Highlights
-
•
Pyogenic liver abscess is related to a mortality rate ranging between 15% and 19%.
-
•
In rare cases, spontaneous rupture of liver abscess may occurr.
-
•
An abdominal wall abscess secondary to spontaneous rupture of a pyogenic liver abscess is an extremely rare event.
-
•
In case of free rupture into the peritoneum, emergency surgery is mandatory.
-
•
A rupture in neighboring tissues or organs can be successfully treated by a combination of systemic antibiotics and fine needle aspiration and/or percutaneous drainage of the abscess.
Keywords: Pyogenic liver abscess, Spontaneous rupture, Abdominal wall abscess, Proteus mirabilis
Abstract
Introduction
Pyogenic liver abscess is a rare cause of hospitalization, related to a mortality rate ranging between 15% and 19%. Treatment of choice is represented by image-guided percutaneous drainage in combination with antibiotic therapy but, in some selected cases, surgical treatment is necessary. In extremely rare cases, spontaneous rupture of liver abscess may occur, free in the peritoneal cavity or in neighboring organs, an event which is generally considered a surgical emergency.
Presentation of case
A 95-years-old woman was hospitalized with fever, upper abdominal pain, mild dyspepsia and massive swelling of the anterior abdominal wall. Computed tomography revealed an oval mass located in the abdominal wall of 12 cm × 14 cm × 7 cm, in continuity with an abscess of the left hepatic lobe. Because Proteus mirabilis was detected in both the liver abscess and the abdominal wall abscess, the patient was diagnosed with a ruptured pyogenic liver abscess. After spontaneous drainage to the exterior of the hepato-parietal abscess, she was successfully treated with antibiotics alone.
Conclusion
Pyogenic liver abscess is a serious and life-threatening illness. Abscess rupture might occur. Many authors consider this complication a surgical emergency, but the site of abscess rupture changes the clinical history of the disease: in case of free rupture into the peritoneum, emergency surgery is mandatory, while a rupture localized in neighboring tissues or organs can be successfully treated by a combination of systemic antibiotics and fine needle aspiration and/or percutaneous drainage of the abscess.
1. Introduction
Pyogenic liver abscess (PLA) is a rare cause of hospitalization, related to a high mortality rate ranging between 15% and 19%. Its incidence is difficult to define, since it varies from one country to another but overall it is estimated at 3.59 per 100,000 and it increases with patient’s age and comorbidities (diabetes, malnutrition, immunosuppression, kidney failure, cirrhosis) [1], [2], [3].
Disease symptoms and signs are not specific neither are laboratory tests: liver function tests may be more or less normal, depending on the extent of abscess, its causes and sepsis severity. The diagnosis is mainly based on imaging. Ultrasound (US) and computed tomography (CT) scans allow diagnosis in over 90% of cases, and often give information about etiology [2].
Treatment of choice is represented by image-guided percutaneous drainage in combination with antibiotic therapy but, in some selected cases, surgical treatment is necessary although associated with increased morbidity and mortality [2], [3].
In extremely rare cases, spontaneous rupture of liver abscess may occur, free in the peritoneal cavity or in neighboring organs, an event which is generally considered a surgical emergency [4], [5].
We present the exceptional case of a frail elderly patient with a remarkably bulky abscess of anterior an abdominal wall, secondary to spontaneous rupture of a PLA, with spontaneous drainage to the exterior and successful treatment with medical therapy alone.
2. Case report
We present the case of a 95-years-old woman, suffering from multiple comorbidities: dementia and severe hypokinetic syndrome, hypertensive heart disease with cardiac conduction abnormalities, chronic obstructive pulmonary disease. She had a negative personal history for previous surgery and hemostasis disorders.
She was hospitalized due to fever, upper abdominal pain, mild dyspepsia and massive swelling of the anterior abdominal wall. On physical examination the patient had a temperature of 38 °C, heart rate 86 beats/min and blood pressure 110/65 mmHg. There was no clinical jaundice. Abdominal examination revealed a painful palpable mass in the epigastric-umbilical regions, of 8 cm in diameter, and covered by hyperemic, hot and tense skin. The patient reported pain in the upper abdominal and periumbilical regions on palpation, in the absence of peritoneal signs.
