Abstract
Camostat mesilate is in widespread clinical use mainly to treat chronic pancreatitis, and drug-induced lung injury has not been previously reported. However, pulmonary infiltration with peripheral blood eosinophilia appeared after taking camostat mesilate for ten days. The histological findings showed eosinophilic infiltration into the alveolar space and interstitum, and drug lymphocyte stimulation test of peripheral blood was positive. Both peripheral blood eosinophilia and pulmonary involvements improved two weeks later with the cessation of this drug. To the best of our knowledge, this case is the first report of camostat mesilate-induced acute eosinophilic pneumonia.
Keywords: Camostat mesilate, Drug cessation, Drug-induced lung injury, Drug lymphocyte stimulation test, Eosinophilic pneumonia
Abbreviations: BALF, bronchoalveolar lavage fluid; DLST, drug lymphocyte stimulation test; GGO, ground glass opacities; HRCT, High-resolution computed tomography
1. Introduction
Any drugs can cause lung injury and their clinical conditions are miscellaneous. Camostat mesilate is in widespread clinical use mainly to treat chronic pancreatitis and the number of people who takes this drug during one year is estimated at about a hundred thousand. Generally this drug is believed to be safe and secure; indeed, camostat mesilate-induced lung injury has not been reported so far. Here we report the first case of camostat mesilate-induced acute eosinophilic pneumonia.
1.1. Case presentation
A 65-year-old man was given camostat mesilate (600 mg/day) for ten days due to pancreatitis after endoscopic retrograde cholangiopancreatography for the evaluations of cholangitis and pancreatic cyst. One week later, he was admitted to our hospital suffering from low grade fever, though abdominal symptom due to pancreatitis had been improved. Chest radiograph revealed bilateral fine nodular opacities in the middle and lower fields (Fig. 1).
Fig. 1.
Chest radiograph obtained on admission showing fine nodular opacities in both middle and lower lung fields.
Arterial blood gas analysis (ambient air) indicated that pH 7.466, PaCO2 32.8 mmHg and PaO2 63.7 mmHg. Results of additional laboratory examination were as follows: white blood cell count 7700/μL (eosinophils 52%), C-reactive protein 18.5 mg/dL (normal range, <0.3 mg/dL), lactate dehydrogenase 250 IU/L (normal range, <225 IU/L), IgE-RIST 166 IU/mL (normal range, <170 IU/mL), surfactant proteins-D 54.6 ng/mL (normal range, <110 ng/mL), Krebs von den Lungen-6 137 U/mL (normal range, <500 U/mL), anti-nuclear antibody < 20 index (normal range, <20 index), cytoplasmic-anti-neutrophil cytoplasmic antibody < 10 EU, and perinuclear-anti-neutrophil cytoplasmic antibody < 10 EU. Sputum culture was negative for pathogenic bacteria and acid-fast bacilli. Pulmonary function test indicated obstructive abnormality (FEV1.0/FVC = 69.6%). High-resolution computed tomography (HRCT) of the lung showed bilateral ground glass opacities (GGO) and septal line thickenings with subpleural distribution in the middle and lower lobes (Fig. 2). The bronchoalveolar lavage fluid (BALF) results were as follows: total cell counts 3.3 × 105/mL, macrophages 22%, eosinophils 77%, and lymphocytes 1%. We found infective etiologies including viral, tuberculous and fungal infections negative. Parasitic analysis for faecal specimen was also negative and there was no history of eating raw meat and fish. Transbronchial lung biopsy specimens revealed the accumulations of eosinophils in the alveolar space and interstitum with the mild edema of alveolar septa (Fig. 3). In addition, the drug lymphocyte stimulation test (DLST) of peripheral blood for camostat mesilate was positive (stimulation index 493%). His temperature decreased and blood eosinophilia and pulmonary involvements were improved two weeks later with the cessation of the drug. Thus, we concluded the case was acute eosinophilic pneumonia induced by camostat mesilate.
Fig. 2.
