Highlights
-
•
Aortic graft infections occur in approximately 0.2–5% of cases of open aortic reconstruction [1–4].
-
•
They are associated with significant morbidity and mortality including graft disruption, haemorrhage or sepsis [1–4].
-
•
Using a cryopreserved allograft aorta in the treatment of prosthetic graft infection is poorly evidenced.
-
•
Further studies are required to compare treatments.Patients presenting acutely even years after aortic surgery should be appropriately imaged to rule out graft related problems.
Keywords: Cadaveric aorta, Aortic graft, Infection, Vascular surgery, Transplant
Abstract
This case report describes a 73-year-old gentleman who underwent explantation of an infected prosthetic aorto-iliac graft and replacement with a cryopreserved thoracic and aorto-iliac allograft. The patient has been followed up a for more than a year after surgery and remains well.
After elective tube graft repair of his abdominal aortic aneurysm (AAA) in 2003, he presented to our unit in 2012 in cardiac arrest as a result of a rupture of the distal graft suture line due to infection. After resuscitation he underwent aorto-bifemoral grafting using a cuff of the original aortic graft proximally. Distally the new graft was anastomosed to his common femoral arteries, with gentamicin beads left in situ.
Post discharge the patient was kept under close surveillance with serial investigations including nuclear scanning, however it became apparent that his new graft was infected and that he would require aortic graft replacement, an operation with a mortality of at least 50%.
The patient underwent the operation and findings confirmed a synthetic graft infection. This tube graft was explanted and a cryopreserved aorta was used to the refashion the abdominal aorta and its bifurcation. The operation required a return to theatre day one post operatively for a bleeding side branch, which was repaired. The patient went on to make a full recovery stepping down from the intensive therapy unit day 6 post operatively and went on to be discharged 32 days after his cryopreserved aorta implantation.
1. Background
Aortic graft infections occur in approximately 0.2–5% of cases of open aortic reconstruction [1], [2], [3]. They are associated with significant morbidity and mortality including graft disruption, haemorrhage and sepsis [4]. The management of graft infection is complex and has included graft excision, extra-anatomical bypass, reconstruction with prosthetic grafts and neo-aortoiliac procedures using the femoral vein. However analysis reveals that these procedures are associated with significant complications, the most important being recurrent graft infection [5].
In-line reconstruction with cryopreserved allograft has been advocated because of the potential resistance to infection [6], [7], [8]. Using fresh allografts has been discontinued because of the tendency for the graft to dilate over a long period of time [9]. Cryopreservation has the role of preserving the integrity of the collagen matrix and this is thought to lead to fewer graft related complications and higher patency rates. They also provide conduits for the reimplantation of renal and mesenteric vessels preventing ischaemia. Allografts do not need to undergo ABO matching, and can also be selected to match the anatomical dimensions while other techniques don’t appreciate this variance [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18].
Though the highlight of this case report is the use of the cryopreserved allograft, the report makes a remarkable read for any medical professional. The chronological order of events shows the perseverance of the medical teams to work together to diagnose and treat a succession of medical problems.
2. Case presentation
An 84-year-old gentleman presented to our unit in June 2012 in cardiac arrest having become acutely unwell whilst attending a BBQ. He had a previous medical history of elective (open) tube graft repair for a AAA 9 years prior, radiotherapy for prostate cancer, ischaemic heart disease (IHD) and hypercholesterolaemia. A diagnosis of graft rupture was made after CT imaging and the patient was resuscitated and taken to theatre for emergency laparotomy.
Given the complexity of the case, 3 consultant vascular surgeons began with a mid-line incision and bilateral longitudinal groin incisions. The aortic neck was clamped infra-renally. The native aortic sac was opened and the old graft visualised. The distal limb of the original tube graft had dehisced from the iliac arteries. The proximal portion of the original graft was preserved and an aorto-bifemoral graft was sutured to a proximal cuff of the original graft. Tunnelling of the graft from the abdomen to the groins was carried out retroperitoneally and it was anastomosed to the common femoral arteries in standard fashion. Gentamicin beads and teicoplanin powder were placed into the aortic sac.
The patient experienced significant blood loss requiring 18 units of packed red blood cells, 8 units of fresh frozen plasma and 1 unit of platelets. He was admitted to ITU and required inotropic support and haemofiltration. 7 days postoperatively a rising WCC and evidence of sepsis required a further laparotomy and the patient was found to have 10–15 cm length of necrotic small bowel. This was resected and not found to have perforated. A small bowel anastomosis and primary closure of the abdomen were conducted. Histopathology revealed transmural infarction with mesenteric blood vessel thrombosis.