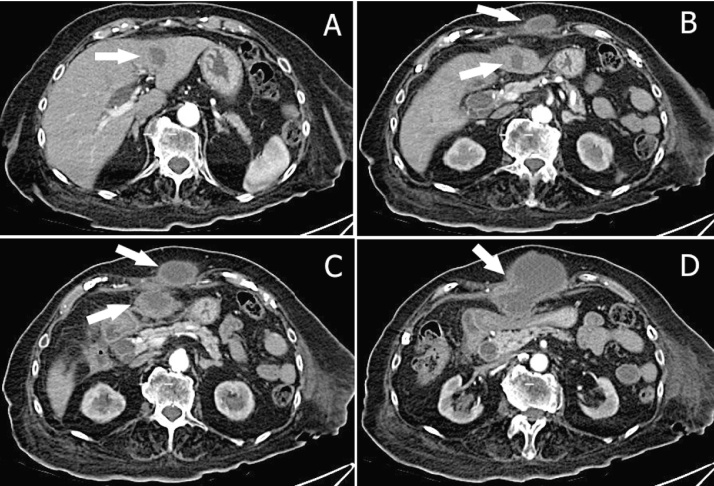
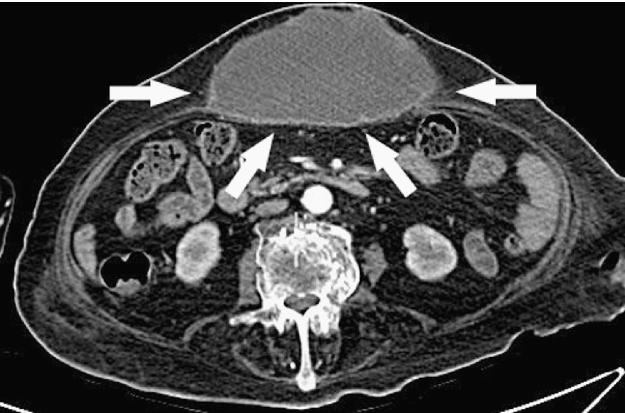
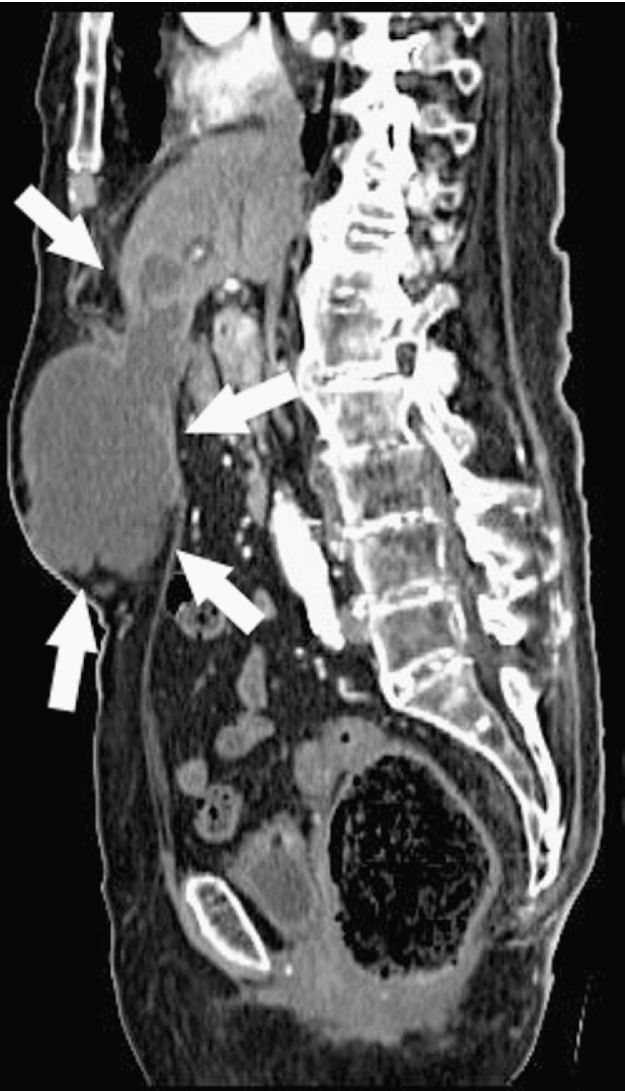
Abdomen US showed a complex-type mass of anterior abdominal wall greater than 12 cm which dislocated posteriorly the stomach; the gallbladder appear normal, without stones. Abdominal CT scan with contrast showed an abscess inside the left hepatic lobe, in the antero-lateral portion of segment 3, 3.5 cm in diameter, in continuity, through a subtle fistulous tract, with an equivalent big necrotic collection extending downward to the umbilical region. The corresponding portion of the abdominal fascia was disrupted; in the subcutaneous space a bulky mass of 12 cm × 14 cm × 7 cm in size was present, with the same necrotic content (Fig. 1, Fig. 2, Fig. 3 ); also there was a dilated intrahepatic bile ducts and common bile duct dilatation with apparent regular outlet in the duodenum.
