Highlights
-
•
There are several endoscopic, laparoscopic and open surgical procedures available for the management of GIST tumors near the GE junction.
-
•
We present a case-report utilizing both endoscopy and laparoscopy to facilitate resection using the non-touch lesion-lifting technique.
-
•
Pathology demonstrated a 4.7 cm GIST. The patient was discharged on post-operative day 3 with no complications.
-
•
We include a full literature review and describe the various combined modalities available for successful resection.
Keywords: GIST tumors, Laparo-endoscopic resection, Gastroesophageal junction tumors, Laparoscopic wedge resection
Abstract
Introduction
The safety and oncologic outcome of laparoscopic gastric GIST resection is well established especially for lesions <5 cm in diameter. The optimal management of GIST tumors near the GE junction remains unclear.
Methods
We present a case-report of a 4.7 cm GIST tumor near the GE junction managed by endoscopically-assisted laparoscopic wedge resection (EAWR). We present a review of the literature highlighting the various combined laparo-endoscopic techniques available.
Results
We used the non-touch lesion-lifting method to laparoscopically resect the GIST tumor under endoscopic guidance. There were no complications and the patient was discharged on postoperative day 3.
Conclusions
Endoscopically-assisted laparoscopic wedge resections are feasible and safe for GIST tumors near the GE junction.
1. Introduction
Gastrointestinal Stromal Tumors (GISTs) are the most frequent tumors of the gastric submucosa [1]. These tumors are derived from the interstitial cells of Cajal and have been shown to harbor gain-of-function mutations in the cell-surface KIT receptor in approximately 90% of cases [2]. Surgical resection with negative margins is the standard of care. The safety and oncologic outcome of laparoscopic gastric GIST resection is well established especially for lesions <5 cm in diameter [3]. Laparoscopy has been shown to decrease intraoperative blood loss, postoperative pain and hospital stay [3]. It has the added advantage of allowing improved visualization during surgery to rule out disease spread to the liver or peritoneum. Although the National Comprehensive Cancer Network Clinical Practice Guidelines for Optimal Management of patients with GIST suggests that laparoscopic techniques should be limited to tumors <5 cm [4], many groups do not consider tumor size as a contraindication for the laparoscopic approach [5]. The management of tumors located near or at the gastro-esophageal (GE) junction poses a particular challenge. The esophagus and vagus nerves are at risk for injury, and the GE junction is at risk for narrowing or dysfunction if the resection is not well-planned.
Several surgical procedures have been proposed for management GE junction GIST tumors. They use a combination of laparoscopic and endoscopic techniques and include: enucleation, endoscopically assisted wedge resection (EAWR), laparoscopically assisted endoscopic resection (LAER), laparoscopic and endoscopic cooperative surgery (LECS) and transgastric resection for tumors located posteriorly. The treatment of GIST tumors at the GE junction has traditionally been an esophagectomy [6]. Recent studies have proposed enucleation with or without fundoplication as a reasonable alternative for small tumors with low mitotic rates [7], [8]. On the other hand, there are several treatment options for tumors near, not at, the GE junction. One of the major limitations of the laparoscopic approach is accurately localizing these lesions and defining their anatomic relationship to the GE junction. This can be easily addressed by adding intraoperative endoscopy. The non-touch lesion-lifting technique, first described by Ohgami et al. in 1996, for laparoscopic wedge resection has gained widespread acceptance and is our method of choice for resecting GIST tumors near the GE junction [1], [9].
In this case-report, we describe the management of a GIST tumor located near the GE junction. We employ both endoscopy and laparoscopy to facilitate resection using the non-touch lesion-lifting technique. Consent in accordance with our institutional regulations was obtained.