After a prolonged period on ITU requiring multi organ support and nutrition he was stepped down to the wards and discharged after 27 days after presentation.
3. Investigations & differential diagnosis
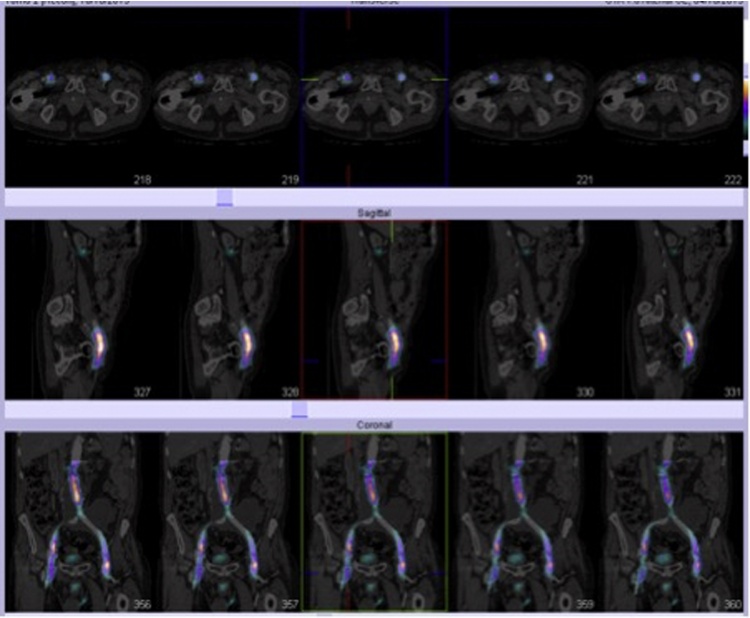
10 months later he was referred by the GP urgently for left groin pain and left inguinal swelling. On examination an umbilical incisional hernia was noted but no signs of common femoral pseudoaneurysms or inguinal herniae. A CT angiogram of the abdominal aorta and lower limbs was requested (Fig. 1). This demonstrated a 10 × 8 cm pelvic collection associated with the aortibifemoral graft extending down to the left groin. The collection was causing obstruction of the left ureter with mild left hydronephrosis. The patient was treated as having likely recurrent graft infection. A 1-month course of antibiotics was prescribed and a white cell technetium scan requested. This was reported as showing no evidence of an infective collection in relation to the graft. Software fusion between the white cell scan and CT angiogram demonstrated no abnormal uptake suggestive of a periprosthetic infection (Fig. 2).
Fig. 1.
CT Angiogram May 2013: a large complex fluid collection arising from the region of the aorto-bifemoral graft extending to the left iliac region measures approximately 10 × 8 cm. The left ureter is obstructed by the pelvic fluid collection with a mild left hydronephrosis.
Fig. 2.
NM White Cell HMPAO Whole body Technecium 99-m July 2013: the fused co-registered images show that the left psoas collection around the aortic graft demonstrates no abnormal uptake suggestive of periprosthetic infection.
The pain persisted a further two months and after discussion with the microbiology team a further 1-month course of doxycycline and metronidazole was commenced. One week after starting his antibiotics he presented to hospital with fevers, chills, wide spread back rash and excruciating pain in his joints and muscles. This was thought to be associated with his recent use of antibiotics, although the differential included progression of graft infection.
CT angiogram of the abdominal aorta and lower limbs showed a persistent collection surrounding the graft, extending into the left psoas and surrounding the anastomosis of the left limb. It also displayed left hydro-ureter and hydro-nephrosis. After multidisciplinary team discussion, the patient underwent CT-guided drainage of the collection (Fig. 3). At this point he was started on IV Gentamicin and Teicoplanin. During aspiration, blood was noted and thought to be from a haematoma. A drain was inserted and organising haematoma aspirated. Samples were sent for microscopy, cytology and sensitivities, which came back negative, and the patient was discharged after 6 days.
Fig. 3.
CT Guided Aspiration August 2013: right lateral position. Puncture of peri-graft collection. Haematoma aspirated. There was some reduction in the volume of low-density within the collection post aspiration.
