Abstract
We describe a case of disseminated Mycobacterium tuberculosis (mTB) with prostatic abscess in a newly diagnosed HIV patient in the United States. The patient is a 34 year-old male who presented with respiratory symptoms and was diagnosed with HIV/AIDS complicated by disseminated mTB infection of the lungs, liver, and prostate. His prostate showed abscess formation on imaging that required drainage however he did not present with any genitourinary complaints. Our literature review revealed that prostatic involvement in mTB in the form of granulomatous prostatitis is uncommon; however, abscess formation is extremely rare and only few such cases have been published. Nearly 50% of the patients with prostatic abscess formation present without symptoms and therefore a high level of suspicion should be maintained; imaging should be performed early and prophylactic antibiotics for non-specific urinary symptoms should be avoided as this may lead to drug resistance of mTB to flouroquinolones.
Keywords: Prostate, Tuberculosis, Disseminated, HIV, Mycobacterium tuberculosis.
Introduction
Disseminated tuberculosis is defined as the spread of Mycobacterium tuberculosis complex (mTB) to one or more organs via hematogenous or lymphatic spread [12]. Even though pulmonary system involvement is most common, extrapulmonary involvement is seen in 10% of cases. Of which 30–40% of the patients with extrapulmonary involvement will present with genitourinary tuberculosis (GU TB) [9]. Amongst the GU organs, prostatic TB is less common (<5%). Overall, the largest study of prostatic TB prevalence was done by Sporer et al. where 100 cases of prostatic TB were identified out of 728 disseminated TB autopsy cases [28]. These cases included prostatic involvement in the form of granulomatous prostatitis and prostatic abscesses. Prostatic abscesses are less common and only 5 such cases have been reported in the United States [10], [11], [15], [24], [29], [30]. In this case report, we present a case of disseminated TB, including a prostatic abscess, in a patient with new diagnosis of HIV. In addition, we review the literature on prostatic abscess formation by mTB to identify prevalence, symptomatology, treatment and prognosis of these patients.
Case report
A 34-year-old Indonesian male presented with a four week history of diffuse abdominal pain, nausea, vomiting, odynophagia, dyspnea on exertion, and a 20-pound unintentional weight loss. The patient had a past medical history significant for intravenous drug abuse, alcohol abuse and 15 packs per year smoking history. The patient had moved to United States from Indonesia 7 years ago. Physical exam revealed a cachectic male with oropharyngeal candidiasis and crackles bilaterally. Diffuse abdominal tenderness on palpation was also noted. Laboratory studies revealed a normal complete blood count (CBC), hyponatremia with sodium of 122 mEq/L, and abnormal liver function tests: AST 442 U/L, ALT 150U/L, and ALP 295 U/L. A fourth-generation HIV antigen/antibody test (Abbott Laboratories) was confirmed positive with a MultiSpot positive for HIV-1 antibodies. Hepatitis C antibody testing was also reactive. Additional testing revealed a CD4 count of 2 cells/mm3 and a HIV-1 titer of 427,000 copies/ml determined by a Quantitative HIV-1 RNA PCR assay (COBAS AmpliPrep/COBAS Taqman Analyzer, v2.0, Roche Diagnostics). Chest imaging was concerning for either multifocal pneumonia and/or possible opportunistic infection (Fig. 1A). Abdominal CT revealed necrotic mesenteric lymphadenopathy (Fig. 1B), micro-abscesses in the liver (Fig. 1C), and a 2.9 × 2.4 × 2.0 cm fluid attenuation collection posterolateral to the prostate suggestive of an abscess (Fig. 1D-E). Urine analysis showed 2+ protein, 1+ urobilinogen, 3 red blood cells, and 2 white