6, 14, 28, 29 However, portal decompression had many limitations and disadvantages. First in the current study like with others,6, 28, 29 these surgical shunts cannot be feasible in one third of the patients because of diffuse venous thrombosis. Second in the study published by Vibert et al.,6 spleno-renal shunt were associated with a long hospital stay (mean = 20 ± 12) days, high morbidity (27%), and early shunt thrombosis (20%). For that reason, surgical shunts cannot be considered as simple surgical procedures and more increasingly are either abandoned or replaced by transjugular intrahepatic porto systemic shunts.30, 31, 32 Although this shunt may be performed on small collateral veins, it is more complicated and the long term results are not known.33 Finally, the main veins should be preserved as much as possible because biliary complications with secondary chronic liver disease are a good indication for liver transplantation and in this situation a patent main vein is essential to restore portal flow to the liver graft.34, 35, 36
However some studies6, 14, 28, 29 showed that despite spleno-renal shunts, approximately half the patients required secondary biliary bypass. It is the authors opinion, there are at least three explanations for this negative result. First, these shunts do not effectively decompress the collateral venous circulation and portal flow preferentially passes into well-organized collateral veins. This could explain why little or no radiological improvement is found in PC following PD.37 Second, sclerosis and fibrosis develop in and around the bile duct due to inflammation and ischemic changes,9, 13 leading to irreversible stenosis. Finally, biliary obstruction is not necessary related to portal cholangiopathy but to the underlying disease or other associated factors. In fact one of the most important results in the current study is that biliary obstruction in patients with chronic pancreatitis (n = 12) was mainly related to chronic pancreatitis (distal bile duct stenosis). This very important point has not been widely acknowledged in previous studies and probably many patients with chronic pancreatitis and PC were falsely diagnosed or treated as portal cholangiopathy. Therefore the authors recommend complete radiological evaluation of the biliary tree before any endoscopic or percutaneous treatment. The current study only reported two patients with hematological diseases and in this situation, biliary obstruction was located in the main bile duct. The current study does not include patients with diffuse portal cholangiopathy including stenosis of the biliary confluence, probably because these patients are more frequently offered non-surgical management. The authors feel that there is no benefit to biliary bypass in this subgroup with multiple biliary stenoses because surgery is more complicated and the long-term results are poor.3
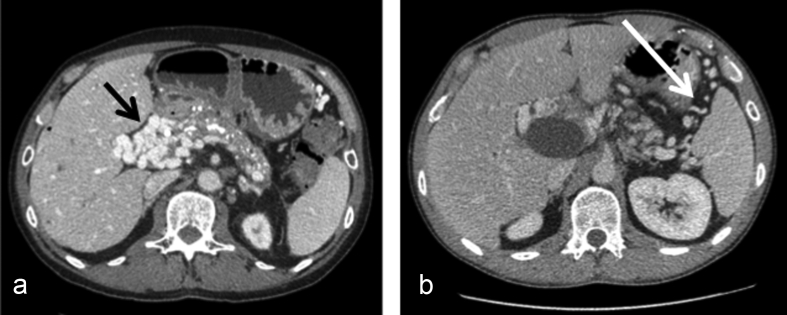
The surgical and postoperative outcome of pedicular and extra-pedicular procedures was similar. According to the authors experience, two types of venous distribution were observed in PC. There was either veins as a vascular mass in a very dense, short (hepatic pedicle) or inflammatory (postsurgical) space (Fig. 3A), that were difficult to control separately, or veins located in a large non-inflammatory space (like within the splenic or mesenteric collateral circulation) separated by non-dense connective tissue that were much easier to control (Fig. 3B). This fact may explain the similar outcomes even though extra-pedicular procedures were major including pancreatic and liver resection.
Figure 3.
