Abstract
Basic epilepsy teachings assert that seizures arise from the cerebral cortex, glossing over infratentorial structures such as the cerebellum that are believed to modulate rather than generate seizures. Nonetheless, ataxia and other clinical findings in epileptic patients are slowly but inevitably drawing attention to this neural node. Tracing the evolution of this line of inquiry from the observed coincidence of cerebellar atrophy and cerebellar dysfunction (most apparently manifested as ataxia) in epilepsy to their close association, this review considers converging clinical, physiological, histological, and neuroimaging evidence that support incorporating the cerebellum into epilepsy pathology. We examine reports of still controversial cerebellar epilepsy, studies of cerebellar stimulation alleviating paroxysmal epileptic activity, studies and case reports of cerebellar lesions directly associated with seizures, and conditions in which ataxia is accompanied by epileptic seizures. Finally, the review substantiates the role of this complex brain structure in epilepsy whether by coincidence, as a consequence of deleterious cortical epileptic activity or antiepileptic drugs, or the very cause of the disease.
Keywords: Ataxia, epilepsy, seizures, atrophy, stimulation
Introduction
The past 20 years have witnessed a considerable shift in the understanding of the cerebellum and of its function and involvement in various conditions. Until quite recently, the cerebellum was thought to be primarily involved in movement planning and execution.1 Despite reluctant wavering in the literature from the original focus on motor control, the collective body of research has coalesced around the astonishing finding that the majority of the human cerebellum is closely associated with networks involved in cognitive processing including memory, language, and emotions.1,2 The cerebellum qmainly communicates with the contralateral cerebrum through two polysynaptic pathways: the input synapsing at the pons level and the output channel relaying information from the cerebellar cortex through the deep cerebellar nuclei to the thalamus and finally various parts of the cerebral cortex.3,4 The polysynaptic characteristic of this circuitry has far-reaching implications on possible disturbances arising from network disruptions, foreshadowing the recently hypothesized and partly confirmed presence of cerebellar fingerprints in Parkinson’s disease,5 dystonia,6 schizophrenia,7 essential tremor,8 and other further syndromes. Significantly less is known about the role of the cerebellum in epileptogenesis. Cerebellar dysfunction has been associated with disinhibition of seizure activity in the cerebral cortex,9–11 and the highly controversial concept of cerebellar epilepsy, with seizures arising directly from cerebellar structures, has baffled experts for more than a century.9,12 The cerebellum itself might not be the initiator, but only another structure experiencing deleterious epileptic activity or the long-term effects of some antiepileptic drugs. Disregarding the ambiguous position of cerebellar atrophy as the primary cause of seizures, a secondary consequence of epilepsy, or mere coincidence, it is not necessarily associated with any critical clinical signature. Even though a significant percentage of patients with epilepsy (up to 54% in a selected sample of institutionalized patients) have gait ataxia,13 cerebellar atrophy is not a fundamental prerequisite for this clinical finding. Hence, our review aims to summarize the available information on the position of this complex structure in epilepsy.
Coincidence
Ataxia is a common symptom in a variety of conditions associated with epilepsy; an overview is provided in Table 1. As mentioned above, the relationship between epilepsy and cerebellar dysfunction is complex. The following paragraph and Table 1 provide an overview of the conditions in which both ataxia and epileptic seizures are observed. It should be noted that in the majority of such cases there is presumably a common cause/shared etiology—metabolic, genetic, or other—concurrently conditioning dysfunction in different regions of the central nervous system. For example, in type 1 inositol 1,4,5-trisphosphate receptor deficiency, a mutation of the competent gene leads to severe ataxia and tonic-clonic seizures due to simultaneous effects on the hippocampal cornu ammonis 1 region, caudate-putamen, cerebral cortex, and cerebellar Purkinje cells.14
Table 1. Ataxia in Conditions Associated with Epilepsy.
| Group of Diseases | Specific Description and Clinical Notes | |
|---|---|---|
| Channelopathies | Sodium channel gene mutation | SCN1A gene mutation: severe myoclonic epilepsy of infants (Dravet syndrome), infants with febrile seizures presenting around 6 months of age with hemiclonic seizures18 |
| SCN2A gene mutation: benign familial neonatal infantile seizures, febrile seizures plus, and intractable epilepsy of infancy, late-onset episodic ataxia, myoclonus19 | ||
| SCN8A gene mutation: infant seizures not triggered by fever, multiple seizure types including focal, tonic, clonic, myoclonic and absence seizures and epileptic spasms; seizures refractory to antiepileptic therapy, motor manifestations: hypotonia, dystonia, hyperreflexia, and ataxia86 | ||
| Potassium channel gene mutation | KCTD7 gene mutation: progressive myoclonus epilepsy, infant seizures, myoclonus, ataxia17 | |
| KCNJ10 gene mutation: epilepsy, ataxia, sensorineural deafness, and tubulopathy (EAST syndrome), tonic-clonic seizures in infancy, later cerebellar ataxia, and hearing loss16 | ||
| Sodium/hydrogen channel gene mutation | SLC9A6 gene mutation: mental retardation, microcephaly, epilepsy, ataxia, Angelman-like syndrome87 | |
| Calcium channel gene mutation | CACNA1A gene mutation: loss-of-function mutations classically present as episodic ataxia type 2 or progressive spinocerebellar ataxia (SCA6), minority of patients carrying CACNA1A mutations develop epilepsy, cognitive impairment, autism, and epileptic encephalopathy with mild cerebellar symptoms88 | |
| Deficiency disorders and metabolically conditioned diseases | Mutation of FOLR1 gene (folic acid transport disorder) | Case study: ataxic syndrome in the second year of life, later a febrile tonic-clonic status, daily tonic and myoclonic seizures.28 |
| Autosomal recessive ataxia with vitamin E deficiency | Case study, caused by mutations of the α-tocopherol transport protein gene located on chromosome 8q1329 | |
| GLUT-1 deficiency syndrome | Persistently low glucose in the cerebrospinal fluid, intellectual disability, epilepsy, movement disorders onset from infancy to adulthood: spasticity, ataxia, dystonia, caused by SLC2A1 gene mutation30 | |
| Inherited glycosylation disorders | Systemic symptoms during infancy – susceptibility to infection, episodes of hepatic impairment, hemocoagulation impairment, childhood seizures and stroke-like episodes and later progressive limb atrophy with severe ataxia and intellectual deficiency, this group represents nearly 70 genetic disorders known to be caused by impaired synthesis of glycoconjugates89 | |
| Wernicke’s encephalopathy | B1 deficiency, ataxia, ophthalmoplegia and confusion, additional symptoms: seizures, peripheral neuropathy, impaired vision and hearing to varying degrees90 | |
| Lysosomal storage disease | Niemann-Pick C | NPC1 gene mutations, deficiency of sphingomyelinase, onset from infancy to adulthood, supranuclear gaze palsy followed by epilepsy development and later progressive gait ataxia91 |
| Lafora disease | Lafora bodies within the cytoplasm of cells, progressive myoclonus epilepsy, intractable tonic-clonic seizures, myoclonus, ataxia, visual hallucinations, and progressive dementia, onset in 2nd decade, fatal within 10 years92 Caused by mutations in either the EPM2A or EPM2B/NHLRC1 gene.93 | |
| Neuronal ceroid lipofuscinoses | Accumulation of autofluorescent lipopigments resembling ceroid and lipofuscin, seizures, dementia, motor function impairment (myoclonus, ataxia, spasticity), vision loss, forms: infantile, late-infantile, juvenile, adult, most common infantile form (from 2 to 4 years of age), starting with epilepsy, later ataxia24 There are more than a dozen genes containing over 430 mutations underlying human NCLs have been identified, CLN1-14.94 | |
| Sialoidoses | Neuraminidase deficiency caused by a mutation in the neuraminidase gene (NEU), located on 6p21.33, cerebellar ataxia, myoclonic epilepsy, myoclonus, macular cherry-red spots, onset from childhood to young adulthood95 | |
| Gaucher disease | GBA gene mutation, hereditary glucocerebrosidase deficiency, type I (non-neuropathic) – hepatosplenomegaly, type II (acute infantile neuropathic) – hepatosplenomegaly and neurological symptoms: eye movement disorders, spasticity, seizures, limb rigidity, typically begins within 6 months of birth, type III (chronic neuropathic) – onset in childhood or even in adulthood, similar to type II but milder symptoms96 | |