2. Case report
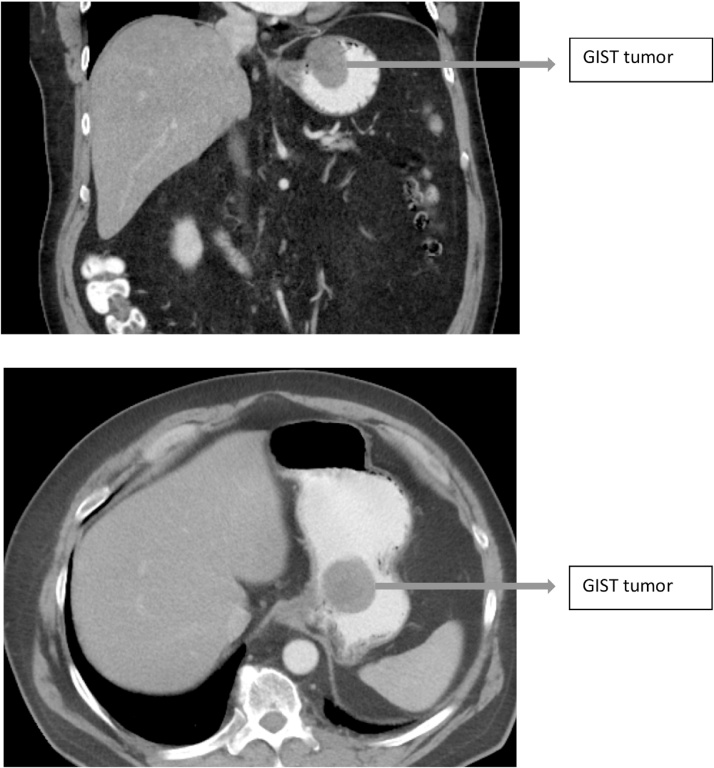
64 year old gentleman referred for a general surgical evaluation of an umbilical hernia. A CT scan of the abdomen was ordered to assess the defect size and its contents. The CT demonstrated an incidental 4.7 cm mass involving the gastric fundus and lesser curvature 2 cm from the GE junction and a fat containing umbilical hernia (Fig. 1). EGD/EUS were performed and demonstrated a 5 cm submucosal hypoechoic nodule located within 2 cm of the gastroesophageal junction along the anterior wall of the stomach. It had multiple cystic areas suggestive of necrosis. Fine-needle aspiration was performed and demonstrated spindle cells, DOG-1 and CD117 (C-kit) positive, consistent with a GIST. A lesion-lifting endoscopically assisted laparoscopic wedge resection was planned.
Fig. 1.
CT scan findings demonstrating a 4.7 cm GIST within 2 cm of the gastroesophageal junction.
The patient was taken to the operating room and placed in a supine position. An incision was made over his umbilical hernia and the hernia sac was dissected down to the fascia. The hernia sac was excised and a 12-mm balloon tipped trocar was inserted into the abdomen. The abdomen was insufflated and a diagnostic laparoscopy was performed. The liver and peritoneum were free of disease. Three more trocars were inserted: a 5-mm right paramedian, 12-mm left mid-clavicular and a 5-mm left anterior axillary trocar. An Endo-Harmonic (Ethicon US, LLC.) was used to mobilize the left lateral segment of the liver and a laparoscopic liver retractor was placed through the right paramedian port. The tumor was located on the anterior lesser curvature of the stomach within 2 cm of the GE junction. The Endo-Harmonic was used to mobilize the esophageal fat pad to define the superior extent of our resection. The gastro-hepatic ligament was incised and the medial border of the stomach was dissected. An endoscope was passed into the stomach and retroflexed identifying the tumor and its relationship to the GE junction. A laparoscopic 60 mm linear stapler was used to lift the tumor anteriorly and close below it. This was done under endoscopic guidance. The endoscope was used to make sure the staple line was below the tumor and away from the GE junction. The resection was completed using 2 Covedien black reloads with Tri-Staple technology (Medtronic-Covedien, Minneapolis US). An air-leak test was performed at the end of the case by endoscopically insufflating the stomach and submerging the staple line under normal saline. There was no leak. The operative time was 90 min with minimal blood loss.