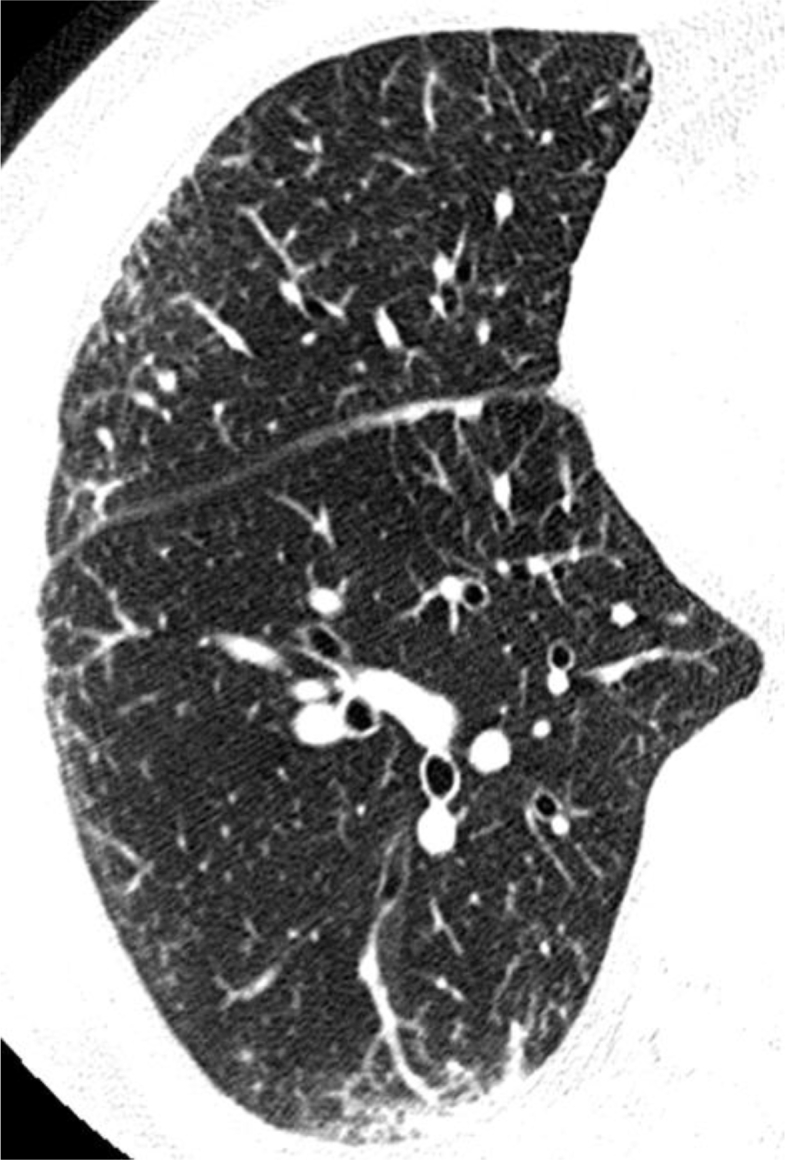
High-resolution computed tomography showed bilateral ground glass opacities and septal line thickenings with subpleural distribution in the middle and lower lobes.
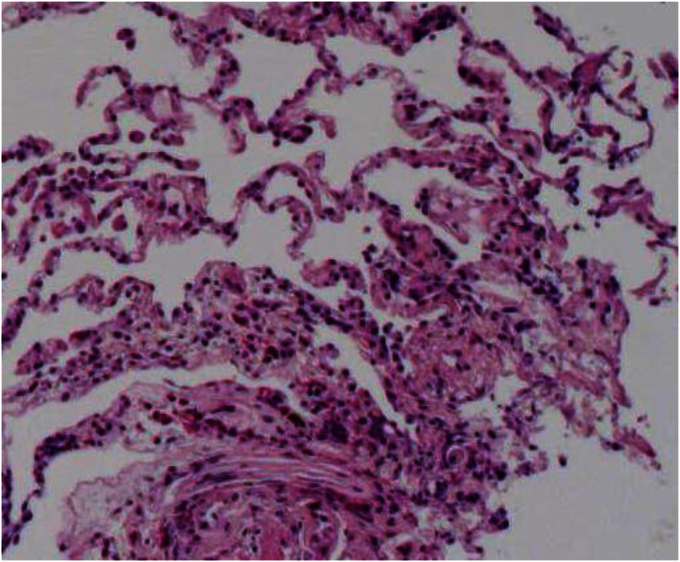
Fig. 3.
Transbronchial lung biopsy specimens revealed the accumulations of eosinophils and macrophages in the alveolar space and interstitum with the mild edema of alveolar septa (Hematoxylin and Eosin staining × 100).
2. Discussion
Recently drug-induced lung injuries have been increasing as various drugs developed, and such clinical conditions are diverse. However, camostat mesilate and its analogous drugs, serine protease inhibitors, have rarely caused lung injury.
The mechanism of pathogenesis is broadly divided into two categories i.e. toxic reaction and allergic reaction [1], [2]. The present case was thought to be allergic reaction. Allen proposed the five criteria for drug-induced eosinophilic lung disease [3]. Clinically the patient should: (A) have no other likely cause of lung disease, (B) have symptoms consistent with the suspect drug, (C) have a time course compatible with drug-induced lung disease, (D) have tissue or BALF findings compatible with drug-induced lung disease, and (E) improve after the drug is discontinued. This case fulfilled these five criteria and so is a definitive case.