| Progressive myoclonus epilepsies | Leigh syndrome | Different inheritance patterns, genes contained in nuclear DNA or genes contained in mitochondrial DNA, mitochondrial syndrome, optic atrophy, ataxia, and dystonia, later epilepsy27 |
| MERRF | Mutation in the MT-TK gene of mitochondrial DNA, progressive course with worsening of the epilepsy and onset of additional symptoms including ataxia, deafness, muscle weakness (myopathy), and dementia97 | |
| Unverricht-Lundborg disease | CSTB gene mutation, progressive myoclonus epilepsy, ataxia is an additional symptom98 | |
| Action myoclonus-renal failure syndrome | SCARB2 gene mutation,99 progressive myoclonus epilepsy associated with renal dysfunction, severe progressive action myoclonus, dysarthria, ataxia, and later infrequent generalized seizures in 2nd or 3rd decade26 | |
| May and White syndrome | Mitochondrial disorder, ataxia, severe action myoclonus, dysarthria, generalized seizures.100 | |
| Immune-mediated conditions | Cerebellar ataxia and epilepsy with anti-GAD antibodies | Adult-onset cerebellar syndrome, ataxia and stiffness, incontinence, retinal pathologies, seizures, and immunological co-morbidities40 |
| Celiac disease | Neurological symptoms in up to 10% of cases, gastrointestinal symptoms preceding years of neurological symptoms, ataxia, myoclonus, tremor, seizures, abnormalities of eye movement31 | |
| Hashimoto encephalopathy | Antibodies to thyroperoxidase, cerebellar ataxia occurs in more than half of patients, diffuse encephalopathy with cognitive abnormalities, tremor, myoclonus, seizures and sleep disturbances acute to subacute with a rapid progression onset in all age groups more common in females than in males90,101 | |
| Hereditary ataxias | DRPLA | Highest frequency in the Japanese population, age of onset 1–60 years (mean age 28,8), early onset: myoclonus, epilepsy and mental retardation, late onset: cerebellar ataxia, choreoathetosis and dementia, caused by a mutation in the ATN1 gene – increased CAG trinucleotide repeat.102,103 |
| SCA2 | ATXN2 gene mutation, onset in the 3rd or 4th decade, truncal ataxia, dysarthria, slowed saccades and less commonly ophthalmoparesis and chorea or parkinsonism104,105 | |
| SCA10 | ATXN10 (E46L) gene mutation, onset from 18 to 45, slowly progressive cerebellar syndrome and epilepsy, sometimes mild pyramidal signs, peripheral neuropathy and neuropsychological disturbances104,106,107,108 | |
| SCA13 | KCNC3 gene mutation, onset in childhood, delayed motor and cognitive development followed by mild progression of cerebellar ataxia41,104,108 | |
| SCA17 | TBP gene mutation, dementia, psychiatric disorders, parkinsonism, dystonia, chorea, spasticity, and epilepsy42,104,107,109 | |
| Friedreich ataxia | FXN gene mutation, ataxia followed by epilepsy, case report, ataxia, weakness and spasticity, sensory impairment, skeletal abnormalities, cardiac difficulties, diabetes43,110 | |
| Ataxia teleangiectasia | ATM gene mutation, progressive cerebellar ataxia beginning between ages 1 and 4 years, oculomotor apraxia, choreoathetosis, telangiectasia of the conjunctivae, immunodeficiency, frequent infections, and an increased risk for malignancy, particularly leukemia and lymphoma111 |
Abbreviations: DRPLA, Dentatorubral-pallidoluysian Atrophy; GAD = Glutamic Acid Decarboxylase; GLUT-1, Glucose Transporter-1; MERRF, Myoclonus Epilepsy with Ragged-red Fibers; SCA = Spinocerebellar Ataxia.
The combination of ataxia and epileptic seizures has been described in several channelopathies. For instance, mutation of the voltage-gated potassium channel gene (KCNJ10) causes EAST syndrome (epilepsy with partial seizures and generalized seizures, ataxia, tubulopathy, and sensorineural deafness).15,16 A mutation in the gene encoding the sodium/hydrogen channel leads to inherited syndromes manifested with microcephaly, ataxia, disturbances of speech, and epilepsy.15–17 A loss-of-function mutation of the genes encoding the sodium channels (SCN1A) is the cause of Dravet syndrome (severe myoclonic epilepsy of infancy, SMEI).18 Another mutation in a sodium channel-encoding gene (SCNA2) has been described in connection with neonatal epilepsy, ataxia episodes, and headaches.19 Similarly, mutations of the voltage-gated calcium channel subunits were described in absence epilepsy and episodic ataxia.20
Symptomatology comprising cerebellar ataxia and epilepsy can be also seen in mitochondrial disorders, genetic syndromes, and myoclonic epilepsies. Ataxia is a part of the symptomatology of Leigh syndrome,21 Unverricht-Lundborg progressive myoclonic epilepsy,22 Lafora disease,23 and several types of lysosomal storage disorders.24 Niemann-Pick disease type C is a progressive neurovisceral metabolic disorder caused by a defect in a recently discovered gene, NPC-1. The biochemical deficit lies in delayed intracellular cholesterol transport. Cerebellar Purkinje cell loss is the most significant specific damage. More recently, significant changes were revealed in concentrations of neurotransmitters.25 Action myoclonus-renal failure syndrome is a kind of progressive myoclonic epilepsy with neurological manifestation of tremor, with later action myoclonus beginning in young adulthood, and rare generalized epileptic seizures. Cerebellar symptoms are present, as is ataxia in all cases in the more advanced stage.26 MERRF (myoclonic epilepsy with ragged-red fibers) is another rare mitochondrial disorder in this category. Diagnostic criteria include myoclonus, generalized epilepsy, cerebellar ataxia, and characteristic muscle biopsy findings (ragged red fibers).27
Progressive ataxia and myoclonic epilepsy were described in a patient with a homozygous mutation in the FOLR1 gene. Members of this gene family bind folic acid and its reduced derivatives and transport 5-methyltetrahydrofolate into cells. Dysfunction leads to failure of folate transportation across the blood-brain barrier. This was described in a 2-year-old child with a morphological defect of myelination failure.28
Epileptic seizures can also be observed in a rare genetic autosomal recessive ataxia with vitamin E deficiency. A case study described an affected patient with neurological disabilities from the age of 5 with epilepsy at 11 years, who was diagnosed at 30 years. At that time, the neurologic findings were dominated by ataxia, areflexia, dysarthria, positive pyramidal irritative phenomena, and epilepsy with generalized seizures. Supplementation with vitamin E suppressed seizures and stopped neurological deficit progression.29
Glucose transporter-1 deficiency syndrome should be considered in intractable epilepsy of childhood. It can present with a variety of symptoms including infantile seizures, developmental delay, movement disorders, and ataxia. Inadequate glucose transport to neurons results in impaired cerebral metabolism and disrupted thalamocortical development. Treatment with a ketogenic diet is well-established and can result in significant improvement in patient quality of life.30
In gluten enteropathy, epileptic seizures can be observed along with ataxia, which is quite common.31,32 Selective loss of Purkinje cells has been described in autoimmune cerebellar ataxia with antigliadin antibodies; however, in this case, without epilepsy.33
Spinocerebellar ataxia type 10 (SCA10), an autosomal dominant neurodegenerative disorder, is the result of a noncoding, pentanucleotide repeat expansion within intron 9 of the ataxin 10 gene. SCA10 patients present with pure cerebellar ataxia; some families also have a high incidence of epilepsy.34 The mean age at onset is 35.5 years, and the mean disease duration is 15.3 years.35 The first sign of disease is usually unbalanced gait and stance with variable degrees of limb ataxia characterized by jerky or uncoordinated movements unexplained by motor weakness or sensory loss. Ataxia is followed by speech difficulties, dysarthria, and ocular abnormalities. The epilepsy usually presents as generalized motor seizures and/or complex partial seizures a few years after the start of cerebellar ataxia. Antiepileptic drugs such as carbamazepine, phenytoin, and valproic acid are effective for most cases.36
Cerebellar ataxia and epilepsy with antiglutamic acid decarboxylase (GAD) antibodies is an autoimmune condition. GAD is the enzyme that catalyzes conversion of glutamate to gamma-aminobutyric acid (GABA).37 GABA is an inhibitory transmitter of Purkinje cells,38 and their loss is a well-known result of seizure activity.39 Recent studies reported that anti-GAD antibodies may be observed in adult-onset cerebellar syndrome, which is mainly characterized by ataxia, stiffness, incontinence, retinal pathologies, seizures, and immunological comorbidities.40
Other spinocerebellar ataxias might also be accompanied by epilepsy, although this is probably a coincidence. There was a case report of mesiotemporal epilepsy in an SCA13 patient and one of nocturnal frontal lobe epilepsy in an SCA17 patient.41,42 There was also a description of epilepsy in patients with Friedreich ataxia.43 A list of all possible conditions exceeds the scope of this article.