blood cells. Multiple routine urinary cultures showed no growth. Three sputum samples were collected and all showed 4+ acid-fast bacilli (AFB) (> 36 AFB organisms per field of view at 400 × magnification) by auramine-rhodamine stain and grew pure Mycobacterium tuberculosis complex in less than 7 days post-collection in the BACTEC ™ Mycobacterial Growth Indicator Tube (MGIT). Following culture, drug sensitivity testing was performed, and the mTB isolate was susceptible to first line drugs, including isoniazid (MIC-0.2 mcg/mL), rifampin (MIC-1.0 mcg/mL), ethambutol (MIC-5.0 mcg/mL), pyrazinamide (MIC-100 mcg/mL) and streptomycin (MIC-2.0mcg/mL). The patient was started on rifampin–300 mg twice daily, isoniazid–300 mg daily, pyrazinamide–1500 mg daily and ethambutol–1200 mg daily (RIPE therapy). Antiretroviral therapy was not initiated at this time. Twelve days later, on follow-up imaging, the prostatic abscess remained unchanged and a CT guided approach was utilized to drain the abscess. Purulent fluid (2 mL) was drained and sent for AFB culture. The purulent fluid revealed 4+ AFB by auramine-rhodamine stain (Fig. 2A) and grew mTB complex. The direct Kinyoun stain is shown in Fig. 2B. Patient clinically improved on RIPE therapy and was discharged with isolation precautions. 3 weeks post discharge, antiretroviral therapy with dolutegravir and Truvada (HAART) was initiated. Post-discharge (28 days), the patient was brought to the emergency department with acute onset of drowsiness and incomprehensible speech. The patient had been compliant with his medications. At this point, differential diagnosis of Immune reconstitution inflammatory syndrome (IRIS), and a new opportunistic infection including toxoplasmosis were considered. MRI of the brain revealed multiple ring enhancing lesions with surrounding edema in the cerebral hemispheres bilaterally, the midbrain, and the cerebellar hemispheres bilaterally as well (Fig. 3A,B). The patient was started on steroids, empiric toxoplasmosis therapy with pyrimethamine, sulfadiazine, and folinic acid. HAART and mTB therapy were continued. A lumbar puncture was performed that showed cerebrospinal fluid glucose of 54, protein of 116, RBC count of 7, and WBC count of 0. The cryptococcal antigen was non-reactive. Toxoplasmosis IgG was >5; however, Toxoplasma gondii PCR was negative. The patient’s hospital stay was also complicated by both hyponatremia and also hypertension attributed to SIADH. The patient gradually improved on the aforementioned therapeutic regimen and nearly two months later, brain MRI showed a decrease in the size of the lesions. The patient’s mental status also improved and the patient was deemed fit for discharge. The patient was lost to follow-up after relocating to another state.
Fig. 1.
(A) CT chest showing patchy involvement of the lung with nodules, one shown here measuring 3.1 cm in greatest dimension. (B) CT abdomen and pelvis showing multiple low attenuation left sided mesenteric lymph nodes measuring 2.5 × 2.4 cm suggestive of necrotic lymphadenopathy (C) multiple microabscess of the liver (D) Mild splenomegaly at 12.5 cm (E) Enlarged prostate with heterogeneous low attenuation area with 2.9 cm × 2.0 cm area of fluid attenuation at the left posterior aspect that appears to arise from the prostate concerning for abscess, axial plane (F) prostatic abscess, sagittal plane.
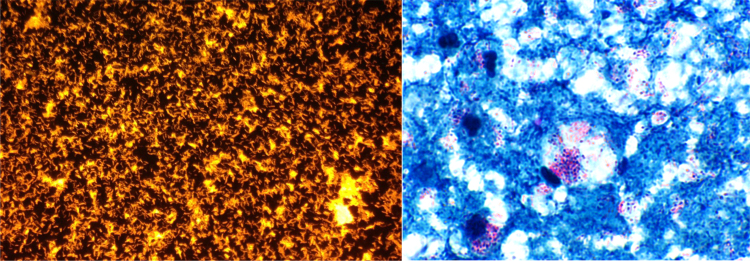
Fig. 2.
(A) 4+ AFB by auramine-rhodamine stain (B) Acid fast Kinyoun stain showing AFB stain positive organisms.
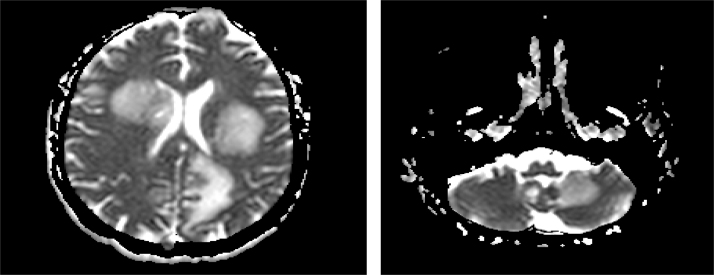
Fig. 3.
A and B. Representative axial images of MRI of the brain showing innumerable ring enhancing lesions with surrounding edema in both cerebral hemispheres (A), bilateral basal ganglia, midbrain and cerebellar hemispheres (B).