A follow up CT Angiogram 15 months after the emergency operation (Fig. 4), showed considerable increase in the size of the collection/haematoma within the left retroperitoneum now extending superiorly to the level of the diaphragm. Retrograde blood flow was noted in the native left external iliac artery which could have been contributing to the collection/haematoma. There was also marked progression of the pelvicalyceal dilatation, which was now severe, with involvement of the ureter down to the level of the collection.
Fig. 4.
CT Angiogram October 2013: considerable increase in size of the left retroperitoneal collection/haematoma which now extends to the groin and the level of the diaphragm. Worsening of the marked left hydronephrosis, the AP diameter of the left renal pelvis now measures 3 cm.
Despite a normal white cell count, negative culture of aspirates and long-term antibiotics, the collection was increasing in size; retrograde bleeding from the native left external iliac artery was suspected to be the cause so a nuclear dynamic radiolabelled red cell injection scan was requested and coiling under local anaesthetic was being considered. The patient was in need of ureteric stenting to decompress the left kidney. The latter of the two had been arranged but cancelled by the anaesthetist because of newly diagnosed aortic stenosis. This now proved a difficult dilemma and the advice of the cardiology team was sought. The patient was deemed to require an aortic valve replacement (AVR), however in the context of potential ongoing aortic graft infection this in itself would be a high risk procedure.
In October 2013 the patient underwent dynamic imaging with radiolabelled red cell injection. These images were fused with the most recent CT scan (Fig. 5). There was no evidence of any acute haemorrhage. In the same month the cardiologists performed a coronary angiogram, which demonstrated mild to moderate proximal left anterior descending stenosis, with an echocardiogram showing an ejection fraction of 60% and severe aortic stenosis. The patient was deemed fit enough to undergo ureteric stenting and left EIA coiling. However, given that the patient remained clinically well and with no definite evidence of infection, intervention was postponed and follow up arranged for 6 months.
Fig. 5.
NM Dynamic imaging following radiolabelled red cell injection October 2013: no abnormal tracer relation is seen suggestive of a haemorrhagic leak around the site of the psoas collection and left groin.
In January 2014 before the arranged follow up date the patient was admitted and transferred from a local hospital with bilateral groin pain, erythema and general malaise. Basic investigations showed a WCC 24 and CRP 43. A CT concluded a reducing collection size around the left psoas, a less dilated left ureter but an increasing collection size in the left groin. The patient quickly proceeded to ultrasound-guided aspiration of the left groin, which found a blood stained aspirate. MC + S and cytology of the aspirate revealed white cells with no growth of organisms found.
The case was re-discussed in a Vascular MDT with a recommendation to perform an aortic graft replacement, albeit with significant risk of mortality. This was discussed with the patient who opted for the surgery to be performed.
4. Treatment
In preparation for the operation he underwent bilateral ureteric stenting with JJ stents to decompress the ureters (Fig. 6).
Fig. 6.
Bilateral Ureteric Stenting January 2014.
The choice of graft was a CryoLife Cryopreserved aorta (Fig. 7). 2 different components were required for this operation:-
-
(i)
CryoLife descending thoracic aorta 14 mm × 8 cm.
-
(ii)
CryoLife aortoiliac 11 mm ×9 cm with branches of 5 mm × 6.5 cm and 5 mm × 8.5 cm.
Fig. 7.
CryoLife Cryopreserved aorta. Descending aorta and aortoiliac components anastomosed in preparation for transplantation.
Both grafts are treated with antimicrobials in the processing including vancomycin, hydrochloric acid, Imipenam, fluconazole, amikacin sulphate, amphoteracin B, gentamicin sulphate and cefotaxime. They are screened for Hepatitis B, Hepatitis C, HIV, Syphilis and HTLV.
579 days after initially presenting to our unit, the patient underwent explantation of the infected aorto-iliac graft and replacement with cryopreserved thoracic and aorto-iliac allograft with harvesting and utilisation of deep femoral veins. Approach was via a midline laparotomy and bilateral groin incisions. Findings included pus in the left groin and blood stained fluid around the abdominal graft. During laparotomy, adhesiolysis was performed and explanation of native aortic neck and graft, below the renal arteries. At the groins the common femoral artery (CFA), profunda femoris artery (PFA) and superficial femoral artery (SFA) as well as the right and left limbs of the existing graft were explanted. The new graft consisted of an end-to-end anastomosis to native aorta and end-to-end anastomosis of the aorto-iliac graft to the new descending thoracic graft. In the groins the deep femoral vein was harvested and used to create an anastomosis to perfuse the arteries of the lower leg.