Consequence
In the following paragraphs, we explore a number of possible explanations for this association, including ataxia as a manifestation of seizure activity, cerebellar atrophy as a frequent finding in patients with epilepsy, permanent and transitional morphological changes of the cerebellum in connection with epilepsy, the possible predictive value of the extent of cerebellar atrophy for epilepsy surgery outcome, and ataxia as a negative side effect of antiepileptic medication.
Ataxia may be the result of otherwise silent epileptiform activity in epileptic patients.44 When it is a form of nonconvulsive seizure activity in children, it is called pseudoataxia or epileptic ataxia. In some cases, ataxia might be the first manifestation of an epileptic disorder. When a child has already been diagnosed and is taking an antiepileptic medication, then the exclusion of drug levels in the toxic range is an obvious prerequisite. Suppression of epileptiform discharges is associated with the disappearance of epileptic ataxia. This condition is particularly known in pediatric neurology.44
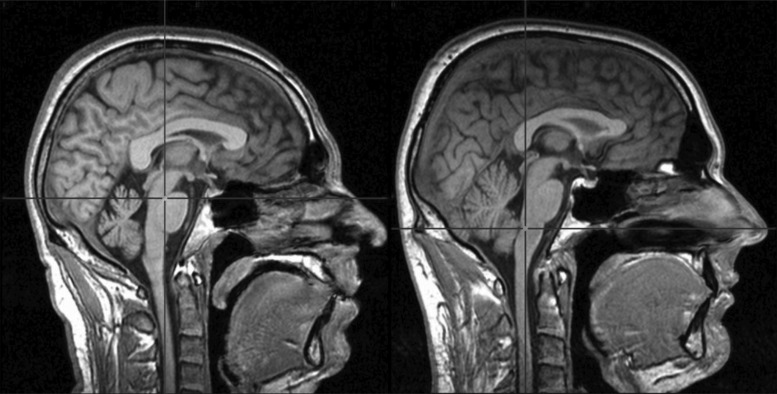
Ataxia can result from cerebellar atrophy, which is a relatively frequent finding in patients with epilepsy. Cerebellar atrophy is a well-known occurrence in patients with epilepsy.45 The precision of evaluation has evolved over time. Older studies determined cerebellar volume by means of pneumoencephalography46; later diagnostics included computed tomography and magnetic resonance imaging scans 47 (Figure 1). The pathogenesis of cerebellar atrophy in patients with epilepsy nevertheless remains unclear. Is atrophy a primary event or only the result of epilepsy? To what extent is it caused by antiepileptic medication?
Figure 1. Difficulties in Assessing the Extent of Cerebellar Atrophy. Magnetic resonance imaging in the medial sagittal plane. Left, 40-year-old patient with temporal lobe epilepsy due to focal cortical dysplasia type II and a 17-year history of predominantly generalized tonic-clonic seizures; right, the average-sized cerebellum of healthy control. Cerebellar size always should be evaluated with respect to intracranial volume.
Some researchers observed significantly smaller cerebellar white matter volumes in patients with newly diagnosed epilepsy compared to healthy controls. The researchers assumed that cerebellar white matter changes meant that the cerebellum is a susceptible structure associated with the development of partial epilepsy.48 Lawson et al. made a similar observation in children and assumed that brain volume reduction is present at epilepsy onset and is not a result of intractable seizures.49
In contrast, some experts have asserted that cerebellar damage does not appear to be a predisposing factor for epilepsy; rather, it is the aftermath of epileptic seizures or anticonvulsant medication in patients with severe and/or long-standing epilepsy.50 Histopathologic changes including Purkinje cell loss are common in people who experienced epileptic status, but it has also been detected in patients with only partial seizures.51 The situation is complex, and the process of cerebellar damage is influenced by more factors. There is a significant correlation between cerebellar volume and the total number of generalized tonic-clonic seizures (GTCSs) and GTCS seizure frequency.50 Possible seizure-related factors of this phenomenon are hypoxic-ischemic nerve cell injury during prolonged seizures and discharges along the cerebrocerebellar connections implicated in the mechanism of Purkinje cell loss.52
There is also evidence of subtle morphological changes in the cerebellum and its connections in idiopathic generalized epilepsy. Li et al. did not observe any cortical abnormalities but demonstrated a tendency towards a negative correlation between illness duration and white matter connectivity from the cerebellum to the cortex, along with cerebellar changes in diffusion tensor imaging.53
Besides the permanent phenomenon of atrophy, transitional functional changes have been observed, particularly peri-ictal cerebellar hyperperfusion resembling crossed cerebellar diaschisis in supratentorial stroke. Conversely, some authors found no relation between the site of the single-photon emission computed tomography (SPECT) seizure localization and the presence or pattern of peri-ictal cerebellar hyperperfusion (uni- or bilateral).54 Another study reported significant hypometabolism in the contralateral cerebellum of patients who had seizure onsets and corresponding hypometabolism in the frontal lobe and both in the temporal and frontal lobes. In contrast, patients who had seizure onsets and corresponding hypometabolism mesially in the temporal lobe tended to have lower values in the ipsilateral cerebellum.55 In a limited sample from a different study, the authors noted that crossed cerebellar diaschisis was a frequent finding of ictal brain SPECT regardless of epileptogenic lesion localization. They optimistically suggested that such information may aid in lateralizing epileptic foci.56 A third study that strictly focused on 33 patients with temporal lobe epilepsy did not demonstrate a significant linkage between the laterality of cerebral and cerebellar ictal hyperperfusion.57 The functional connection between the cerebellum and hippocampus is proven. Studies have revealed a dynamic interplay between these structures during temporal lobe seizures. Hippocampal epileptiform activity modulates cerebellar activity and vice versa. Excitation or inhibition of the cerebellum can significantly decrease hippocampal seizure duration; however, the exact pathways are not yet clear.58
Measuring cerebellar atrophy in patients with epilepsy might be useful for predicting resective epilepsy surgery outcome. Some reports have suggested worse outcomes for temporal lobe resection in patients with intractable epilepsy with cerebellar atrophy.54,59 Our own observations confirm that the extent of cerebellar atrophy is greater in a group of patients with persistent seizures than those who are seizure-free after surgery.