Discussion
We report a rare case of disseminated mTB with a prostatic abscess in the United States in a newly diagnosed patient with AIDS. The patient group most commonly afflicted with disseminated mTB is the HIV/AIDS population (50–70%) [20]. As discussed, involvement of the prostate is less common than involvement of other GU organs [14]. When present, prostatic mTB commonly manifests as granulomatous prostatitis. Literature review of prostatic TB cases shows that prostatic abscess formation is exceedingly rare. Worldwide, approximately 21 such cases have been reported in the literature with most case reports from India and Spain and only 5 such cases from United States (Table 1) [1], [3], [4], [7], [8], [16], [17], [19], [23], [25], [26], [27].
Table 1.
Outline of published literature on prostatic abscess cases due to mTB.
| Year | Country | Immune status | Number of patients/Organs affected | Symptoms | Imaging | Treatment | Follow up | Case |
|---|---|---|---|---|---|---|---|---|
| 1988 | United States | AIDS | 1 case prostatic, pulmonary and nodal TB | Cough, dyspnea | US showed a prostatic lesion | Transrectal puncture and TB therapy | None described | [24] |
| 1994 | United States | AIDS (All had CD4 count <200 cells/μl) | 7 cases/unknown | Fever, irritative voiding symptoms | TRUS | Variable-surgical approach and anti-tuberculosis therapy | Not provided | [29] |
| 1995 | Spain | AIDS | prostatic abscess, disseminated TB | unknown | TRUS | Drainage and anti-tuberculosis therapy | Recovered | [8] |
| 1996 | India | AIDS | 2 patients; 1 with vague urinary symptoms | post-mortem | prostatic abscesses | not applicable | not applicable | [18] |
| 1996 | United States | AIDS | Not provided | Not provided | Not provided | Not provided | Not provided | [30] |
| 1997 | United States | BCG therapy | prostatic abscess, disseminated TB | Not provided | Not provided | Not provided | Not provided | [10] |
| 2000 | Australia | HIV (Previous history of pulmonary TB) (CD4 count-101 cells/μl) |
prostate | fever, dysuria, perineal pain, diarrhea | 3 cm prostatic abscess | RIPE (antiretroviral were stopped due to interactions with cytochrome p450 system | resistant to rifampin; developed a rectoprostatic fistula; treatment continued with other drugs | [5] |
| 2001 | Pakistan | Immuno-competent | 2 cases of isolated Prostatic TB | acute urinary retention | At cystoscopy, prostate was enlarged | RIPE for 9 months | Recovered | [26] |
| 2002 | United States | Known HIV (CD4 count-40 cells/μl) | prostatic TB | fever, night sweats, chills, dysuria | CT showed hypodense areas in bilateral kidneys, multiple 1- 1.5 cm intraprostatic collections with enhancing rims; enlarged prostate (5 cm) | Transurethral prostatectomy was done; RIPE and HAART | none | [11] |
| 2003 | India | Immuno-competent | prostate | urinary retention | heterogenous parenchymal echotexture along with multiple irregular cavitations in the prostate | drugs and prostatectomy | none | [3] |
| 2005 | United States | BCG therapy | 1 case prostatic abscess | perineal pain, dysuria, tenesmus, strangury | Digital rectal examination aroused suspicion of prostate infection | transurethral prostatic resection produced white copius secretions; RIPE therapy | Recovered | [2] |
| 2006 | India | Immuno-competent | prostatic | pyrexia of unknown origin | CT showed prostatic abscess; 1.9 cm on TRUS | TRUS guided drainage; TB drugs started; one month later still fevers; prostate enlarged and extraprostatic extension; now drained | Recovered | [17] |
| 2008 | Spain | Immuno-competent | prostatic abscess | fever, fatigue, weight loss | infection in the right lobe of the prostate | RIP for two months and IR for next 10 months | normal | [27] |
| 2009 | India | Immuno-compromised (alcoholism) | cutaneous, lung and prostate | painful non healing ulcers of lower lip and scrotum, cough low grade fever, anorexia, dysuria | Not provided | RIPE | skin lesions improved in 2 weeks; no additional follow up | [23] |
| 2010 | Malaysia | HIV (CD4 count-91cells/μl) |
prostatic abscess | poor urinary flow, frequency, urgency | Transrectal US showed irregular cystic lesion (4.5 cm) | RIPE | lost to follow up | [19] |
