Abstract
Hypercholesterolemia is a main risk factor for atherosclerosis development. Arterial macrophages, or foam cells, take-up and process lipoprotein particles deposited in arteries, and store much of the cholesterol carried by these particles in their cytoplasm. However, the effects of exposure to different cholesterol levels on foam cells remain poorly understood. Given the remarkable plasticity of macrophages in response to environmental variables, studies on macrophage biology should ideally be performed in the environment where they exert their physiological functions, namely atherosclerotic lesions in the case of foam cells. We used a mouse model of atherosclerosis, the apolipoprotein E-deficient mouse, to study in vivo the transcriptional response of foam cells to short- and long-term elevations in plasma cholesterol, induced by feeding mice a western type diet. The microarray data sets from this study have been deposited in NCBI's Gene Expression Omnibus under the accession number GSE70619. Here we provide detailed information on the experimental set-up, on the isolation of RNA by laser capture microdissection, and on the methodology used for RNA amplification and analysis by microarray and quantitative real-time PCR.
Keywords: Atherosclerosis, Foam cell, Gene expression, Hypercholesterolemia
| Specifications | |
|---|---|
| Organism/cell line/tissue | Mus musculus/foam cells within atherosclerotic lesions |
| Sex | Female |
| Sequencer or array type | Affymetrix mouse genome 430 2.0 arrays |
| Data format | Raw |
| Experimental factors | Transcriptional profiling of lesional foam cells in response to short- and long-term WD feeding |
| Experimental features | Lesional macrophages were isolated by LCM. Purified RNA was amplified by IVT, and used for microarray and qPCR analysis |
| Consent | Data are publicly available |
| Sample source location | Houston, USA |
1. Direct link to deposited data
The data are available at the NCBI's Gene Expression Omnibus (GEO) repository: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE70619
2. Experimental design, materials and methods
2.1. Experimental design
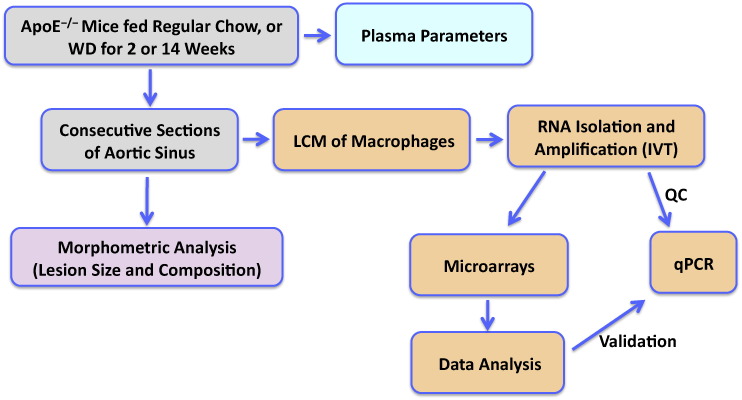
Many of the variables that contribute to the progression of atherosclerosis can be controlled in mice in a way that cannot be controlled in humans. We used apolipoprotein E-knockout (apoE−/−) mice to investigate the transcriptional response of foam cells within atherosclerotic lesions to exposure to different cholesterol levels [1]. Plasma cholesterol in apoE−/− mice is related to the cholesterol content in their diet. Thus, we divided 15 mice into 3 groups of 5 mice each, and to increase plasma cholesterol we switched the diet of two of the groups from regular mouse chow to a cholesterol-rich western diet (WD). Mice were fed WD for a short period (2 weeks) or for a longer period (14 weeks) prior to sacrifice at 22 weeks of age. The shorter 2 weeks WD feeding allowed studying foam cells under hypercholesterolemia in the absence of significant changes in atherosclerotic plaque size, whereas the longer increase in plasma cholesterol significantly enhanced atherosclerosis development. At the end of the study blood was drawn for lipid profiling. Upon sacrifice, the hearts were removed, bisected by a parallel cut approximately 1 mm under the tips of the atria, and the upper parts, which contain the aortic sinus, were immediately embedded in O.C.T. and stored frozen at − 80 °C. The experimental outline is summarized in Fig. 1.