Ataxia might also represent a negative side effect of antiepileptic medication. Ataxia is especially frequent with the use of phenytoin and benzodiazepines.60 To varying degrees, ataxia is described as an adverse side effect of all antiepileptic drugs (Table 2). The issue has been best studied with phenytoin; high levels are toxic to the cerebellar cortex and Purkinje cells and may lead to their permanent damage and/or cerebellar atrophy.61,62 Its long-term administration is particularly hazardous for ataxia development, and clinical impairment occurs in half of treated patients.63 Ataxia might also be a side effect of gabapentin, but this drug is also useful in treating ataxia.64 For instance, in cortical cerebellar atrophy where GABA content reduction reflects the loss of Purkinje cells, the GABAergic properties of gabapentin have a favorable impact.64 Both effects are likely the result of interference with cerebellar GABAergic neurons, particularly Purkinje cells, the output neurons of the cerebellar cortex. In valproate treatment, tremor is more often observed as an adverse event, and ataxia could be a sign of hyperammonemic encephalopathy.65 Another reversible form of neurotoxicity was also reported with valproate, mimicking multiple system atrophy with parkinsonism and cerebellar symptoms.66
Table 2. Antiepileptic Drugs that Induce Cerebellar Ataxia1.
| 1a – Overview of all of the case and cohort studies found in the literature and included in the present review, percentage of affected patients 1b – Data on ataxia described as an adverse reaction in randomized placebo-controlled studies, percentage of affected patients | ||
|---|---|---|
| 1a | 1b | |
| Clobazam | 6.3% | |
| Clonazepam | 50% | |
| Eslicarbazepine | 7.5% | |
| Gabapentin | 8.7% | 10.1% |
| Lacosamide | 9.3% | |
| Lamotrigine | 5.5% | 18.5% |
| Levetiracetam | 1.5% | |
| Oxcarbazepine | 29.9% | |
| Phenytoin | 37.9% | |
| Pregabalin | 9.7% | |
| Retigabine | 15% | 10.4% |
| Tiagabine | 7.2% | 6.1% |
| Topiramate | 1.3% | 6.6% |
| Valproate | 3.6% | 3% |
| Vigabatrin | 6.8% | 3.6% |
| Zonisamide | 10.6% | 12.7% |
Data from (60).
There is very limited information on the exact mechanism by which most antiepileptic drugs induce ataxia. In addition to affecting the GABAergic system, analogies dealing with the role of ion channel dysfunction in ataxias indicate that interactions with ion channels can also be expected.67
Cause
The relationship between cerebellar damage and seizures has been known for a surprisingly long time. The role of the cerebellum was studied in detail as early as in the late 19th century by Hughlings Jackson.68 He described a particular form of seizure disorder as “tetanus-like seizures” secondary to a tubercular abscess of the middle lobe of the cerebellum.69 In this case, paroxysmal manifestations are caused strictly by the cerebellar lesion, and it became apparent that epileptic seizures arise not only in the cerebral cortex, but also in subcortical structures and the cerebellum.70,71 Tumorous lesions of the cerebellum (ganglioglioma,72 ganglioneurocytoma, low-grade astrocytoma,73 and hamartoma) and in artificial stimulation of the cerebellum cause ipsilateral paroxysmal symptoms including facial grimacing, ipsi- and contralateral head and eye deviation, nystagmus, and alterations of tone and posture in the ipsi- and contralateral limbs. In a case report of ganglioglioma of the cerebellar peduncle in a 4-year-old female with a history of seizures from the age of 2 months, ictal manifestation was described as follows: clonic twitching of the ipsilateral hemiface and eyelid often associated with deviation of the eyes to the side of the lesion, dystonic extension of the ipsilateral arm, increased respiratory rate, and groaning, followed by short-lasting apnea. Episodes usually lasted less than 20 s, and consciousness appeared to be preserved. The seizures occurred almost continuously. The role of the brainstem nuclei remains unclear, but widespread brainstem dysfunction seemed unlikely. In all cases, there was normal scalp electroencephalography (EEG) or only mild and nonspecific background abnormality. Ictal EEG recordings with implanted depth electrodes in the cerebellum demonstrated focal seizure discharges in the region of the tumorous mass.74 Seizures are typically drug resistant and are possibly cured by lesion resection.72,75–77 In a similar case of cerebellar ganglioglioma that presented with abnormal movements, myoclonus was not considered epileptic but was described as symptomatic. Also in this case, electrophysiological observation provided evidence that the electrical activity (slow waves) associated with myoclonus was confined to the cerebellar mass, and tumor resection led to seizure disappearance.78 Tumorous lesions of the cerebellum, in the majority of cases mentioned above, contain neuronal elements, but are not a prerequisite as with cerebellar astrocytomas.70,73 Astrocytes by themselves participate in epileptogenesis, at least in forming the extracellular environment by buffering potassium cations and maintaining the balance between excitatory and inhibitory neurotransmitters.79
Cerebellar damage can also indirectly cause epileptic seizures. The cerebellum has an inhibitory effect on paroxysmal epileptic activity.9,10 Some studies have demonstrated a higher incidence and intensity of epileptic seizures in patients and animal models with cerebellar damage.10,11 This is consistent with the hypothesis that the damaged cerebellum fails to inhibit epileptic seizures. Experimental electrical stimulation of cerebellar structures can affect epileptic activity within the cerebral cortex. The effect of such stimulations depends on many variables such as stimulation frequency and region.9,70 The mechanism of action is supposed to be thalamic inhibition, but the circuits and influences are less clearly defined.80 The exact mechanism of seizure activity potentiation in cerebellar lesions is still elusive and remains a matter of speculation since tangible, direct data are beyond the reach of current technology. In epileptic patients, the histologic findings of Purkinje cell loss,51,52 as well as structures exerting inhibitory effects on the dominantly excitatory cerebellar nuclei, support the hypothesized disinhibition of their output pathways—the stated cerebellar nuclei with propagation to the thalamocortical pathway. Furthermore, this pathology will lead to erroneous performance of the cerebellar adaptive filter (i.e., the signal patterns leading to unfavorable outcomes), and epileptic activity will no longer be recognized as such, so the defective algorithms of the atrophied cerebellar cortex will not suppress them or “filter them out.” On the other hand, the nature of epileptic activity inhibition in cerebellar stimulation seems more complicated. Although crude electric stimulation of the cerebellar cortex will produce aberrant information flow from the stimulated cells, their simple hyperactivity will inhibit the excitatory output of deep cerebellar nuclei and downstream activity in the thalamocortical pathway. Nonetheless, stimulation of the cerebellar cortex has yielded inconsistent results.81 In putative contradiction to the above reasoning, a recent report hypothesized that decreased interictal regional activity of deep cerebellar nuclei is likely connected with an epileptogenic state. The expected anticonvulsive effect of deep nuclei stimulation is explained by its modulation, not simply by excitation or inhibition.82 Buijink et al. reached a similar conclusion when examining brain atrophy in patients with familial cortical myoclonic tremor with epilepsy. This heritable disease is characterized by progressive myoclonus of the distal limbs, infrequent epileptic seizures, signs of cortical hyperexcitability, and Purkinje cell loss. The authors concluded that pathologic cerebellar changes, reflected as marked cerebellar atrophy, can decrease cerebellar inhibition of dentatothalamic-cortical tracts and hypothesized that reduced inhibitory Purkinje cell output to the dentate nucleus causes this decreased inhibition.83
The 1960s and 1970s were a period of experimentation investigating the role of the cerebellum in epileptogenesis. In 1972, Hutton demonstrated the impact of cerebellar lesions on cortical, penicillin-induced epileptogenic focus in cats. The cerebellum was electrically stimulated, and several ablations were performed prior to evaluating the interspike interval of cortical discharges. Stimulation of the vermis and right paramedian lobes during the early stages of focal seizures usually resulted in spike inhibition or reduced spike frequency. The effect of gross surface cerebellar stimulation was similar. Stimulation of the contralateral dentate nucleus did not produce a regularly reproducible effect. Stimulation of the contralateral interpositus nuclei was slightly more effective, while stimulation of the fastigial, ipsilateral interpositus, and dentate nuclei did not have significant effects on focal cortical seizures. Ablation of the paramedian lobes and vermis facilitated seizure activity.11 Recently, cerebellar-directed optogenetic intervention in a mouse model of temporal lobe epilepsy showed that excitation of the midline cerebellar Purkinje neurons decreases seizure frequency. Interventions targeting the cerebellum might therefore offer a potential therapy for medically intractable epilepsies.84
Our knowledge of the impact of cerebellar stimulation on epileptic seizures is not limited to animal models. According to a systematic review by Fontas et al., three clinical double-blind studies used similar surgical implantation techniques and stimulation targets and parameters. Two of them failed to demonstrate any significant seizure reduction, while the third showed a significant poststimulation decrease in seizure frequency.80 Cerebellar stimulation can also be carried out noninvasively by means of transcranial magnetic stimulation (rTMS). In a small sample of six patients with refractory epilepsy, high-frequency rTMS over the cerebellar cortex was associated with decreased seizure frequency during treatment of patients with both single and multiple epileptic foci. However, seizure frequency returned to baseline levels soon after the end of rTMS.85 The results of cerebellar stimulation for treating intractable epilepsy are promising but not yet fully convincing.80
Summary
Some patients with epilepsy display more or less pronounced ataxia as a manifestation of structural cerebellar impairment. Sometimes this is a simple coincidence, but in many cases ataxia results from epilepsy or represents an adverse effect of a specific antiepileptic medication. Both situations can lead to significant cerebellar atrophy, which is then reflected in the development of clinical cerebellar symptoms. The question remains whether such atrophy is the start of a vicious circle in which a damaged cerebellum loses its inhibitory effect on cerebral epileptic activity with subsequent worsening of the disease course. The ability of the cerebellum to indirectly affect epileptic activity within the cerebral cortex was repeatedly demonstrated in both animal experiments and in clinical epileptology. The direct role of the cerebellum in human epileptogenesis and the ability of cerebellar structures to generate epileptic activity appear increasingly realistic given the growing number of relevant publications. The recognition of the role of the cerebellum in epileptogenesis has already been put to practical use, and attempts have been made to control epilepsy by stimulating the cerebellar cortex and nuclei. The discovery of the most suitable structures for such interventions should be a subject for further research.