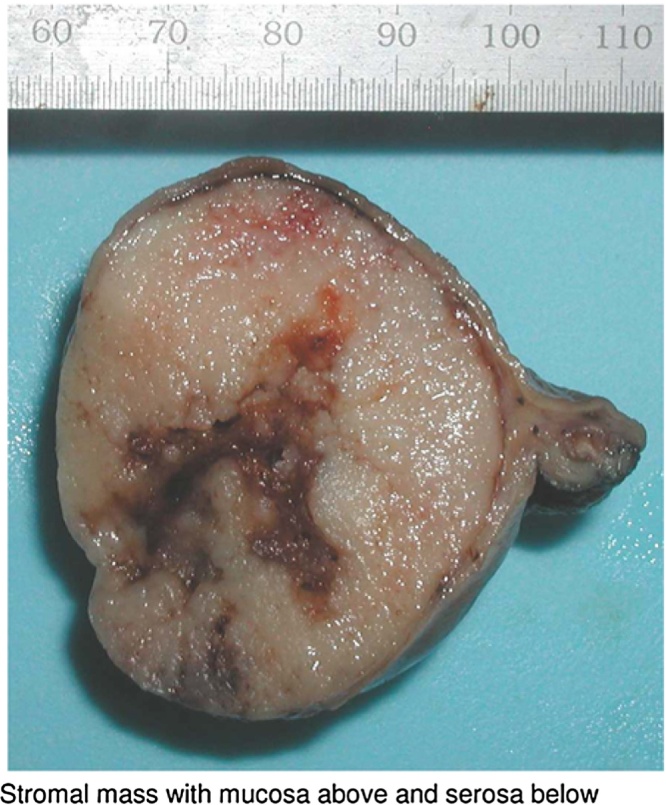
The patient underwent a water soluble followed by thin Barium upper GI study on the first postoperative day which did not show a leak. He was placed on a clear liquid diet and advanced to a regular diet the following day. He was discharged on the third post-operative day. Pathology demonstrated a 4.7 cm GIST with negative margins and a mitotic rate of <5/50 HPF (Fig. 2). His case was presented at our institutional tumor board and no adjuvant treatment was recommended. He underwent a surveillance CT scan 6 months after surgery which showed no evidence of disease. He remains symptom free without pain or iron-deficiency anemia.
Fig. 2.
Final pathology specimen − (submucosal lesion).
3. Discussion and literature review
There are several endoscopic, laparoscopic and open surgical procedures available for the management of GIST tumors near the GE junction. Over the last decade, multiple reports have described both the feasibility and safety of laparoscopy in treating these tumors [10], [11]. Many groups have reported successful laparoscopic resection of lesions >5 cm with equivalent oncologic results [5], [12]. It is our practice to approach all cases laparoscopically, at least initially. Laparoscopy provides better visualization to rule out occult metastases that are otherwise not picked up on pre-operative imaging. Once inside the abdomen, a combination of laparoscopy and endoscopy determines the feasibility of laparoscopic resection.
Several combined techniques have been described and it is important for both the surgeon and gastroenterologist to understand them and know when to use them. We prefer endoscopically assisted wedge resections (EAWR) as is described in this case-report. The endoscope is retroflexed and the surgeon can visualize the staple line as the stapler is closed achieving negative margins and without narrowing the GE junction. EAWR has the added advantage of minimizing tumor manipulation as the stapler can be used to lift the tumor before resection. Other authors have described using traction sutures to lift the tumor up before firing the stapler [1]. This may be useful, especially for smaller intra-gastric tumors. Larger, predominantly extra-luminal masses are more easily handled with the stapler alone. Tumors located posteriorly are more challenging; however, they can be addressed through fundic rotation techniques. By entering into the lesser sac and dividing the short gastric vessels, the gastric fundus can be rotated to the right or left facilitating resection under endoscopic guidance [13], [14]]. Bioabsorbable staple line reinforcement or over-sewing of the staple line can be used to reduce the leak rate.
Laparoscopically assisted endoscopic resection (LAER) is useful for small lesions with a predominant intra-gastric component. The gastroenterologist proceeds with a submucosal resection while the surgeon observes and occasionally assists laparoscopically by pushing the lesion or stretching the stomach wall. If a perforation develops, it can be closed by stapler application or suturing [15].