CT scan in two patients with chronic pancreatitis and portal cavernoma. We can differentiate between veins agglomerated (black arrow) as a mass in short/inflammatory space (like the hepatic pedicle), difficult to control (A) and those non agglomerated (white arrow) situated in a large non inflammatory space (extrapedicular) easy to control (B)
As expected, surgery was more complicated in patients with previous surgical scars. Postoperative adhesions can induce inflammation and the development of a collateral venous circulation between the splanchnic and caval systems resulting in difficult haemorrhagic dissection. Two of the 3 patients with severe liver fibrosis had the most severe outcome in the current study. This subgroup of patients had a long history of chronic biliary obstruction treated by multiple endoscopic or percutaneous treatments and a prior attempt at surgical treatment. It is difficult to determine if this unfavorable outcome was directly related to liver fibrosis or to the extensive past surgery. In the authors experience, cirrhosis/fibrosis made dissection more difficult and probably impaired the quality of the veins, increasing the surgical risk. The authors recommend a preoperative liver biopsy in these high risk patients to exclude severe fibrosis, and if present, the surgical indication should be reconsidered and other therapeutic options such as liver transplantation should be discussed.
Although the current study was non-comparative, the authors feel that portal decompression should not systematically be performed but based on the indications (anti-haemorrhagic or therapeutic effect), and risk factors for complications such in those with previous surgical scars and chronic liver disease. In this subgroup of patients the surgical indication must be clearly defined.
In conclusion, with experienced surgeons, SMS is safe in patients with PC with no mortality and an acceptable rate of complications. In the current study, the main etiology of PC was chronic pancreatitis and biliary obstruction was mainly related to the underlying disease and not to portal cholangiopathy. Portal decompression should be discussed in patients with risk factors including previous surgical scars and chronic liver disease.
Source of funding
None.
Conflicts of interest
None to declare.
Acknowledgments
The authors would like to thank D. Roche for her editorial assistance and correction of the article.
Footnotes
The manuscript was presented as a poster presentation in EAHPBA, Manchester, United Kingdom, April 2015 and as a talking poster in IHPBA, Sao Paulo, Brazil, April 2016.
References
- 1.Gibson J., Richards R. Cavernous transformation of the portal vein. J Pathol Bacteriol. 1955;70:81–95. doi: 10.1002/path.1700700108. [DOI] [PubMed] [Google Scholar]
- 2.Diaz E., Nahon S., Charachon A., Traissac L., Lenoble M., Challier E. Portal vein thrombosis associated with a myeloproliferative disorder, prothrombin G20210A mutation, antiphospholipid syndrome, with repermeation during anticoagulant therapy. Gastroenterol Clin Biol. 2001 May;25:549–551. [PubMed] [Google Scholar]
- 3.Condat B., Vilgrain V., Asselah T., O'Toole D., Rufat F., Zappa M. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003 Jun;37:1302–1308. doi: 10.1053/jhep.2003.50232. [DOI] [PubMed] [Google Scholar]