Footnotes
Funding: None.
Financial Disclosure: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 2.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the Dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–260. doi: 10.1007/s11065-010-9142-x. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 3.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filip P, Lungu OV, Bareš M. Dystonia and the cerebellum: a new field of interest in movement disorders. Clin Neurophysiol. 2013;124:1269–1276. doi: 10.1016/j.clinph.2013.01.003. doi: 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Mothersill O, Knee-Zaska C, Donohoe G. Emotion and theory of mind in schizophrenia-investigating the role of the cerebellum. Cerebellum. 2016;15:357–368. doi: 10.1007/s12311-015-0696-2. doi: 10.1007/s12311-015-0696-2. [DOI] [PubMed] [Google Scholar]
- 8.Filip P, Lungu OV, Manto MU, Bareš M. Linking essential tremor to the cerebellum: physiological evidence. Cerebellum. 2015 Nov 3; doi: 10.1007/s12311-015-0740-2. [Epub ahead of print] doi: 10.1007/s12311-015-0740-2. [DOI] [PubMed] [Google Scholar]
- 9.Wong JC, Escayg A. Illuminating the cerebellum as a potential target for treating epilepsy. Epilepsy Curr. 2015;15:277–278. doi: 10.5698/1535-7511-15.5.277. doi: 10.5698/1535-7511-15.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dow RS, Fernandez-Guardiola A, Manni E. The influence of the cerebellum on experimental epilepsy. Electroencephalogr Clin Neurophysiol. 1962;14:383–398. doi: 10.1016/0013-4694(62)90115-3. doi: 10.1016/0013-4694(62)90115-3. [DOI] [PubMed] [Google Scholar]
- 11.Hutton JT, Frost JD, Jr, Foster J. The influence of the cerebellum in cat penicillin epilepsy. Epilepsia. 1972;13:401–408. doi: 10.1111/j.1528-1157.1972.tb04580.x. doi: 10.1111/j.1528-1157.1972.tb04580.x. [DOI] [PubMed] [Google Scholar]
- 12.Mesiwala AH, Kuratani JD, Avellino AM, Roberts TS, Sotero MA, Ellenbogen RG. Focal motor seizures with secondary generalization arising in the cerebellum. Case report and review of the literature. J Neurosurg. 2002;97:190–196. doi: 10.3171/jns.2002.97.1.0190. doi: 10.3171/jns.2002.97.1.0190. [DOI] [PubMed] [Google Scholar]
- 13.Young GB, Oppenheimer SR, Gordon BA, et al. Ataxia in institutionalized patients with epilepsy. Can J Neurol Sci. 1994;21:252–258. doi: 10.1017/s0317167100041238. doi: 10.1017/S0317167100041238. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Nakagawa T, Inoue T, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 15.Pena SD, Coimbra RL. Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy. Clin Genet. 2015;87:e1–3. doi: 10.1111/cge.12542. doi: 10.1111/cge.12542">10.1111/cge.12542">10.1111/cge.12542. [DOI] [PubMed] [Google Scholar]
- 16.Cross JH, Arora R, Heckemann RA, et al. Neurological features of epilepsy, ataxia, sensorineural deafness, tubulopathy syndrome. Dev Med Child Neurol. 2013;55:846–856. doi: 10.1111/dmcn.12171. doi: 10.1111/dmcn.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhan SM, Murphy LM, Robinson JF, et al. Linkage analysis and exome sequencing identify a novel mutation in KCTD7 in patients with progressive myoclonus epilepsy with ataxia. Epilepsia. 2014;55:e106–111. doi: 10.1111/epi.12730. doi: 10.1111/epi.12730. [DOI] [PubMed] [Google Scholar]
- 18.Berkovic SF. Genetics of epilepsy in clinical practice. Epilepsy Curr. 2015;15:192–196. doi: 10.5698/1535-7511-15.4.192. doi: 10.5698/1535-7511-15.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Anttonen AK, Liukkonen E, et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010;75:1454–1458. doi: 10.1212/WNL.0b013e3181f8812e. doi: 10.1212/WNL.0b013e3181f8812e. [DOI] [PubMed] [Google Scholar]
- 20.Imbrici P, Jaffe SL, Eunson LH, et al. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–2692. doi: 10.1093/brain/awh301. doi: 10.1093/brain/awh301. [DOI] [PubMed] [Google Scholar]
- 21.Leshinsky-Silver E, Shuvalov R, Inbar S, Cohen S, Lev D, Lerman-Sagie T. Juvenile Leigh syndrome, optic atrophy, ataxia, dystonia, and epilepsy due to T14487C mutation in the mtDNA-ND6 gene: a mitochondrial syndrome presenting from birth to adolescence. J Child Neurol. 2011;26:476–481. doi: 10.1177/0883073810384615. doi: 10.1177/0883073810384615. [DOI] [PubMed] [Google Scholar]
- 22.Joensuu T, Tegelberg S, Reinmaa E, et al. Gene expression alterations in the cerebellum and granule neurons of Cstb(-/-) mouse are associated with early synaptic changes and inflammation. PLoS One. 2014;9:e89321. doi: 10.1371/journal.pone.0089321. doi: 10.1371/journal.pone.0089321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortolano S, Vieitez I, Agis-Balboa RC, Spuch C. Loss of GABAergic cortical neurons underlies the neuropathology of Lafora disease. Mol Brain. 2014;7:7. doi: 10.1186/1756-6606-7-7. doi: 10.1186/1756-6606-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mole SE, Williams RE. Neuronal ceroid-lipofuscinoses. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(®) [Internet] Seattle, WA: University of Washington; 1993. [cited 2015 Aug 23] Available from: http://www.ncbi.nlm.nih.gov/books/NBK1428/ [Google Scholar]
- 25.Yadid G, Sotnik-Barkai I, Tornatore C, et al. Neurochemical alterations in the cerebellum of a murine model of Niemann–Pick type C disease. Brain Res. 1998;799((2)):250–256. doi: 10.1016/s0006-8993(98)00449-1. doi: 10.1016/S0006-8993(98)00449-1. [DOI] [PubMed] [Google Scholar]
- 26.Badhwar A, Berkovic SF, Dowling JP, et al. Action myoclonus-renal failure syndrome: characterization of a unique cerebro-renal disorder. Brain. 2004;127:2173–2182. doi: 10.1093/brain/awh263. doi: 10.1093/brain/awh263. [DOI] [PubMed] [Google Scholar]
- 27.Finsterer J, Zarrouk Mahjoub S. Epilepsy in mitochondrial disorders. Seizure. 2012;21:316–321. doi: 10.1016/j.seizure.2012.03.003. doi: 10.1016/j.seizure.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Dueñas B, Toma C, Ormazábal A, et al. Progressive ataxia and myoclonic epilepsy in a patient with a homozygous mutation in the FOLR1 gene. J Inherit Metab Dis. 2010;33:795–802. doi: 10.1007/s10545-010-9196-1. doi: 10.1007/s10545-010-9196-1. [DOI] [PubMed] [Google Scholar]