Fig. 1.
Abdomen CT scans show: (A) the liver abscess in the left hepatic lobe (arrow); (B,C) “two abscesses”, one of the anterior abdominal wall and one of left lobe (arrows); (D) single trans-fascial abscess resulting from spontaneous rupture of the liver abscess in the abdominal wall (arrow).
Fig. 2.
Abdomen CT scan shows: bulky abscess of the anterior abdominal wall of 12 cm × 14 cm × 7 cm (arrows).
Fig. 3.
Axial abdomen CT scan shows: trans-fascial abscess resulting from spontaneous rupture of the liver abscess in the abdominal wall (arrows).
Her laboratory tests showed: white blood cells 14.5 × 1000/uL (4.10 × 1000/uL), hemoglobin 10.3 g/dL (12.5–15.5 g/dL), C-reactive protein 12,53 mg/dL (0–0.5 mg/dL); liver function and coagulation tests were normal. The tumor markers and serology for amebiasis or tuberculosis were negative.
Ultrasound-guided fine needle aspiration of the parietal and liver abscesses was performed: culture test identified Proteus mirabilis in both samples and a specific antibiotic therapy with third-generation cephalosporins and metronidazole was set up, after an introductory empirical therapy with amoxicillin and clavulanic acid. There was no need to place a percutaneous drainage, because of spontaneous rupture of the abscess on to the surface of the abdominal wall.
As result of this course and after an adequate antibiotic therapy, the inflammatory markers improved significantly, white blood cells 5.9 × 1000/uL (4.10 × 1000/uL) and C-reactive protein 0.75 mg/dL (0–0.5 mg/dL); likewise, there was a remission of clinical picture with apyrexia, disappearance of abdominal pain and abdominal wall swelling and closing of the fistula, after repeated daily dressings.
The patient resumed feeding gradually, without disorders, and hospital discharge took place after 39 days. Considering the age and fragility of the patient no further examination was performed. After 12 months, the patient is alive and in good clinical condition.
3. Discussion
In Western countries, 80% of liver abscess are caused by bacteria: the most frequently isolated species of them are Streptococcus pyogenes (29.5%), Escherichia coli (18.1%), Staphylococcus species (10.5%) and Klebsiella pneumoniae (9.2%); in the meantime, in Asia, above all in Taiwan, the most common bacteria is Klebsiella pneumoniae (69–78%) [1], [2], [3], [6], [7], [8].
The invasion of the liver parenchyma occurs through the bile ducts (cholelithiasis, obstructive biliary tree tumors, stenosis, congenital malformation), portal venous system (appendicitis or diverticulitis), arterial blood (endocarditis or pyelonephritis) or contiguity, in particular through the bed of the gallbladder. It can also result from a penetrating or non-penetrating liver trauma, a secondary infection of amoebic abscesses, cavities hydatid cysts, metastases and primary liver tumors or a complication of liver transplantation or hepatic artery embolization [2], [9].
Right hepatic lobe is affected more often than the left one (2:1), while bilateral involvement occurs in 5% of cases [9].