The operation was completed taking a total of 10 h. The estimated blood loss was 10.5 L and the patient required 20 units of packed red cells, 12 unit of fresh frozen plasma, 2 units of platelets, 3 units of cryoprecipitate, one unit of novoseven and one unit of factor 8.
Day 1 post operatively it became apparent that the patient was bleeding; after return to theatre a midline laparotomy was performed and an incision into the aortic sac revealed a large collection of blood and haematoma. The bleeding point was found to be a side branch of the cryopreserved allograft and this was repaired. The left groin was also explored and suture line of the anastomosis was found to bleeding and this anastomosis was refashioned. His abdomen was left open to be re-explored at a later date. Day 3 after his graft replacement he returned to theatre, where a dry abdomen was found and the abdominal wall closed with porcine biological mesh.
He continued his recovery on ITU requiring inotropic support and haemofiltration. He was extubated on day 6 and stepped down to the ward on day 9. 32 days after his operation he was discharged from hospital. Microbiology of the old graft grew Staphylococcus haemolyticus.
The patient had repeated course of antibiotics including oral and intravenously during the time he had a suspected graft infection. This was never confirmed with microbiological evidence or through imaging. It was finally clinically suspected and findings confirmed intra-operatively (with frank pus found around the synthetic graft) during the explanation of his graft and implantation of cryopreserved aorta. This inevitably led to further antibiotics treatment prophylactically around his major surgery. The repeated courses of antibiotics make a patient susceptible to Methicillin-resistant Staphylococcus aureus (MRSA). Fortunately the patient was never suspect or diagnosed with this.
5. Outcome and follow-up
He was kept under close surveillance as an outpatient, seen in clinic one month post operative and underwent removal of ureteric stents at 2 months. A CT scan at 3 months showed critical stenosis of the left groin anastomosis (Fig. 8).
Fig. 8.
CT Angiogram April 2014: There is a critical stenosis at the left groin anastomosis and the collection associated with the graft is smaller.
He proceeded to angiography where findings included 1 cm stenosis within the left groin anastomosis. This underwent successful angioplasty via retrograde puncture of the left SFA. A duplex at 4 months showed minimal residual stenosis.
In December 2014 he had his one-year follow up appointment. In late 2015 he successfully received his aortic root and aortic valve replacement for aortic stenosis.
6. Discussion
One of the primary outcomes in saving life and limb during graft infection management is minimising the incidence of recurrent infection [19].
A multi-centred study published in 2014 from the USA reviewed all cases from 14 of the 20 centres conducting cryopreserved allograft implantation. Since 2002 a total of 220 patients were included. The study covered 25% of all cryopreserved allografts in the USA since their introduction. It is the largest study to date and concludes lower early and long-term morbidity and mortality than previously reported treatment options. These include low rates of aneurysm formation, allograft rupture (4%), recurrent infection (4%) and limb loss (0%) [19]. A small study of 21 patients using cryopreserved aorta found a 0% graft reinfection rate, 30 day mortality of 0% and mean follow up at 48 months [20]. The allografts are useful in cases with infected fields, mycotic aneurysms and aorta-enteric fistula [20], [21], [22], [23]. Six USA studies have revealed no aneurysm and dilatation in the allograft at follow up [21], [22], [23], [24], [25], [26].
Other options for treatment of aortic prosthetic graft infection include extra anatomical bypass, neo-aortioiliac procedures using the femoral vein or unpreserved allografts, but these are associated with high morbidity [5], [11], [12]. Aortic stump blowout in 25% of patients, amputation in 16% and a 1-year survival of 73% were the reported figures by one of the largest studies of extra anatomic bypass [12]. Using prosthetic grafts or neo-aortoiliac system has shown better outcomes. As well as avoiding stump blow out, one study reveals prosthetic grafts are associated with 0% early limb loss and neo-aortoiiac system with 6% early limb loss and 1-year survival of 83% [14], [16]. Recurrent infection rates of all these treatments have ranged from 10 to 20% in different series [5], [12], [13], [14], [15], [16].
The short literature review above describes the evidence for the different options available for the management of arterial graft infection in vascular surgery. Evidence for comparison is limited due to a small number of cases.
In our case of graft infection, we appreciated that we had one attempt at performing an operation and that due to the high morbidity and mortality of the case, it was likely any major vascular surgery after the implantation of a cryopreserved aorta would have been futile. Infection is always a risk in any surgery, but as mentioned above the primary outcomes in saving life and limb during graft infection management is minimising the incidence of recurrent infection. Literature above finds that the cryopreserved aorta has a lower rate of recurrent infection.