Laparoscopic and Endoscopic Cooperative Surgery (LECS) was introduced by Hiki et al. in 2008 for the management of intragastric GISTs and early gastric cancer [16]. The procedure has 2 components: an endoscopic submucosal dissection (ESD) around the tumor followed by a laparoscopic seromuscular dissection. The defect is finally closed using a linear cutting stapler. The advantages of this technique is that the line of resection is endoscopically planned and marked out prior to any dissection preserving the volume of remaining stomach without compromising the EG junction. The disadvantages include performing a gastrotomy, increasing the risk of intra-abdominal infection or tumor seeding into the peritoneal cavity [17]. In addition, both a gastroenterologist and a surgeon have to be present in the room for the lengthy procedure. “CLEAN-NET” or “combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique” is a modification to LECS in which the submucosal dissection is avoided [17]. These techniques are more common in Asian countries where early gastric cancer is prevalent and ESD is very common.
Endoscopically assisted transgastric resection (EATR) is an alternative to the fundic rotational techniques for the management of posterior tumors near the GE junction. Laparoscopic trocars are inserted into the stomach under endoscopic guidance. The lesion can be dissected endoscopically and the defect over sewn or it can be lifted and stapled [18], [19].
Table 1 is a literature review of the various combinations used over the last decade.
Table 1.
Literature review of combined laparo-endoscopic modalities for resecting GIST tumors of the stomach.
| Article | Gender | Age (years) | Procedure | Pathology | Location | Hospital stay |
|---|---|---|---|---|---|---|
| Endoscopic-assisted laparoscopic resection for gastric subepithelial tumors. [5] | 22 Males 16 Females |
Mean age 67 Range 41–86 | laparoscopic wedge resections and laparoscopic subtotal gastrectomies were performed. If the tumor was not identified at laparoscopy by inspection or palpation, a perioperative endoscopy was performed to confirm its position and ensure adequate resection. (14 patients had intraoperative endoscopic assessment (EAWR) | 32 GISTs 3 Neuroendocrine tumors 3 others |
16 in fundus 18 in body 4 in antrum 19 anterior, 19 posterior 12 on lesser curvature, 14 on greater curvature |
Median of 3 days |
| Laparo-endoscopic transgastric resection of gastric submucosal tumors. [20] | 6 Males 8 Females | Mean age 56.8 ± 13 | EATR | 10 GISTs 3 Leiomyomas 1 Schwannoma | 1 in duodenum 2 in fundus 2 on lesser curvature 4 at GE junction 6 in antrum |
1.8 ± 1.5 days |
| A case of gastric adenocarcinoma of fundic gland type resected by combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET). [17] | Male | 80s | Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) | Gastric cancer of fundic gland type (GAFT) | Greater curvature of the proximal fornix | 19 days |
| Single port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. [21] | Female | 75 | LECS | GIST | Anterior gastric wall, near EGJ | 5 days |
| Long term outcomes of combined endoscopic/laparoscopic intragastric enucleation of presumed gastric stromal tumors. [8] | 8 Males 8 Females |
Mean age 62 | EATR | 9 GISTs 5 Leiomyomas 1 Schwannoma 1 Ectopic pancreatic cyst |
8 Posterior body/greater curvature 5 GE junction 2 Incisura 1 Fundus |
Mean 4.4 days |
| Defining a subgroup treatable for laparoscopic and endoscopic cooperative surgery in undifferentiated early gastric cancer: the role of lymph node metastasis. [22] | 11 Males 7 Females |
14 patients < 60 4 patients over 60 | LECS | Early gastric cancer | Not specified | Not specified |
| Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor with complete situs inversus: report of a case. [23] | Male | 78 | LECS | GIST | Upper stomach near GE Junction | 12 days |
| Laparoscopic wedge resection of the stomach for gastrointestinal stromal tumor (GIST): non-touch lesion lifting method. [1] | 15 Males 27 females |
Median 66 Range 37–78 |
EAWR | 42 GISTs | 30 upper third of stomach 12 middle third of stomach |
Median 7 days (6–14) |
| Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for lateral spreading mucosal gastric cancer. [24] | Female | 70 | LECS | Early mucosal gastric cancer | Greater curvature of the fornix | Not specified |
| Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. [25] | 10 Males 10 Females |
Mean age 59.3 ± 11.9 years | LECS | 16 GISTs 1 Inflammation from parasite 1 Leiomyoma 1 Glomus tumor 1 Abberant pancreas |
8 in upper third of stomach (2 tumors located within 10 and 15 mm from EG junction) 8 in middle third of stomach 4 in lower part of stomach |
Average 11.6 days (range 6–13 days) |
| Laser-supported diaphanoscopy: a new technique in laparoscopic-endoscopic rendezvous procedures allowing better and moretissue-sparing tumor resection than wedge resection. [26] |
6 Males 4 Females |
Mean age 64.7 Range 34–86 | Tumor was marked with Endolight during endoscopy and was then resected laparoscopically (EAWR) | 10 GISTs | 5 anterior and 5 posterior gastric wall | Not specified |
| Fundic Rotation Technique: A useful procedure for laparoscopic exogastric resection of gastric submucosal tumors located on the posterior wall near the esophagogastric junction. [13] | 3 Males 2 Females |
Range 57–72 years | EATR | 5 GISTs | Within 1 inch of GE junction | Mean 8.2 days |
| Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. [27] | 4 Males | Mean age 58.5 | Laparoscopically assisted endoscopic full-thickness resection (LAER) | 1 Lipoma 1 GIST 1 Ectopic pancreas 1 Schwannoma | 1 Middle anterior greater curvature 1 Upper anterior lesser curvature 1 Middle posterior greater curvature 1 Middle lesser curvature |
7–8 days |
| Combined Endolaparoscopic Intragastric Excision for Gastric Neoplasms. [28] | 7 Males 5 Females |
Mean age 73 Range 47–83 |
Laparoscopically assisted transgastric resection (LATR) Complete transgastric submucosal dissection. the non-full-thickness resection defect is closed intra-gastrically under laparoscopic guidance |
8 GISTs 1 Adenomatous polyp with focal intramucosal adenocarcinoma 1 Leiomyoma 1 Well-differentiated adenocarcinoma 1 Pancreatic heterotopia |
1 at incisura 2 in body 2 in fundus 3 in antrum 4 in cardia |
Mean 5.2 days |
| Simultaneous Use of Laparoscopy and Endoscopy for Minimally Invasive Resection of Gastric Subepithelial Masses — Analysis of 93 Interventions. [15] |
43 Males 47 Females | Range 27–83 |
EAWR − 55 patients. EATR − 34 patients. LAER − 1 patient |
62 GISTs 9 Leiomyomas 5 Neurinomas 5 Lipomas 3 Neuroendocrine tumors 3 Hyperplastic polyps |
Not specified | 4–19 days |
| Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. [16] | 7 Females | Range 34–66 | LECS | 6 GISTs 1 Shwannoma | 1 on anterior GE junction 1 in upper anterior stomach 1 in lower anterior stomach 1 in middle posterior stomach 1 in posterior remnant stomach 2 in upper posterior stomach |
7.4 ± 8.1 days |
| Combined endoscopic and laparoscopic approach for palliative resection of metastatic melanoma of the stomach. [29] | Male | 58 | EGD then laparoscopy using EndoGIA 45 mm stapler to resect tumor. 3-0 polypropylene sutures were used to over-sew the suture line (EAWR). | Metastatic melanoma | Anterior wall of stomach | 2 days |
EAWR: Endoscopically assisted wedge resection. EATR: Endoscopically assisted transgastric resection. CLEAN-NET: Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique. LECS: Laparoscopic and endoscopic cooperative surgery. LAER: Laparoscopic assisted endoscopic resection. LATR: Laparoscopic assisted transgastric resection. Similar to LAER but through gastric ports.
It is our preference to start the surgery with a diagnostic laparoscopy. We dissect the esophageal fat pad and the lesser curvature. We perform intraoperative endoscopy. We then proceed with EAWR if there is adequate room to fire a stapler without narrowing the GE junction. We believe that avoiding a gastrotomy makes more oncologic sense and rotating the fundus, avoids difficult transgastric resections. Larger multi-institutional studies with long term follow-up are needed to establish EAWR as the treatment of choice for GIST tumors located near the GE junction. Such studies will establish the ease and safety of this technique and may demonstrate a superior oncologic outcome when compared to other methods that involve a gastrotomy and risk peritoneal seeding.