- 4.Resseguier A.S., André M., Orian Lazar E.A., Bommelaer G., Tournilhac O., Delèvaux I. Natural history of portal cavernoma without liver disease. A single centre retrospective study of 32 cases. Rev Med Interne. 2015 Sep 17;37:394–398. doi: 10.1016/j.revmed.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Ben Chaabane N., Melki W., Safer L., Bdioui F., Halara O., Saffar H. Cholestatic jaundice secondary to portal cavernoma: case report. Ann Chir. 2006 Nov;131:543–546. doi: 10.1016/j.anchir.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Vibert E., Azoulay D., Aloia T., Pascal G., Veilhan L.A., Adam R. Therapeutic strategies in symptomatic portal biliopathy. Ann Surg. 2007 Jul;246:97–104. doi: 10.1097/SLA.0b013e318070cada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhiman R.K., Saraswat V.A., Valla D.C., Chawla Y., Behera A., Varma V. Portal cavernoma cholangiopathy: consensus statement of a working party of the Indian national association for study of the liver. J Clin Exp Hepatol. 2014 Feb;4(Suppl. 1):S2–S14. doi: 10.1016/j.jceh.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilawari J.B., Chawla Y.K. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272–276. doi: 10.1136/gut.33.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khuroo M.S., Yattoo G.N., Zargar S.A., Javid G., Dar M.Y., Khan B.A. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–813. [PubMed] [Google Scholar]
- 10.Bejanin H., Choury A., Fritsch J., Buffet C., Baumann R. Bile duct obstruction by portal cavernoma. Hepatology. 1994 Apr;19:1060. [PubMed] [Google Scholar]
- 11.Bayraktar Y., Balkanci F., Ozenc A., Arslan S., Koseoglu T., Ozdemir A. The “pseudo-cholangiocarcinoma sign” in patients with cavernous transformation of the portal vein and its effect on the serum alkaline phosphatase and bilirubin levels. Am J Gastroenterol. 1995;90:2015–2019. [PubMed] [Google Scholar]
- 12.Nagi B., Kochhar R., Bhasin D., Singh K. Cholangiopathy in extrahepatic portal venous obstruction. Radiological appearances. Acta Radiol. 2000;41:612–615. doi: 10.1080/028418500127345992. [DOI] [PubMed] [Google Scholar]
- 13.Dhiman R.K., Puri P., Chawla Y., Minz M., Bapuraj J.R., Gupta S. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–652. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary A., Dhar P., Sarin S.K., Sachdev A., Agarwal A.K., Vij J.C. Bile duct obstruction due to portal biliopathy in extrahepatic portal hypertension: surgical management. Br J Surg. 1998;85:326–329. doi: 10.1046/j.1365-2168.1998.00591.x. [DOI] [PubMed] [Google Scholar]
- 15.Gorgul A., Kayhan B., Dogan I., Unal S. Disappearance of the pseudo-cholangiocarcinoma sign after TIPSS. Am J Gastroenterol. 1996;91:150–154. [PubMed] [Google Scholar]
- 16.Choudhuri G., Tandon R.K., Nundy S., Misra N.K. Common bile duct obstruction by portal cavernoma. Dig Dis Sci. 1988 Dec;33:1626–1628. doi: 10.1007/BF01535956. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petren T. Die extrahepatischen Gallenwegsvenen und ihre pathologische anatomische Bedetung. Verh Anat Ges. 1932;41:139–143. [Google Scholar]
- 19.Saint J.H. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operations on the extrahepatic biliary tract. Br J Surg. 1961;48:489–498. doi: 10.1002/bjs.18004821104. [DOI] [PubMed] [Google Scholar]
- 20.Qadan M., Clarke S., Morrow E., Triadafilopoulos G., Visser B. Sump syndrome as a complication of choledochoduodenostomy. Dig Dis Sci. 2012 Aug;57:2011–2015. doi: 10.1007/s10620-011-2020-4. Epub 2011 Dec 14. [DOI] [PubMed] [Google Scholar]
- 21.Plessier A., Rautou P.E., Valla D.C. Management of hepatic vascular diseases. J Hepatol. 2012;56(Suppl. 1):S25–S38. doi: 10.1016/S0168-8278(12)60004-X. [DOI] [PubMed] [Google Scholar]
- 22.Thervet L., Faulques B., Pissas A., Bremondy A., Monges B., Salducci J. Endoscopic management of obstructive jaundice due to portal cavernoma. Endoscopy. 1993 Aug;25:423–425. doi: 10.1055/s-2007-1009120. [DOI] [PubMed] [Google Scholar]