We must also remember that the graft that was excised was a unique synthetic graft. It had originally been a tube graft after elective repair in 2009. Following emergency surgery for rupture in 2012 this tube graft was refashioned to add an aorto-bifemoral graft. This meant it was longer than advocated synthetic grafts. This was an anatomical challenge and the choice of a cryopreserved aorta over came this and provided options for re-implantation of native arteries. As described above in the patients own femoral veins were harvested and used as autografts in the distil anastomosis of the cryopreserved aorta. An option not available in use of synthetic grafts.
The reasons described guided the surgical team in their pre-operative planning. The aim of avoiding further surgery, recurrence of infection, the anatomical challenge and the use of autograft material all meant that the use of the cryopreserved aorta was far superior choice to synthetic graft use or other surgical options mentioned in the literature if not the only surgical option available.
Conflicts of interest
Nil.
Funding
Nil.
Ethical approval
Nil – Not required for case report.
Consent
Yes consent obtained.
Author contribution
All authors involved in:
-
-
Conceptions and design, acquisition of data or analysis and interpretation of data.
-
-
Drafting the article or revising it critically for important intellectual content.
-
-
Final approval of the version published.
Guarantor
All authors.
References
- 1.O’Connor S., Andrew P., Batt M., Becquemin J.P. A systematic review and meta-analysis of treatments for aortic graft infection. J. Vasc. Surg. 2006;44:38–45. doi: 10.1016/j.jvs.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 2.Hallett J.W., Marshall D.M., Petterson T.M., Gray D.T., Bower T.C., Cherry K.J., Jr. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J. Vasc. Surg. 1997;25:277–286. doi: 10.1016/s0741-5214(97)70349-5. [DOI] [PubMed] [Google Scholar]
- 3.Johnson K.W. Multicenter prospective study of nonruptured abdominal aortic aneurysms: II. Variables predicting morbidity and mortality. J. Vasc. Surg. 1989;9:427. doi: 10.1067/mva.1989.vs0090437. [DOI] [PubMed] [Google Scholar]
- 4.McCready R.A., Bryant M.A., Divelbiss J.L., Chess B.A., Chitwood R.W., Paget D.S. Arterial infections in the new millennium: an old problem revisited. Ann. Vasc. Surg. 2006;20:590–595. doi: 10.1007/s10016-006-9107-y. [DOI] [PubMed] [Google Scholar]
- 5.O’Hara P.J., Hertzer N.R., Beven E.G., Krajewski L.P. Surgical management of infected abdominal aortic grafts: review of a 25-year experience. J. Vasc. Surg. 1986;3:725–731. [PubMed] [Google Scholar]
- 6.Bacourt F., Koskas F. Axillobifemoral artery bypass and aortic exclusion for vascular septic lesions: a multicentered retrospective study of 98 cases. Ann. Vasc. Surg. 1992;6:119–126. doi: 10.1007/BF02042731. [DOI] [PubMed] [Google Scholar]
- 7.Quinones-Baldrich W.J., Hernandez J.J., Moore W.S. Long-term results following surgical management of aortic graft infection. Arch. Surg. 1991;126:507–511. doi: 10.1001/archsurg.1991.01410280111018. [DOI] [PubMed] [Google Scholar]
- 8.Brown K.E., Heyer K., Rodriguez H., Eskandari M.K., Pearce W.H., Morasch M.D. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J. Vasc. Surg. 2009;49:660–666. doi: 10.1016/j.jvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Kieffer E., Gomes D., Chiche L., Fleron M.H., Koskas F., Bahnini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J. Vasc. Surg. 2004;39:1009–1017. doi: 10.1016/j.jvs.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Vardanian A.J., Chau A., Quinones-Baldrich W., Lawrence P.F. Arterial allograft allows in-line reconstruction of prosthetic graft infection with low recurrence rate and mortality. Am. Surg. 2009;75:1000–1003. [PubMed] [Google Scholar]
- 11.Liekweg W.G., Jr., Greenfield L.J. Vascular prosthetic infections: collected experience and results of treatment. Surgery. 1977;81:335. [PubMed] [Google Scholar]