4. Conclusions
Laparoscopic wedge resection is widely accepted for the surgical management of gastric GISTs. The non-touch lesion-lifting technique can be performed safely near the GE junction when combined with intraoperative gastroscopy. Fundic rotation techniques can be used for posterior lesions. A simplified and validated algorithm for the management of gastric GISTs remains needed.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
No animals were used in this study.
Conflicts of interest
None of the authors have any conflicts of interest to disclose.
Funding
No sources of funding available.
Ethical approval
Ethical approval has been given.
Consent
Written consent in accordance with the ethics committee has been obtained.
Author contribution
Hishaam Ismael: Study concept and writing the paper.
Yury Ragoza: Data collection.
James Caccitolo: Assisted in surgery and reviewed the paper.
Steven Cox: Data interpretation and paper.
Guarantor
Hishaam Ismael MD.
References
- 1.Kiyozaki H., Saito M., Chiba H. Laparoscopic wedge resection of the stomach for gastrointestinal stromal tumor (GIST): non-touch lesion lifting method. Gastric Cancer. 2014;17(April (2)):337–340. doi: 10.1007/s10120-013-0272-8. [DOI] [PubMed] [Google Scholar]
- 2.Correa-Cote J., Morales-Uribe C., Sanabria A. Laparoscopic management of gastric gastrointestinal stromal tumors. World J. Gastrointest. Endosc. 2014;6(July (7)):296–303. doi: 10.4253/wjge.v6.i7.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh Y.X., Chok A.Y., Zheng H.L. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann. Surg. Oncol. 2013;20(October (11)):3549–3560. doi: 10.1245/s10434-013-3051-1. [DOI] [PubMed] [Google Scholar]
- 4.Demetri G.C., Benjamin R.S., Blanke C.D. NCCN task force report: management of patients with gastrointestinal stromal tumor (GIST) −update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(Suppl 2):S1-S29. quiz S30. [PubMed] [Google Scholar]
- 5.Davilla J.S., Momblan D., Gines A. Endoscopic-assisted laparoscopic resection for gastric subepithelial tumors. Surg. Endosc. 2016;30:199–203. doi: 10.1007/s00464-015-4183-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F.B., Shi H.C., Shu Y.S. Diagnosis and surgical treatment of esophageal gastrointestinal stromal tumors. World J. Gastroenterol. 2015;21(May (18)):5630–5634. doi: 10.3748/wjg.v21.i18.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coccolini F., Catena F., Ansaloni L. Esophagogastric junction gastrointestinal stromal tumor: resection vs: enucleation. World J. Gastroenterol. 2010;16(September (35)):4374–4376. doi: 10.3748/wjg.v16.i35.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mino J.S., Guerron A.D., Monteriro R. Long-term outcomes of combined endoscopic/laparoscopic intragastric enucleation of presumed gastric stromal tumors. Surg. Endosc. 2015;(August (15)) doi: 10.1007/s00464-015-4416-2. [DOI] [PubMed] [Google Scholar]
- 9.Ohgami M., Otani Y., Kubota T. Laparoscopic curative surgery for early gastric cancer. Nippon Rinsho. 1999;54:1307–1311. [PubMed] [Google Scholar]
- 10.Huguet K.L., Rush R.M., Tessier D.J. Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch. Surg. 2008;143:587–590. doi: 10.1001/archsurg.143.6.587. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura J., Nakajima K., Omori T. Surgical strategy for gastrointestinal stromal tumor: laparoscopic vs open resection. Surg. Endosc. 2007;21:875–878. doi: 10.1007/s00464-006-9065-z. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T., Nakajima K., Miyazaki Y. Surgical strategy for the gastric gastrointestinal stromal tumors (GISTs) larger the 5 cm: laparoscopic surgery is feasible, safe and oncologically acceptable. Surg. Laparosc. Endosc. Percutan. Tech. 2015;25(April (2)):114–118. doi: 10.1097/SLE.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 13.Matusi H., Nabeshima K., Okamoto Y. Fundic rotation technique: a useful procedure for laparoscopic exogastric resection of gastric submucosal tumors located on the posterior wall near the esophagogastric junction. Tokai J. Exp. Clin. Med. 2011;36(4):152–158. [PubMed] [Google Scholar]