- 29.Müller KI, Bekkelund SI. Epilepsy in a patient with ataxia caused by vitamin E deficiency. BMJ Case Rep. 2011;2011:pii. doi: 10.1136/bcr.01.2011.3728. bcr0120113728. doi: 10.1136/bcr.01.2011.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen S, Keough K, Gibson J. Clinical reasoning: novel GLUT1-DS mutation: refractory seizures and ataxia. Neurology. 2015;84:e111–e114. doi: 10.1212/WNL.0000000000001467. doi: 10.1212/WNL.0000000000001467. [DOI] [PubMed] [Google Scholar]
- 31.Javed S, Safdar A, Forster A, et al. Refractory coeliac disease associated with late onset epilepsy, ataxia, tremor and progressive myoclonus with giant cortical evoked potentials-a case report and review of literature. Seizure. 2012;21:482–485. doi: 10.1016/j.seizure.2012.04.003. doi: 10.1016/j.seizure.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia KP, Brown P, Gregory R, et al. Progressive myoclonic ataxia associated with coeliac disease. The myoclonus is of cortical origin, but the pathology is in the cerebellum. Brain. 1995;118:1087–1093. doi: 10.1093/brain/118.5.1087. doi: 10.1093/brain/118.5.1087. [DOI] [PubMed] [Google Scholar]
- 33.Nanri K, Shibuya M, Taguchi T, Hasegawa A, Tanaka N. Selective loss of Purkinje cells in a patient with anti-gliadin-antibody-positive autoimmune cerebellar ataxia. Diagn Pathol. 2011;6:14. doi: 10.1186/1746-1596-6-14. doi: 10.1186/1746-1596-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFarland KN, Liu J, Landrian I, et al. Repeat interruptions in spinocerebellar ataxia type 10 expansions are strongly associated with epileptic seizures. Neurogenetics. 2014;15:59–64. doi: 10.1007/s10048-013-0385-6. doi: 10.1007/s10048-013-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teive HA, Munhoz RP, Raskin S, et al. Spinocerebellar ataxia type 10: frequency of epilepsy in a large sample of Brazilian patients. Mov Disord. 2010;25:2875–2878. doi: 10.1002/mds.23324. doi: 10.1002/mds.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin X, Ashizawa T. Recent progress in spinocerebellar ataxia type-10 (SCA10) Cerebellum. 2005;4:37–42. doi: 10.1080/14734220510007897. doi: 10.1080/14734220510007897. [DOI] [PubMed] [Google Scholar]
- 37.Georgieva Z, Parton M. Cerebellar ataxia and epilepsy with anti-GAD antibodies: treatment with IVIG and plasmapheresis. BMJ Case Rep. 2014;2014:pii. doi: 10.1136/bcr-2013-202314. bcr2013202314. doi: 10.1136/bcr-2013-202314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonnum F, Storm-Mathisen J, Walberg F. Glutamate decarboxylase in inhibitory neurons. A study of the enzyme in Purkinje cell axons and boutons in the cat. Brain Res. 1970;20:259–275. doi: 10.1016/0006-8993(70)90293-3. doi: 10.1016/0006-8993(70)90293-3. [DOI] [PubMed] [Google Scholar]
- 39.Dam M, Bolwig T, Hertz M, Bajorec J, Lomax P, Dam AM. Does seizure activity produce Purkinje cell loss. Epilepsia. 1984;25:747–751. doi: 10.1111/j.1528-1157.1984.tb03486.x. doi: 10.1111/j.1528-1157.1984.tb03486.x. [DOI] [PubMed] [Google Scholar]
- 40.Dogan VB. An anti-GAD autoantibody-associated cerebellar syndrome case: a curable cause of ataxia. Neurol Sci. 2015;36:1929–1931. doi: 10.1007/s10072-015-2280-4. doi: 10.1007/s10072-015-2280-4. [DOI] [PubMed] [Google Scholar]
- 41.Bürk K, Strzelczyk A, Reif PS, et al. Mesial temporal lobe epilepsy in a patient with spinocerebellar ataxia type 13 (SCA13) Int J Neurosci. 2013;123:278–282. doi: 10.3109/00207454.2012.755180. doi: 10.3109/00207454.2012.755180. [DOI] [PubMed] [Google Scholar]
- 42.Belluzzo M, Musho-Ilbeh S, Monti F, Pizzolato G. A case of nocturnal frontal lobe epilepsy in a patient with spinocerebellar ataxia type 17. Seizure. 2012;21:805–806. doi: 10.1016/j.seizure.2012.08.006. doi: 10.1016/j.seizure.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Golomb MR, Illner A, Christensen CK, Walsh LE. A child with Friedreich’s ataxia and epilepsy. J Child Neurol. 2005;20:248–250. doi: 10.1177/08830738050200031201. doi: 10.1177/08830738050200031201. [DOI] [PubMed] [Google Scholar]
- 44.Bennett HS, Selman JE, Rapin I, Rose A. Nonconvulsive epileptiform activity appearing as ataxia. Am J Dis Child. 1982;136:30–32. doi: 10.1001/archpedi.1982.03970370032007. doi: 10.1001/archpedi.1982.03970460018003. [DOI] [PubMed] [Google Scholar]
- 45.Spielmeyer W. The anatomic substratum of the convulsive state. Arch Neurol Psychiatry. 1930;23:869–875. doi: 10.1001/archneurpsyc.1930.02220110025002. [Google Scholar]
- 46.Selhorst JB, Kaufman B, Horwitz SJ. Diphenylhydantoin-induced cerebellar degeneration. Arch Neurol. 1972;27:453–455. doi: 10.1001/archneur.1972.00490170085012. doi: 10.1001/archneur.1972.00490170085012. [DOI] [PubMed] [Google Scholar]
- 47.Kessler C, Henningsen H, Reuther R, Christian W. Cerebellar atrophy in epileptic patients: computer tomography study. Fortschr Neurol Psychiatr. 1985;53:437–441. doi: 10.1055/s-2007-1001989. doi: 10.1055/s-2007-1001989. [DOI] [PubMed] [Google Scholar]
- 48.Park KM, Han YH, Kim TH, et al. Cerebellar white matter changes in patients with newly diagnosed partial epilepsy of unknown etiology. Clin Neurol Neurosurg. 2015;138:25–30. doi: 10.1016/j.clineuro.2015.07.017. doi: 10.1016/j.clineuro.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Bye AME. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia. 2000;41:1456–1462. doi: 10.1111/j.1528-1157.2000.tb00122.x. doi: 10.1111/j.1528-1157.2000.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 50.Hagemann G, Lemieux L, Free SL, et al. Cerebellar volumes in newly diagnosed and chronic epilepsy. J Neurol. 2002;249:1651–1658. doi: 10.1007/s00415-002-0843-9. doi: 10.1007/s00415-002-0843-9. [DOI] [PubMed] [Google Scholar]
- 51.Salcman M, Defendini R, Correll J, Gilman S. Neuropathological changes in cerebellar biopsies of epileptic patients. Ann Neurol. 1978;3:10–19. doi: 10.1002/ana.410030104. doi: 10.1002/ana.410030104. [DOI] [PubMed] [Google Scholar]