PLA treatment envisages antibiotic specific therapy, percutaneous drainage and treatment of the underlying condition or its cause. Empirical antibiotic therapy should be started immediately to reduce sepsis systemic effects and should be addressed to typically responsible bacteria, aerobic Gram-negative and Gram-positive cocci (piperacillins, tazobactam, amoxicillin-clavulanate, third-generation cephalosporins), in combination with an aminoglycoside and an anaerobic drug such as metronidazole, in case the antibiotic which has been chosen is not active against anaerobes or if there is a chance that abscess has amoebic origin. The length of the antibiotic therapy is generally between 2 and 6 weeks. A small PLA, <3–5 cm, especially if multiple, can be treated only with antibiotics, although there is no general consent. Surgical drainage is rarely indicated, however, some authors suggest it, in case percutaneous treatment fails, in the event of >5 cm large abscesses, and/or multilocular PLA, or when surgical treatment of PLA underlying cause is required [1], [2], [10], [11], [12].
With the improvement of diagnostic tests and treatment options, many cases may be diagnosed at early stages of the disease and effectively treated. As a result, PLA-related complications, such as abscess rupture, rarely occur [4].
PLA rupture represents a very uncommon complication but, in some patients, it might increase morbidity and mortality. Two studies from Taiwan showed the rupture rates PLA of 1.2% and 5.7%, respectively. The highest rate is associated with Klebsiella infection, and these patients have been shown to be significantly correlated with a greater abscess (>8 cm), diabetes, formation of gas (gas increases the tension within the abscess cavity and thus the risk of rupture of the abscess) and the involvement of the left hepatic lobe (given its smaller size). Moreover, a higher rupture frequency was detected in patients with liver cirrhosis and septic metastases. Authors presumed that patients suffering from liver cirrhosis – with impairment of phagocytic functions and bactericides, as they have a smaller number of Kupffer cells and reduced function – show a more severe local inflammation that increases the trend to abscess rupture [4], [5], [11].
In general, rupture of liver abscess is considered a surgical emergency. However, this depends on other factors too, such as rupture site and other underlying conditions. The sites of rupture are subphrenic and perihepatic regions, peritoneum, pleura, skin, mediastinum and pericardium. A rupture in the bowel, pericardium or in the mediastinum is rare. A rupture leading to peritonitis requires prompt surgical intervention, while localized rupture can be managed with drainage, either percutaneous or surgical, in addition to an appropriate antimicrobial treatment [4], [5].
Elderly patients suffering from PLA usually experience outcomes similar to those in young adults. Their hospital stay may be longer, they are more easily exposed to multiple comorbidities and more likely to have a biliary disease or an underlying malignancy, while young adults are more likely to be male with alcoholic or cryptogenic etiology [3], [13].
We performed an extensive literature search about the rupture in the abdominal wall of a PLA and we have identified only two other cases (Table 1). In the case described by Kawoosa et al. [9], the patient was a young woman with a history of previous laparotomy for PLA, with a multiple PLA of the right hepatic lobe caused by Klebsiella pneumoniae, spontaneously ruptured into right upper quadrant wall and treated with percutaneous drainage and antibiotics. In the case described by Belabbes et al. [14] the patient was an old woman, with no comorbidities, with a bulky abscess of the left hepatic lobe (not identified a pathogen), that spontaneously ruptured into epigastric-umbilical wall and treated with percutaneous drainage and antibiotics. Similar to this is the our case in which the etiologic agent was identified in Proteus mirabilis, rarely responsible for PLA (0.78%).
Table 1.
Patient characteristics from the reports of abdominal wall abscess secondary to pyogenic liver abscess.
| Authors | Age | Gender | Liver abscess | Liver lobe | Abdominal regions | Pathogen | Antibiotics | Percutaneous drainage | Surgery |
|---|---|---|---|---|---|---|---|---|---|
| Current Report | 95 | Female | Solitary | Left | Epigastric/Umbilical | Proteus mirabilis | Cephalosporins/Metronidazole | No | No |
| Belabbes et al. [14] | 78 | Female | Solitary | Left | Epigastric/Umbilical | NA | Cephalosporins/Metronidazole | Yes | No |
| Kawoosa et al. [9] | 32 | Female | Multiple | Right | Right Hypochondriac | Klebsiella pneumoniae | Imipenem/Metronidazole | Yes | No |
NA: not available.