Fig. 1.
Experimental workflow. ApoE−/− mice were fed regular mouse chow or a western type diet (WD) for 2 or 14 weeks to increase plasma cholesterol. Lesional macrophages were selectively isolated using LCM. RNA was purified, amplified by IVT, and used for gene expression profiling. IVT, in vitro transcription. LCM, laser capture microdissection. QC, quality control.
2.2. Laser capture microdissection of macrophages
Like in humans, atherosclerotic lesions of apoE−/− mice are cellularly heterogeneous, and the small size of murine arteries represents an added challenge for the isolation of specific cell populations. Laser capture microdissection (LCM) is a technique that allows to identify and isolate specific cells from sections of heterogeneous tissues, and can therefore be used to harvest individual cell populations within mouse atheroma [2]. To this end, we performed consecutive 7-μm sections through the aortic sinuses of mice in our 3 experimental groups. Sections were sequentially mounted on 3 slides. The first and third slides, which were used for LCM, were immediately fixed through a battery of alcohols, cell nuclei were stained with toluidine blue with the HistoGene LCM Frozen Section Staining Kit (Applied Biosystems), and stored O/N at room temperature in a slide box containing fresh desiccant. The second slide was stained for macrophages with anti-Lamp2/Mac3 antibody (Santa Cruz Biotechnology), and used as a template to identify macrophages within lesions. This slide was also used to quantify lesion area, and the total and relative areas of macrophages within lesions. LCM of macrophages was performed with a Veritas Microdissection System (Applied Biosystems). We used an infrared laser to perform ~ 2000 shots on macrophage-rich areas of approximately 20 sections of each sample. While the duration and intensity of the laser pulses were adjusted for each individual sample, normally the laser power was set to ~ 65 mV and the pulse duration was ~ 2500 μs, which typically resulted in a spot size of between 15 and 20 μm. Individual cells or small clusters were collected on CapSure HS LCM Caps (Applied Biosystems).
2.3. RNA purification and amplification
Immediately after LCM, RNA from captured macrophages was purified with the PicoPure RNA Isolation Kit (Applied Biosystems) and stored at − 80 °C. While this RNA is suitable for analysis of gene expression by quantitative polymerase chain reaction (qPCR), the amount of RNA that can be isolated considerably limits the number of genes that can be analyzed. Thus, we used the RiboAmp HS RNA Amplification Kit (Applied Biosystems) to perform two rounds of RNA amplification by in vitro transcription (IVT). Each round consisted of a reverse transcription using oligo(dT) primers tagged with the T7 promoter sequence and synthesis of double-stranded DNA, followed by an IVT reaction with a T7 RNA polymerase to yield amplified complementary RNA (cRNA). The quality of the cRNA was assessed by A260/A280 ratios and by an electropherogram with the Agilent 2100 Bioanalyzer. Of note, the IVT reactions do not amplify ribosomal RNA. Thus, standard quality controls such as 28S/18S ribosomal RNA ratio or the RNA integrity number (RIN) cannot be used to characterize amplified RNA. Instead, upon electrophoresis the amplified cRNA typically appears as a broad band, and we confirmed that the intensity distribution of the bands were similar among samples. Additional quality controls were performed using qPCR to confirm the enrichment in macrophage markers in the LCM populations with respect to RNA isolated and amplified from whole aortic sinuses, as well as depletion in endothelial and smooth muscle cell markers in the same samples [1], [2].