Pietra and coworkers indicated that drug-induced eosinophilic lung disease has two patterns; acute and chronic form [4]. It was also reported that the opacities of chronic patterns were persistent despite the cessation of causative agents and the administration of oral prednisolone [5], [6]. In this case, clinical symptoms appeared after prescribing camostat mesilate for ten days. HRCT findings showed mainly GGO and septal line thickening with subpleural distribution in the middle and lower lobes. Dominant histological findings are characterized by accumulations of eosinophils and macrophages and infiltrations of eosinophis, lymphocytes and plasma cells in the alveolar septa. All these findings are consistent with acute form. Indeed, the clinical condition of the present case was improved only by the cessation of camostat mesilate. Therefore, we speculate that early diagnosis and therapeutic intervention are very important for favorable outcome.
Suzuki and coworkers advocates that drug provocation test was the most useful for the detection of responsible agents [7], [8]. Although DLST has several technical problems such as lymphocyte stimulation by drug itself (mitogenic activity), lymphocyte inhibition [9], criteria for stimulation index, and the effects of intermediate metabolites of the drug, we embraced the research by Pitchler and coworkers that if stimulation index was more than 300%, DLST was positive [10]. As the stimulation index of this case was ∼500%, we determined the result of DLST was positive. Thus, we diagnosed this case as camostat mesilate-induced acute eosinophilic pneumonia.
The causative agents of drug-induced eosinophilic lung disease were wide-ranging. In fact, about 160 drugs which can cause eosinophilic pneumonia have been reported by 2015 in the web site of PNEUMOTOX ONLINE (http://www.pneumotox.com); however, camostat mesilate-induced lung injury has not been reported so far. Further, there was no mention of camostat mesilate-induced lung injury in the website of Pharmaceuticals and Medical Devices Agency (PMDA) of Japan (http://www.pmda.go.jp) nor PubMed (http://www.ncbi.nlm.nih.gov/pubmed). To the best of our knowledge, this case is the first report of camostat mesilate-induced acute eosinophilic pneumonia. The possibility of drug-induced lung injury by camostat mesilate should be taken into account.
Acknowledgement
We appreciate Mrs. Yoriko Inoue for her technical assistance.
References
- 1.Cooper J.A., Jr., White D.A., Matthay R.A. Drug-induced pulmonary disease. Part 1: cytotoxic drugs. Am. Rev. Respir. Dis. 1986;133:321–340. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
- 2.Cooper J.A., Jr., White D.A., Matthay R.A. Drug-induced pulmonary disease. Part 2: noncytotoxic drugs. Am. Rev. Respir. Dis. 1986;133:488–505. doi: 10.1164/arrd.1986.133.3.488. [DOI] [PubMed] [Google Scholar]
- 3.Allen J.N. Drug-induced eosinophilic lung disease. Clin. Chest Med. 2004;25:77–88. doi: 10.1016/S0272-5231(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 4.Pietra G.G. Pathologic mechanisms of drug-induced lung disorders. J. Thorac. Imaging. 1991;6:1–7. doi: 10.1097/00005382-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rossi S.E., Erasmus J.J., McAdams H.P., Sporn T.A., Goodman P.C. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000;20:1245–1259. doi: 10.1148/radiographics.20.5.g00se081245. [DOI] [PubMed] [Google Scholar]
- 6.Muller N.L., White D.A., Jiang H., Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br. J. Cancer. 2004;91:S24–S30. doi: 10.1038/sj.bjc.6602064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y., Miwa S., Shirai M., Ohba H., Murakami M., Fujita K., Suda T., Nakamura H., Hayakawa H., Chida K. Drug lymphocyte stimulation test in the diagnosis of adverse reactions to antituberculosis drugs. Chest. 2008;134:1027–1032. doi: 10.1378/chest.07-3088. [DOI] [PubMed] [Google Scholar]
- 8.Matsuno O., Okubo T., Hiroshige S., Takenaka R., Ono E., Ueno T., Nureki S., Ando M., Miyazaki E., Kumamoto T. Drug-induced lymphocyte stimulation test is not useful for the diagnosis of drug-induced pneumonia. Tohoku J. Exp. Med. 2007;212:49–53. doi: 10.1620/tjem.212.49. [DOI] [PubMed] [Google Scholar]
- 9.Kloppenburg M., Brinkman B.M., de Rooij-Dijk H.H., Miltenburg A.M., Daha M.R., Breedveld F.C., Dijkmans B.A., Verweij C. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob. Agents Chemother. 1996;40:934–940. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichler W.J., Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–820. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]