We think that this liver abscess can be secondary to a subclinic enteric (ileal or colonic) infection with microbial translocation of a strain of Proteus mirabilis, event possible especially in an elderly patient. Other causes, as a possible superimposed infection of a hematoma of the upper abdominal wall or an acute omphalitis are less likely, also considering the absence of any other symptom of a primary disease of the abdominal wall.
The unusual evolution of this abscess of the left lobe of the liver invites to anatomical considerations. The topographic characteristic of its progression toward the navel was clearly showed by CT scans, demonstrating also that the round ligament, physiologically provided with a rich lymphatic and blood vessels network, is the preferred path of diffusion [15], [16], [17]. This is supported by some arguments. First, the absence of intraperitoneal adhesions, possible after previous surgery or diseases of the upper abdomen, exclude other possible ways of diffusion; second, the position of the liver abscess in the antero-medial portion of segment 3, is close to the insertion of the round ligament and of its roots in the liver parenchyma; third, in an adult patient, it is more likely the diffusion of a liver abscess to the round ligament than the contrary, in absence of signs of primary abdominal wall infection. Therefore we propose the following cascade of pathological events: liver abscess, most probably due to colonization from an accidental intestinal infection, secondary involvement of the round ligament and then of the abdominal wall. Spontaneous rupture of the abdominal wall abscess to the exterior, favored by thin, dehydrated, slightly vascularized and under tension skin, complete the pathophysiology.
Finally, it is important to know some intra-abdominal diseases such possible causes of the abdominal wall abscesses to put in differential diagnosis: perforated tumors of the digestive tract, particularly gastric and colonic (cecum, transverse colon and sigmoid colon); acute inflammatory diseases such as acute sigmoid diverticulitis, acute cholecystitis and acute appendicitis; and in patients operated on following the development of fistulas or acute ischemic damage from recent laparotomy, usually with the aspect of phlegmon.
4. Conclusion
PLA is a serious and life-threatening illness, which rare in Western countries but still common in other areas such as Taiwan. An immediate diagnosis and proper treatment, mostly based on systemic antibiotics and percutaneous drainage of the abscess, allow complete recovery. However, abscess rupture might occur, an event that greatly increases morbidity and mortality rates. Many authors consider this complication a surgical emergency, but this is not entirely correct since the site of abscess rupture changes the clinical history of the disease: in case of free rupture into the peritoneum, emergency surgery is mandatory, while a rupture localized in neighboring tissues or organs, regardless of what tissues or organs they might be, can be successfully treated by a combination of systemic antibiotics and fine needle aspiration and/or percutaneous drainage of the abscess.
Conflict of interest
The authors declare that they have no conflict of interest.
Sources of funding
The authors have no financial ties to disclose.
Ethical approval
Not necessary.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
Zizzo: Study Design, Data Collection, Statistical Analysis, Data Interpretation, Manuscript Preparation, Literature Search, Writing. Zaghi: Study Design, Data Collection, Data Interpretation, Manuscript Preparation. Manenti: Data Collection, Data Interpretation, Manuscript Preparation. Luppi: Study Design, Manuscript Preparation. Ugoletti: Data Collection, Manuscript Preparation. Bonilauri: Study Design.
Guarantor
Dr Stefano Bonilauri, Chief of C.S. General and Emergency Surgery, Azienda Ospedaliera – IRCCS Arcispedale Santa Maria Nuova, Avenue Risorgimento 80, 42123 Reggio Emilia (Italy).
Contributor Information
Maurizio Zizzo, Email: zizzomaurizio@gmail.com.
Claudia Zaghi, Email: Claudia.Zaghi@asmn.re.it.
Antonio Manenti, Email: antonio.manenti@unimore.it.
Davide Luppi, Email: Davide.Luppi@asmn.re.it.
Lara Ugoletti, Email: gigiodoctorchir@gmail.com.