2.4. Gene expression analysis
For broad gene expression profiling the LCM-RNAs from foam cells in the different dietary groups were hybridized to Mouse Genome 430 2.0 Arrays from Affymetrix. A common step when using the Affymetrix platform is the use of an IVT reaction to generate biotinylated cRNA. Given that the LCM RNA was already submitted to two rounds of IVT, for cRNA labeling we employed the TURBO Labeling Biotin Kit (Applied Biosystems) to biotinylate 15 μg of each cRNA. This system uses a chemical reaction to form a coordinate bond between the base guanine and a biotin complex, and does not involve further RNA amplification. Labeled cRNA was then fragmented and hybridized to microarray chips, and subsequent steps such as washing, staining and scanning were performed following standard Affymetrix protocols at the Genomic and RNA Profiling Core at Baylor College of Medicine. The microarray data were filtered with dChip software to only include in the analysis the probes with ≥ 50% presence call and with expression levels of ≥ 25 in ≥ 50% samples. The filtered data were transferred to MeV software [3], log2 transformed, and analyzed by ANOVA. Pairwise comparisons were performed with the Welch's t-test, assuming unequal variances. Differences were considered significant when P < 0.01. To determine the main biological pathways affected in foam cells in response to WD feeding for 2 or 14 weeks, data were analyzed with Pathway Express [4]. A P value was generated based on the number of genes differentially expressed in each pathway with respect to the number expected to change by chance. Pathway Express was also used to generate a gamma P value that, in addition to classical statistics, takes into consideration relevant biological parameters such as the fold-change and the topology of the genes in the pathways. A detailed description of the results of the analyses can be found in [1].
Quantitative-PCR was used to confirm the enrichment in macrophages in the LCM populations and to validate the microarray data. Reverse transcription was performed with SuperScript III (Invitrogen), using random hexamers as primers for the cRNA. The classical antisense RNA amplification by IVT is usually 3′ biased, i.e. the product is shorter than the parent mRNA. This is because oligo(dT) primers are used to insert the T7 polymerase promoter and, as a result, transcription is more robust in mRNA regions closer to the 3′ end, where the reaction is initiated. Thus, primers were designed at the 3′-terminus region of the mRNA, including the 3′-untranslated region. Other than these considerations, standard PCR protocols and quality controls were used for analysis of gene expression by qPCR.
3. Discussion
Foam cells are a hallmark of atherosclerotic lesions, and play central roles in the regulation of both lipid homeostasis at the arterial wall and local inflammation. Given the high plasticity of macrophages in response to environmental variables, it seems most reasonable to study their function under the environment where they exert their physiological role. This may be quite challenging when we study atherosclerosis in mice, as the small size of their arteries and the cellular heterogeneity of atherosclerotic lesions make it difficult to isolate specific cell populations. Here we describe the use of LCM for isolation of foam cells resident within murine atherosclerotic lesions that were exposed to different cholesterol levels, as well as the methodology and quality controls used for RNA amplification and gene expression analysis. Given the high relevance of inflammation to the pathogenesis of atherosclerosis, the emphasis of our initial study was on the analysis of genes that regulate inflammatory and immune responses [1]. By thoroughly describing the experimental design and procedures, this article may facilitate the work of other researchers who might be interested in analyzing the data from a different perspective.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by NIH grants R01HL104251 (to AP) and R01DK097160 (to VKY), and by an American Heart Association Scientist Development Grant 14SDG19690016 to YHG.
Contributor Information
Young-Hwa Goo, Email: gooy@mail.amc.edu.
Antoni Paul, Email: paula@mail.amc.edu.
References
- 1.Goo Y.H., Son S.H., Yechoor V.K., Paul A. Transcriptional profiling of foam cells reveals induction of guanylate-binding proteins following western diet acceleration of atherosclerosis in the absence of global changes in inflammation. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul A., Yechoor V., Raja R., Li L., Chan L. Microarray gene profiling of laser-captured cells: a new tool to study atherosclerosis in mice. Atherosclerosis. 2008;200:257–263. doi: 10.1016/j.atherosclerosis.2007.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeed A.I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M., Sturn A., Snuffin M., Rezantsev A., Popov D., Ryltsov A., Kostukovich E., Borisovsky I., Liu Z., Vinsavich A., Trush V., Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 4.Draghici S., Khatri P., Tarca A.L., Amin K., Done A., Voichita C., Georgescu C., Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]