- 52.Crooks R, Mitchell T, Thom M. Patterns of cerebellar atrophy in patients with chronic epilepsy: a quantitative neuropathological study. Epilepsy Res. 2000;41:63–73. doi: 10.1016/s0920-1211(00)00133-9. doi: 10.1016/S0920-1211(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Du H, Xie B, et al. Cerebellum abnormalities in idiopathic generalized epilepsy with generalized tonic-clonic seizures revealed by diffusion tensor imaging. PLoS One. 2010;5:e15219. doi: 10.1371/journal.pone.0015219. doi: 10.1371/journal.pone.0015219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohnen NI, O’Brien TJ, Mullan BP, So EL. Cerebellar changes in partial seizures: clinical correlations of quantitative SPECT and MRI analysis. Epilepsia. 1998;39:640–650. doi: 10.1111/j.1528-1157.1998.tb01433.x. doi: 10.1111/j.1528-1157.1998.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 55.Savic I, Altshuler L, Passaro E, Baxter L, Engel J., Jr Localized cerebellar hypometabolism in patients with complex partial seizures. Epilepsia. 1996;37:781–787. doi: 10.1111/j.1528-1157.1996.tb00652.x. doi: 10.1111/j.1528-1157.1996.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 56.Won JH, Lee JD, Chung TS, Park CY, Lee BI. Increased contralateral cerebellar uptake of technetium-99m-HMPAO on ictal brain SPECT. J Nucl Med. 1996;37:426–429. [PubMed] [Google Scholar]
- 57.Shin WC, Hong SB, Tae WS, Seo DW, Kim SE. Ictal hyperperfusion of cerebellum and basal ganglia in temporal lobe epilepsy: SPECT subtraction with MRI coregistration. J Nucl Med. 2001;42:853–858. [PubMed] [Google Scholar]
- 58.Yu W, Krook-Magnuson E. Cognitive collaborations: bidirectional functional connectivity between the cerebellum and the hippocampus. Front Syst Neurosci. 2015;9:177. doi: 10.3389/fnsys.2015.00177. doi: 10.3389/fnsys.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Specht U, May T, Schulz R, et al. Cerebellar atrophy and prognosis after temporal lobe resection. J Neurol Neurosurg Psychiatry. 1997;62:501–506. doi: 10.1136/jnnp.62.5.501. doi: 10.1136/jnnp.62.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Gaalen J, Kerstens FG, Maas RP, Härmark L, van de Warrenburg BP. Drug-induced cerebellar ataxia: a systematic review. CNS Drugs. 2014;28:1139–1153. doi: 10.1007/s40263-014-0200-4. doi: 10.1007/s40263-014-0200-4. [DOI] [PubMed] [Google Scholar]
- 61.Awada A, Amene P, al Jumah M, al Beladi K. [Residual cerebellar ataxia following acute phenytoin intoxication] Rev Neurol (Paris) 1999;155:306–308. doi: RNE-04-1999-155-4-0000-0000-101019-ART95. [PubMed] [Google Scholar]
- 62.Gupta M, Patidar Y, Khwaja GA, Chowdhury D, Batra A, Dasgupta A. Persistent cerebellar ataxia with cerebellar cognitive affective syndrome due to acute phenytoin intoxication: a case report. Neurol Asia. 2013;18:107–111. [Google Scholar]
- 63.Shanmugarajah P, Hoggard N, Howell S, et al. Phenytoin and cerebellar ataxia: not all down to toxicity? J Neurol Neurosurg Psychiatry. 2013;84:e2–e2. doi: 10.1136/jnnp-2013-306573.103. [Google Scholar]
- 64.Gazulla J, Errea JM, Benavente I, Tordesillas CJ. Treatment of ataxia in cortical cerebellar atrophy with the GABAergic drug gabapentin. A preliminary study. Eur Neurol. 2004;52:7–11. doi: 10.1159/000079252. doi: 10.1159/000079252. [DOI] [PubMed] [Google Scholar]
- 65.Verma R, Kori P. Valproate-induced encephalopathy with predominant pancerebellar syndrome. Indian J Pharmacol. 2012;44:129–130. doi: 10.4103/0253-7613.91886. doi: 10.4103/0253-7613.91886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shill HA, Fife TD. Valproic acid toxicity mimicking multiple system atrophy. Neurology. 2000;55:1936–1937. doi: 10.1212/wnl.55.12.1936. doi: 10.1212/WNL.55.12.1936. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura K, Yoshida K, Miyazaki D, Morita H, Ikeda S. Spinocerebellar ataxia type 6 (SCA6): clinical pilot trial with gabapentin. J Neurol Sci. 2009;278:107–111. doi: 10.1016/j.jns.2008.12.017. doi: 10.1016/j.jns.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Horsley V. Dr. Hughlings Jackson’s views of the functions of the cerebellum, as illustrated by recent research: being the Hughlings Jackson Lecture for 1906. Br Med J. 1907;1:803–808. doi: 10.1136/bmj.1.2414.803. doi: 10.1136/bmj.1.2414.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCrory PR, Bladin PF, Berkovic SF. The cerebellar seizures of Hughlings Jackson. Neurology. 1999;52:1888–1890. doi: 10.1212/wnl.52.9.1888. doi: 10.1212/WNL.52.9.1888. [DOI] [PubMed] [Google Scholar]
- 70.Boop S, Wheless J, Van Poppel K, McGregor A, Boop FA. Cerebellar seizures. J Neurosurg Pediatr. 2013;12:288–292. doi: 10.3171/2013.5.PEDS1394. doi: 10.3171/2013.5.PEDS1394. [DOI] [PubMed] [Google Scholar]
- 71.Badawy RA, Lai A, Vogrin SJ, Cook MJ. Subcortical epilepsy. Neurology. 2013;80:1901–1907. doi: 10.1212/WNL.0b013e3182929f4f. doi: 10.1212/WNL.0b013e3182929f4f. [DOI] [PubMed] [Google Scholar]
- 72.Martins WA, Paglioli E, Hemb M, Palmini A. Dysplastic cerebellar epilepsy: complete seizure control following resection of a ganglioglioma. Cerebellum. 2015 Jul 25; doi: 10.1007/s12311-015-0705-5. [Epub ahead of print] doi: 10.1007/s12311-015-0705-5. [DOI] [PubMed] [Google Scholar]
- 73.Strazzer S, Zucca C, Fiocchi I, Genitori L, Castelli E. Epilepsy and neuropsychologic deficit in a child with cerebellar astrocytoma. J Child Neurol. 2006;21:817–820. doi: 10.1177/08830738060210091701. doi: 10.1177/08830738060210091701. [DOI] [PubMed] [Google Scholar]
- 74.Harvey AS, Jayaka P, Duchowny M, et al. Hemifacial seizures and cerebellar ganglioglioma: an epilepsy syndrome of infancy with seizures of cerebellar origin. Ann Neurol. 1996;40:91–98. doi: 10.1002/ana.410400115. doi: 10.1002/ana.410400115. [DOI] [PubMed] [Google Scholar]
- 75.Hanai S, Okazaki K, Fujikawa Y, et al. Hemifacial seizures due to ganglioglioma of cerebellum. Brain Dev. 2010;32:499–501. doi: 10.1016/j.braindev.2009.06.005. doi: 10.1016/j.braindev.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 76.Dagcinar A, Hilmi Kaya A, Ali Taşdemir H, Kuruoglu E, Sabancilar Z, Sav A. A fourth ventricular ganglioneurocytoma representing with cerebellar epilepsy: a case report and review of the literature. Eur J Paediatr Neurol. 2007;11:257–260. doi: 10.1016/j.ejpn.2007.02.005. doi: 10.1016/j.ejpn.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Gan YC, Connolly MB, Steinbok P. Epilepsy associated with a cerebellar arachnoid cyst: seizure control following fenestration of the cyst. Childs Nerv Syst. 2008;24:125–134. doi: 10.1007/s00381-007-0439-x. doi: 10.1007/s00381-007-0439-x. [DOI] [PubMed] [Google Scholar]