Stefano Bonilauri, Email: Stefano.Bonilauri@asmn.re.it.
References
- 1.Alkofer B., Dufay C., Parienti J., Lepennec V., Dargere S., Chiche L. Are pyogenic liver abscesses still a surgical concern? A Western experience. HPB Surg. 2012;2012:316013. doi: 10.1155/2012/316013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lardière-Deguelte S., Ragot E., Amroun K. Hepatic abscess: diagnosis and management. J. Vasc. Surg. 2015;152(4):231–243. doi: 10.1016/j.jviscsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Meddings L., Myers R.P., Hubbard J. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am. J. Gastroenterol. 2010;105(1):117–124. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 4.Jun C.H., Yoon J.H., Wi J.W. Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J. Dig. Dis. 2015;16(1):31–36. doi: 10.1111/1751-2980.12209. [DOI] [PubMed] [Google Scholar]
- 5.Chong V.H., Zainal-Abidin Z., Hassan H., Chong C.F. Rare complications of pyogenic liver abscess. Singapore Med. J. 2010;51(10):e169–72. [PubMed] [Google Scholar]
- 6.Brook I., Frazier E.H. Microbiology of liver and spleen abscesses. J. Med. Microbiol. 1998;47(12):1075–1080. doi: 10.1099/00222615-47-12-1075. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y.C., Lin C.H., Chang S.N., Shi Z.Y. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000-2011. J. Microbiol. Immunol. Infect. 2014 doi: 10.1016/j.jmii.2014.08.028. pii: S1684-1182(14)00212-6. [DOI] [PubMed] [Google Scholar]
- 8.Mangukiya D.O., Darshan J.R., Kanani V.K., Gupta S.T. A prospective series case study of pyogenic liver abscess: recent trands in etiology and management. Indian. J. Surg. 2012;74(5):385–390. doi: 10.1007/s12262-011-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawoosa N.U., Bashir A., Rashid B. Spontaneous cutaneous rupture of a pyogenic liver abscess. Indian. J. Surg. 2010;72(4):339–342. doi: 10.1007/s12262-010-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampropoulos C.E., Papaioannou I., Antoniou Z. Multiple, large pyogenic liver abscesses treated conservatively: a case-report and review of the literature. GE J. Port. Gastrenterol. 2013;20(1):21–24. [Google Scholar]
- 11.Motoyama T., Ogasawara S., Chiba T. Successful non-surgical treatment of ruptured pyogenic liver abscess. Intern. Med. 2013;52(23):2619–2622. doi: 10.2169/internalmedicine.52.0980. [DOI] [PubMed] [Google Scholar]
- 12.Skouras C., Mole D.J. Benign liver lesions. Surgery. 2014;32(12):648–654. [Google Scholar]
- 13.Lung J., Khan A., Malone M.L. Pyogenic liver abscess in a frail older adult. J. Am. Geriatr. Soc. 2014;62(2):408–409. doi: 10.1111/jgs.12664. [DOI] [PubMed] [Google Scholar]
- 14.Belabbes S., El Barni R., El Kharras A. Anterior abdominal wall abscess revealing a pyogenic liver abscess: a case report. Research. 2014;1:1256. [Google Scholar]
- 15.Ames J.T., Federle M.P. Septic thrombophlebitis of the portal venous system: clinical and imaging findings in thirty-three patients. Dig. Dis. Sci. 2011;56(7):2179–2184. doi: 10.1007/s10620-010-1533-6. [DOI] [PubMed] [Google Scholar]
- 16.Arakura N., Ozaki Y., Yamazaki S. Abscess of the round ligament of the liver associated with acute obstructive cholangitis and septic thrombosis. Intern. Med. 2008;48(21):1885–1888. doi: 10.2169/internalmedicine.48.2396. [DOI] [PubMed] [Google Scholar]
- 17.Warren L.R., Chandrasegaram M.D., Madigan D.J., Dolan P.M., Neo E.L., Worthley C.S. Falciform ligament abscess from left sided portal pyaemia following malignant obstructive cholangitis. World J. Surg. Oncol. 2012;10:278. doi: 10.1186/1477-7819-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]