- 12.Campbell W.B., Tambeeur L.J., Green V.R. Local complications after arterial bypass grafting. Ann. R. Coll. Surg. Engl. 1994;76:127. [PMC free article] [PubMed] [Google Scholar]
- 13.Reilly L.M., Stoney R.J., Goldstone J., Ehrenfeld W.K. Improved management of aortic graft infection: the influence of operation sequence and staging. J. Vasc. Surg. 1987;5:421–431. doi: 10.1067/mva.1987.avs0050421. [DOI] [PubMed] [Google Scholar]
- 14.Clagett G.P., Valentine R.J., Hagino R.T. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J. Vasc. Surg. 1997;25:25. doi: 10.1016/s0741-5214(97)70347-1. [DOI] [PubMed] [Google Scholar]
- 15.Nevelsteen A., Lacroix H., Suy R. Autogenous reconstruction of the lower extremity deep veins: an alternative treatment of prosthetic infection after reconstructive surgery of aortoiliac disease. J. Vasc. Surg. 1995;22:129. doi: 10.1016/s0741-5214(95)70106-0. [DOI] [PubMed] [Google Scholar]
- 16.Bandyk D.F., Novotney M.L., Back M.R., Johnson B.L., Schmacht D.C. Expanded application of in situ replacement for prosthetic graft infection. J. Vasc. Surg. 2001;34:411–420. doi: 10.1067/mva.2001.117147. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W., Lin P.H., Bush R.L., Terramani T.T., Matsuura J.H., Cox M. In situ reconstruction with cryopreserved arterial allografts for management of mycotic aneurysms or aortic prosthetic graft infections: a multi-institutional experience. Tex. Heart Inst. J. 2006;33:14–18. [PMC free article] [PubMed] [Google Scholar]
- 18.Noel A.A., Gloviczki P., Cherry K.J., Safi H., Goldstone J., Morasch M.D. Abdominal aortic reconstruction in infected fields: early results of the United States cryopreserved aortic allograft registry. J. Vasc. Surg. 2002;35:847–852. doi: 10.1067/mva.2002.123755. [DOI] [PubMed] [Google Scholar]
- 19.Harlander-Locke M.P., Harmon L.K., Lawrence P.F., Oderich G.S., McCready R.A., Morasch M.D., Feezor R.J. The use of cryopreserved aortioiliac allograft for aortic reconstruction in the United States. J. Vasc. Surg. 2014;59(3):669–674. doi: 10.1016/j.jvs.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Vardanian A., Chau A., Quinones-Baldrich W., Lawrence P. Arterial allograft allows in-line reconstruction of prosthetic graft infection with low recurrence rate and mortality. Am. Surg. 2009;75:1000–1003. [PubMed] [Google Scholar]
- 21.Noel A., Gloviczki P., Cherry K., Safi H., Jr., Goldstone J., Morasch M., Johansen K., Members of the United States Cryopreserved Aortic Allograft Registry Abdominal aortic reconstruction in infected fields: early results of the United States cryopreserved aortic allograft registry. J. Vasc. Surg. 2002;35:847–852. doi: 10.1067/mva.2002.123755. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W., Lin P., Bush R., Terramani T., Matsuura J., Cox M., Peden E., Guerrero M., Silberfein E., Dardik A., Rosenthal D., Lumsden A. In situ reconstruction with cryopreserved arterial allograft. Tex. Heart Inst. J. 2006;33:14–18. [PMC free article] [PubMed] [Google Scholar]
- 23.McCready R., Bryant M., Divelbiss J., Chess B., Chitwood R., Paget D. Arterial infections in the new millennium: an old problem revisited. Ann. Vasc. Surg. 2006;20(September (5)):590–595. doi: 10.1007/s10016-006-9107-y. [DOI] [PubMed] [Google Scholar]
- 24.Brown K., Heyer K., Rodriguez H., Eskandari M., Pearce W., Morasch M. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J. Vasc. Surg. 2009;49:660–666. doi: 10.1016/j.jvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Dosluoglu H., Kittredge J., Cherr G. Use of cryopreserved femoral vein for in situ replacement of infected femorofemoral prosthetic artery bypass. Vasc. Endovasc. Surg. 2008;42(1):74–78. doi: 10.1177/1538574407308204. [DOI] [PubMed] [Google Scholar]
- 26.McCready R., Bryant M., Divelbiss J., Wack M., Mattison H. Case study: chronic femoropopliteal prosthetic graft infection with exposed graft. Vasc. Endovasc. Surg. 2009:1–4. doi: 10.1177/1538574408326265. (epub) [DOI] [PubMed] [Google Scholar]