| 2010 | India | Immuno-competent | prostatic abscess | fever, urinary frequency, dysuria, perineal pain | MRI showed a prostatic abscess (7.7 cm) | drainage and 6 months RIPE | doing well in 15 year follow up | [25] |
| 2012 | Korea | status post-BCG therapy | prostate | urinary frequency, dysuria, perineal discomfort | Oval shaped low density lesion | drainage and RIPE | no abscess after 12 months | [7] |
| 2012 | Portugal | Known history of HIV (unknown CD4 count) | disseminated TB- CNS, spleen, kidney and prostate | fever, asthenia, weight loss | CT showed splenomegaly with multiple nodules and renal and prostate bacesses (heterogeneous areas with areas that were hypodense); Brain CT showed multiple suspicious hypodensities; leptomeningeal involvement | HRZE therapy and systemic corticotherapy; intrathecal corticotherapy; second line drugs later used (levofloxacin, amycacin, cycloserin) | patient died 10 months into therapy and continued to have neurologic degradation | [1] |
| 2014 | Germs (United States) | Immune-competent- vague long standing urinary symptoms | disseminated- prostatic, peritoneal, pulm and likely renal TB | 2 weeks of progressively worsening abdominal pain, distension, fever, dysuria, dyschezia, weight loss | CT revealed ascites, diffuse peritonitis, multiple prostatic masses (largest 3.5 cm) and focal pyelo in left kidney | transurethral aspiration- RIPE; side effect so discontinued pyrazinaminde. And completed 9 months of therapy | clinical well in 4 year f/u | [15] |
| 2015 | BMJ | Immune-compromised (alcoholism) | prostate | fever, weight loss, sweats, abdominal pain | CT showed prostatic abscesses and necrotic celiac, aortic, hepatic and thoracic adenopathy | RIPE (12 months) | Cured | [4] |
| 2015 | United States | AIDS (CD4 count-8 cells/μl) | prostatic abscess, chest, brain | high fever, urinary retention, hypogastralgia | 5.2 cm abscess in prostate | drainage and RIPE | Recovered | [21] |
Even though majority of the patients with GU mTB present with symptoms of dysuria, changes in urinary flow, urgency or frequency, nearly 25% of the patients present with no GU symptoms [22]. In the review of literature, amongst the patients with prostatic abscesses, 11/21 patients presented with urinary symptoms (52%). In our patient, no GU complaints were documented and the prostatic abscess was an incidental finding on CT of the abdomen and pelvis. Prostate specific antigen (PSA) testing is not usually helpful since only rare instances of PSA elevation have been noted in patients with GU involvement Lanjewar and Maheshwari, 1994. Commonly, sterile pyuria and hematuria are the most common findings. A high rate of suspicion should be maintained in HIV/immunocompromised patients presenting with urinary symptoms. It is important to not treat dysuria symptoms in these patients with empiric antibiotic therapy such as with fluoroquinolones for presumable bacterial UTI as this can lead to drug resistance to mTB as they have activity against mTB [13].
Since mTB is acquired person through person through aerosolized droplets, the infection initially affects the pulmonary system. In an immunocompetent patient, granuloma formation around the bacilli within the lung parenchyma can contain mTB replication and prevent disease activation. However, in immunocompromised patients, specifically AIDS patients, granuloma formation is inadequate due to altered functionality of CD4 T cells, macrophages, dendritic cells, neutrophils and fibroblasts [6]. These immune-microenvironment alterations allow for uncontrolled mTB replication, higher chances of getting active infection and distant spread. GU spread occurs through lymphatic spread, hematogenous seeding, descending infection to urinary organs or tract, direct extension from neighboring TB focus in the genital tract, and rarely ascending infection through the urethra in cases of BCG therapy. One or successive hematogenous seedings are the most common mode of transmission Sporer and Auerbach, 1978. Typically an extended (long) latency period of approximately 22 years exists between the first pulmonary infection and presentation of GU mTB Figueiredo and Lucon, 2008. Incongruously, in this case, the patient presented with pulmonary involvement and was diagnosed with disseminated TB including hepatic, splenic, lymphatic and prostatic involvement simultaneously.