- 14.Ke Z.W., Chen D.L., Cai J.L. Extraluminal laparoscopic-wedge resection of submucosal tumors on the posterior wall of the gastric fundus close to the esophagocardiac junction. J. Laparoendosc. Adv. Surg. Tech. A. 2009;19:741–744. doi: 10.1089/lap.2009.0166. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm D., Delius V., Burian M. Simultaneous use of laparoscopy and endoscopy for minimally invasive resection of gastric subepithelial masses—analysis of 93 interventions. World J. Surg. 2008;32:1021–1028. doi: 10.1007/s00268-008-9492-1. [DOI] [PubMed] [Google Scholar]
- 16.Hiki N., Yamamoto Y., Fukunaga T. Laparoscopic and endoscopic cooperative surgery for gastrointestinal tumor dissection. Surg. Endosc. 2007;22:1729–1735. doi: 10.1007/s00464-007-9696-8. [DOI] [PubMed] [Google Scholar]
- 17.Kato M., Uraoka T., Isobe Y. A case of gastric adenocarcinoma of fundic gland type resected by combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique, (CLEAN-NET) Clin. J. Gastroenterol. 2015;8(December (6)):393–399. doi: 10.1007/s12328-015-0619-2. Epub 2015 Nov 28. [DOI] [PubMed] [Google Scholar]
- 18.Gayer C., Edelman D., Curtis B. Combined endoscopic and laparoscopic approach to gastroesophageal tumor. JSLS. 2011;15(April–June (2)):228–231. doi: 10.4293/108680811X13071180406790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singaporewalla R.M., Ganesan B.H., Lee T.D. Laprondoscopic removal of a benign gastric stromal tumor at the cardia. JSLS. 2006;10(January–March (1)):117–121. [PMC free article] [PubMed] [Google Scholar]
- 20.Barajas-Gamboa J.S., Acosta G., Savides T.J. Laparo-endoscopic transgastric resection of gastric submucosal tumors. Surg. Endosc. 2015;29:2149–2157. doi: 10.1007/s00464-014-3910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obuchi T., Sasaki A., Baba S. Single-port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. Surg. Today. 2015;45:641–646. doi: 10.1007/s00595-014-0870-z. [DOI] [PubMed] [Google Scholar]
- 22.Li H., Chen L., Huo Z. Defining a subgroup treatable for laparoscopic and endoscopic cooperative surgery in undifferentiated early gastric cancer: the role of lymph node metastasis. J. Gastrointest. Surg. 2015;19:1763–1768. doi: 10.1007/s11605-015-2897-x. [DOI] [PubMed] [Google Scholar]
- 23.Mori M., Shuto K., Hirano A. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor with complete situs inversus: report of a case. Surg. Case Rep. 2015;1:72. doi: 10.1186/s40792-015-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunobe S., Hiki N., Gotoda T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338–342. doi: 10.1007/s10120-012-0146-5. [DOI] [PubMed] [Google Scholar]
- 25.Tsujimoto H., Yaguchi Y., Kumano I. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J. Surg. 2012;36:327–330. doi: 10.1007/s00268-011-1387-x. [DOI] [PubMed] [Google Scholar]
- 26.Patrzyk M., Ludwig K., Heidecke C.D. Laser-supported diaphanoscopy: a new technique in laparoscopic-endoscopic rendezvous procedures allowing better and more tissue-sparing tumor resection than wedge resection. Surg. Endosc. 2011;25:2023–2028. doi: 10.1007/s00464-010-1468-1. [DOI] [PubMed] [Google Scholar]
- 27.Abe N., Takeuchi H., Yanagida O. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg. Endosc. 2009;23:1908–1913. doi: 10.1007/s00464-008-0317-y. [DOI] [PubMed] [Google Scholar]
- 28.Wong D., Wong S., Leung A. Combined endolaparoscopic intragastric excision for gastric neoplasms. JLAST. 2009;19(6):765–770. doi: 10.1089/lap.2009.0067. [DOI] [PubMed] [Google Scholar]
- 29.Date R.S., Griffiths E.A., Pritchard S.A. Combined endoscopic and laparoscopic approach for palliative resection of metastatic melanoma of the stomach. World J. Surg. Oncol. 2006;4(20) doi: 10.1186/1477-7819-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]