- 23.Saraswat V.A., Rai P., Kumar T., Mohindra S., Dhiman R.K. Endoscopic management of portal cavernoma cholangiopathy: practice, principles and strategy. J Clin Exp Hepatol. 2014 Feb;4(Suppl. 1):S67–S76. doi: 10.1016/j.jceh.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahl S., Zimmermann S., Genz I., Glasbrenner B., Pross M., Schulz H.U. Risk factors for failure of endoscopic stenting of biliary strictures in chronic pancreatitis: a prospective follow-up study. Am J Gastroenterol. 2003 Nov;98:2448–2453. doi: 10.1111/j.1572-0241.2003.08667.x. [DOI] [PubMed] [Google Scholar]
- 25.Dumortier J., Vaillant E., Boillot O., Poncet G., Henry L., Scoazec J.Y., Partensky C. Diagnosis and treatment of biliary obstruction caused by portal cavernoma. Endoscopy. 2003;35:446–450. doi: 10.1055/s-2003-38779. [DOI] [PubMed] [Google Scholar]
- 26.Mutignani M., Shah S.K., Bruni A., Perri V., Costamagna G. Endoscopic treatment of extrahepatic bile duct strictures in patients with portal biliopathy carries a high risk of haemobilia: report of 3 cases. Dig Liver Dis. 2002;34:587–591. doi: 10.1016/s1590-8658(02)80093-7. [DOI] [PubMed] [Google Scholar]
- 27.Perlemuter G., Béjanin H., Fritsch J., Prat F., Gaudric M., Chaussade S. Biliary obstruction caused by portal cavernoma: a study of 8 cases. J Hepatol. 1996 Jul;25:58–63. doi: 10.1016/s0168-8278(96)80328-x. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal A.K., Sharma D., Singh S., Agarwal S., Girish S.P. Portal biliopathy: a study of 39 surgically treated patients. HPB. 2011 Jan;13:33–39. doi: 10.1111/j.1477-2574.2010.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varma V., Behera A., Kaman L., Chattopadhyay S., Nundy S. Surgical management of portal cavernoma cholangiopathy. J Clin Exp Hepatol. 2014 Feb;4(Suppl. 1):S77–S84. doi: 10.1016/j.jceh.2013.07.005. Epub 2013 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi X., Han G., Yin Z., He C., Wang J., Guo W. Transjugular intrahepatic portosystemic shunt for portal cavernoma with symptomatic portal hypertension in non-cirrhotic patients. Dig Dis Sci. 2012 Apr;57:1072–1082. doi: 10.1007/s10620-011-1975-5. [DOI] [PubMed] [Google Scholar]
- 31.Fanelli F., Angeloni S., Salvatori F.M., Marzano C., Boatta E., Merli M. Transjugular intrahepatic portosystemic shunt with expanded-polytetrafuoroethylene-covered stents in non-cirrhotic patients with portal cavernoma. Dig Liver Dis. 2011 Jan;43:78–84. doi: 10.1016/j.dld.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Luo X., Nie L., Zhou B., Yao D., Ma H., Jiang M. Transjugular intrahepatic portosystemic shunt for the treatment of portal hypertension in noncirrhotic patients with portal cavernoma. Gastroenterol Res Pract. 2014;2014:659726. doi: 10.1155/2014/659726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camerlo A., Fara R., Barbier L., Grégoire E., Le Treut Y.P. Which treatment to choose for portal biliopathy with extensive portal thrombosis? Dig Surg. 2010;27:380–383. doi: 10.1159/000314610. [DOI] [PubMed] [Google Scholar]
- 34.Filipponi F., Urbani L., Catalano G., Iaria G., Biancofiore G., Cioni R. Portal biliopathy treated by liver transplantation. Transplantation. 2004 Jan 27;77:326–327. doi: 10.1097/01.TP.0000101795.29250.10. [DOI] [PubMed] [Google Scholar]
- 35.Hajdu C.H., Murakami T., Diflo T., Taouli B., Laser J., Teperman L. Intrahepatic portal cavernoma as an indication for liver transplantation. Liver Transpl. 2007 Sep;13:1312–1316. doi: 10.1002/lt.21243. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S., Taneja S. Liver transplantation for portal cavernoma cholangiopathy. J Clin Exp Hepatol. 2014 Feb;4(Suppl. 1):S85–S87. doi: 10.1016/j.jceh.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutson D.G., Pereiras R., Zeppa R., Levi J.U., Schiff E.R., Fink P. The fate of esophageal varices following selective distal splenorenal shunt. Ann Surg. 1976 May;183:496–501. doi: 10.1097/00000658-197605000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]