Common predisposing factors to prostatic abscess formation in disseminated mTB include immunosuppression whether it is in the form of impaired cell mediated immunity, acquired immunodeficiency syndrome (AIDS) as in this case, prolonged immunosuppressants, or steroid use. Other patient populations at risk for disseminated mTB, even though much more infrequent, include patients with previous BCG therapy for bladder carcinoma and immunocompetent patients. During the literature review, we noted different patterns of mTB dissemination in these three different patient populations. Most of the immunocompetent patients with prostatic abscesses had isolated prostate involvement by mTB (5/6). Only one case report documented involvement of the prostate as part of disseminated TB, in an immunocompetent host. In the BCG therapy population, 2/3 patients had isolated prostate involvement with one developing miliary TB [7]. Lastly, amongst the HIV/immunocompromised population, 4 patients had disseminated disease alongside prostatic abscess formation while 11 patients had prostate only involvement [1], [8], [23], [24]. It can be theorized that the cases with disseminated TB including prostatic abscess are likely under-reported in literature because extent of visceral organ involvement even when present may not be well-delineated or documented in such cases. These findings raise a crucial clinical management issue- since immunocompromised patients, particularly AIDS patients are at high risk for developing prostatic abscess, it is crucial to get a transrectal ultrasound, CT or MRI of the abdomen and pelvis to rule-out abscess formation. In our patient, even though the prostatic abscess was identified early, RIPE therapy could not penetrate the abscess wall and despite weeks of treatment, the prostatic abscess imaging remained unchanged. The standard of care therapy for these patients should include prostatic abscess drainage in addition to mTB therapy as was done in our case. Majority of the cases in the literature review were treated similarly and did well. Only one patient died during RIPE therapy and that was attributed to central nervous system involvement by mTB.
Conclusion
Here, we presented a case of prostatic abscess formation as part of disseminated TB in a patient with no GU symptoms and a new AIDS diagnosis. In AIDS patients, it is important to keep a high index of suspicion for prostatic abscess formation, perform imaging early, avoid prophylactic antibiotics without culture proven urinary tract infection, and treat with drainage and mTB therapy.
Conflict of interest
None of the authors have a conflict of interest to disclose.
Contributor Information
Upasana Joneja, Email: upasana.joneja@jefferson.edu.
William R. Short, Email: william.short@uphs.upenn.edu.
Amity L. Roberts, Email: amity.roberts@jefferson.edu.
References
- 1.André M.R.P.A., Antunes F. Tuberculosis Infection in the 21ést century: can we win? J Clin Case Rep. 2012;2:160. [Google Scholar]
- 2.Aust T.R., Massey J.A. Tubercular prostatic abscess as a complication of intravesical bacillus Calmette-Guerin immunotherapy. Int J Urol. 2005;12:920–921. doi: 10.1111/j.1442-2042.2005.01183.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava N., Bhargava S.K. Primary tuberculosis of the prostate. Indian J Radiol Imaging. 2003;13:236–237. [Google Scholar]
- 4.Lyon TBSg. Cavalli Z., Ader F., Chidiac C., Ferry T. Asymptomatic prostatic tuberculous abscess in an immunocompetent 54-year-old man with peritoneal tuberculosis. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-212342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan W.C., Thomas M. Prostatic abscess: another manifestation of tuberculosis in HIV-infected patients. Aust N Z J Med. 2000;30:94–95. doi: 10.1111/j.1445-5994.2000.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 6.Diedrich C.R., Flynn J.L. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79:1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doo S.W., Kim J.H., Yang W.J., Kim S.I., Lee D.W., Hong S.S. A case of tuberculous prostatitis with abscess. World J Men's Health. 2012;30:138–140. doi: 10.5534/wjmh.2012.30.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte Ojeda J.M., Garcia Luzon A., Carrero V.M., Vazquez S., Calahorra L., Passas J. Tuberculous prostatic abscess in a patient with AIDS. Actas Urol Esp. 1995;19:655–661. [PubMed] [Google Scholar]
- 9.Figueiredo A.A., Lucon A.M. Urogenital tuberculosis: update and review of 8961 cases from the world literature. Rev Urol. 2008;10:207–217. [PMC free article] [PubMed] [Google Scholar]
- 10.Foster D.R. Miliary tuberculosis following intravesical BCG treatment. Br J Radiol. 1997;70:429. doi: 10.1259/bjr.70.832.9166085. [DOI] [PubMed] [Google Scholar]
- 11.Gebo K.A. Prostatic tuberculosis in an HIV infected male. Sex Transm Infect. 2002;78:147–148. doi: 10.1136/sti.78.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson M.S., Puckett M.L., Shelly M.E. Renal tuberculosis. Radiographics. 2004;24:251–256. doi: 10.1148/rg.241035071. [DOI] [PubMed] [Google Scholar]
- 13.Ginsburg A.S., Woolwine S.C., Hooper N., Benjamin W.H., Jr., Bishai W.R., Dorman S.E. The rapid development of fluoroquinolone resistance in M. Tuberculosis. N Engl J Med. 2003;349:1977–1978. doi: 10.1056/NEJM200311133492023. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N., Mandal A.K., Singh S.K. Tuberculosis of the prostate and urethra: a review. Indian J Urol. 2008;24:388–391. doi: 10.4103/0970-1591.42623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson M.G., Caplan-Shaw C.E., McMacken M. Tuberculous prostate abscesses in an immunocompetent patient: a dramatic presentation of disseminated tuberculosis. Germs. 2014;4:41–45. doi: 10.11599/germs.2014.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S., Kashyapi B.D., Bapat S.S. A rare presentation of tuberculous prostatic abscess in young patient. Int J Surg Case Rep. 2015;10:80–82. doi: 10.1016/j.ijscr.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S., Kekre N.S., Gopalakrishnan G. Diagnosis and conservative treatment of tubercular rectoprostatic fistula. Ann R Coll Surg Engl. 2006;88:26. doi: 10.1308/147870806X83242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanjewar D.N., Maheshwari M.B. Prostatic tuberculosis and AIDS. Natl Med J India. 1994;7:166–167. [PubMed] [Google Scholar]
- 19.Lee P.Y., Ong T.A., Norlida A.D. Tuberculosis prostatic abscess in an immunocompromised patients. Malays Fam Phys. 2010;5:145–147. [PMC free article] [PubMed] [Google Scholar]
- 20.Lenk S., Schroeder J. Genitourinary tuberculosis. Curr Opin Urol. 2001;11:93–98. doi: 10.1097/00042307-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Liang K., Guo W. Prostatic abscess caused by Mycobacterium tuberculosis and Escherichia coli in a patient with acquired immunodeficiency syndrome. Am J Med Sci. 2015;350:153–154. doi: 10.1097/MAJ.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 22.Medlar E.M., Spain D.M., Holliday R.W. Post-mortem compared with clinical diagnosis of genito-urinary tuberculosis in adult males. J Urol. 1949;61:1078–1088. doi: 10.1016/S0022-5347(17)69186-9. [DOI] [PubMed] [Google Scholar]
- 23.Mittal R., Sudha R., Veeraraghavan M., Murugan S., Adikrishnan S., Krishnakanth M. Disseminated tuberculosis with involvement of prostate-a case report. Indian J Tuberc. 2010;57:48–52. [PubMed] [Google Scholar]
- 24.Moreno S., Pacho E., Lopez-Herce J.A., Rodriguez-Creixems M., Martin-Scapa C., Bouza E. Mycobacterium tuberculosis visceral abscesses in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1988;109:437. doi: 10.7326/0003-4819-109-5-437_1. [DOI] [PubMed] [Google Scholar]
- 25.Puri R., Jain P., Sud R., Kumar M., Hussain S., Basnotra A. EUS-guided drainage of an isolated primary tubercular prostatic abscess. Gastrointest Endosc. 2010;71:425–428. doi: 10.1016/j.gie.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Rafique M., Rafique T., Rauf A., Bhutta R.A. Tuberculosis of prostate. JPMA. 2001;51:408–410. [PubMed] [Google Scholar]
- 27.Saenz-Abad D., Letona-Carbajo S., Benito-Arevalo J.L., Sanioaquin-Conde I., Ruiz-Ruiz F.J. Prostatic tuberculosis: case report. Sao Paulo Med J. 2008;126:227–228. doi: 10.1590/S1516-31802008000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sporer A., Auerbach O. Tuberculosis of prostate. Urology. 1978;11:362–365. doi: 10.1016/0090-4295(78)90232-7. [DOI] [PubMed] [Google Scholar]
- 29.Trauzzi S.J., Kay C.J., Kaufman D.G., Lowe F.C. Management of prostatic abscess in patients with human immunodeficiency syndrome. Urology. 1994;43:629–633. doi: 10.1016/0090-4295(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 30.Wolf L.E. Tuberculous abscess of the prostate in AIDS. Ann Intern Med. 1996;125:156. doi: 10.7326/0003-4819-125-2-199607150-00029. [DOI] [PubMed] [Google Scholar]