- 78.Koh KN, Lim BC, Hwang H, et al. Cerebellum can be a possible generator of progressive myoclonus. J Child Neurol. 2010;25:728–731. doi: 10.1177/0883073809342273. doi: 10.1177/0883073809342273. [DOI] [PubMed] [Google Scholar]
- 79.Hamberger A, Bock E, Nordborg C, et al. Biochemical correlates to cortical dysplasia, gliosis, and astrocytoma infiltration in human epileptogenic cortex. Neurochem Res. 1993;18((4)):511–518. doi: 10.1007/BF00967255. doi: 10.1007/BF00967255. [DOI] [PubMed] [Google Scholar]
- 80.Fountas KN, Kapsalaki E, Hadjigeorgiou G. Cerebellar stimulation in the management of medically intractable epilepsy: a systematic and critical review. Neurosurg Focus. 2010;29:E8. doi: 10.3171/2010.5.FOCUS10111. doi: 10.3171/2010.5.FOCUS10111. [DOI] [PubMed] [Google Scholar]
- 81.Kros L, Eelkman Rooda OH, De Zeeuw CI, Hoebeek FE. Controlling cerebellar output to treat refractory epilepsy. Trends Neurosci. 2015;38:787–799. doi: 10.1016/j.tins.2015.10.002. doi: 10.1016/j.tins.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Rijkers K, Moers-Hornikx VM, Hemmes RJ, et al. Sustained reduction of cerebellar activity in experimental epilepsy. BioMed Res Int. 2015;2015:718591. doi: 10.1155/2015/718591. doi: 10.1155/2015/718591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buijink AWG, Broersma M, van der Stouwe AMM, et al. Cerebellar atrophy in cortical myoclonic tremor and not in hereditary essential tremor – a voxel-based morphometry study. Cerebellum. 2015;30:1–9. doi: 10.1007/s12311-015-0734-0. doi: 10.1007/s12311-015-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro. 2014;1:pii. doi: 10.1523/ENEURO.0005-14.2014. e.2014. doi: 10.1523/eneuro.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brighina F, Daniele O, Piazza A, Giglia G, Fierro B. Hemispheric cerebellar rTMS to treat drug-resistant epilepsy: case reports. Neurosci Lett. 2006;397:229–233. doi: 10.1016/j.neulet.2005.12.050. doi: 10.1016/j.neulet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 86.Larsen J, Carvill GL, Gardella E, et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology. 2015;84:480–489. doi: 10.1212/WNL.0000000000001211. doi: 10.1212/WNL.0000000000001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gilfillan GD, Selmer KK, Roxrud I, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82:1003–1010. doi: 10.1016/j.ajhg.2008.01.013. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damaj L, Lupien-Meilleur A, Lortie A, et al. CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur J Hum Genet. 2015;23:1505–1512. doi: 10.1038/ejhg.2015.21. doi: 10.1038/ejhg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freeze H, Eklund EA, Ng B, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11:453–466. doi: 10.1016/S1474-4422(12)70040-6. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nachbauer W, Eigentler A, Boesch S. Acquired ataxias: the clinical spectrum, diagnosis and management. J Neurol. 2015;262:1385–1393. doi: 10.1007/s00415-015-7685-8. doi: 10.1007/s00415-015-7685-8. [DOI] [PubMed] [Google Scholar]
- 91.Tsao CY. Cerebellar ataxia, vertical supranuclear gaze palsy, sensorineural deafness, epilepsy, dementia, and hallucinations in an adolescent male. Semin Pediatr Neurol. 2014;21:106–108. doi: 10.1016/j.spen.2014.04.012. doi: 10.1016/j.spen.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 92.Harirchian MH, Shandiz EE, Turnbull J, Minassian BA, Shahsiah R. Lafora disease: a case report, pathologic and genetic study. Indian J Pathol Microbiol. 2011;54:374–375. doi: 10.4103/0377-4929.81645. doi: 10.4103/0377-4929.81645. [DOI] [PubMed] [Google Scholar]
- 93.Delgado-Escueta AV. Advances in lafora progressive myoclonus epilepsy. Curr Neurol Neurosci Rep. 2007;7((5)):428–433. doi: 10.1007/s11910-007-0066-7. doi: 10.1007/s11910-007-0066-7. [DOI] [PubMed] [Google Scholar]
- 94.Mole SE, Cotman SL. Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochimica et Biophysica Acta (BBA) Mol Basis Dis. 2015;1852((10, Part B)):2237–2241. doi: 10.1016/j.bbadis.2015.05.011. doi: 10.1016/j.bbadis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vieira de Rezende Pinto WB, Sgobbi de Souza PV, Pedroso JL, Barsottini OG. Variable phenotype and severity of sialidosis expressed in two siblings presenting with ataxia and macular cherry-red spots. J Clin Neurosci. 2013;20:1327–1328. doi: 10.1016/j.jocn.2012.12.014. doi: 10.1016/j.jocn.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 96.Grabowski GA, Zimran A, Ida H. Gaucher disease types 1 and 3: phenotypic characterization of large populations from the ICGG Gaucher Registry. Am J Hematol. 2015;90(Suppl 1):S12–S18. doi: 10.1002/ajh.24063. doi: 10.1002/ajh.24063. [DOI] [PubMed] [Google Scholar]
- 97.DiMauro S, Hirano M. MERRF. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(®) [Internet] Seattle, WA: University of Washington; 1993. [cited 2015 Nov 23] Available from: http://www.ncbi.nlm.nih.gov/books/NBK1520/ [Google Scholar]
- 98.Lehesjoki AE, Gardiner M. Progressive myoclonus epilepsy: Unverricht-Lundborg disease and neuronal ceroid lipofuscinoses. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies [Internet] 4th ed. Bethesda, MD: National Center for Biotechnology Information (US); 2012. [cited 2015 Aug 23] Available from: http://www.ncbi.nlm.nih.gov/books/NBK98154/. doi: 10.1093/med/9780199746545.001.0001. [PubMed] [Google Scholar]
- 99.Amrom D, Andermann F, Andermann E. GeneReviews. Seattle, WA: University of Washington; 1993. Action myoclonus – renal failure syndrome. [Internet] [cited 2016 Mar 30, 1993. Available from: http://www.ncbi.nlm.nih.gov/books/NBK333437/ [Google Scholar]
- 100.Vaamonde J, Muruzabal J, Tuñón T, et al. Abnormal muscle and skin mitochondria in family with myoclonus, ataxia, and deafness (May and White syndrome) J Neurol Neurosurg Psychiatry. 1992;55:128–132. doi: 10.1136/jnnp.55.2.128. doi: 10.1136/jnnp.55.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mocellin R, Walterfang M, Velakoulis D. Hashimoto’s encephalopathy: epidemiology, pathogenesis and management. CNS Drugs. 2007;21:799–811. doi: 10.2165/00023210-200721100-00002. doi: 10.2165/00023210-200721100-00002. [DOI] [PubMed] [Google Scholar]
- 102.Whaley NR, Fujioka S, Wszolek ZK. Autosomal dominant cerebellar ataxia type I: a review of the phenotypic and genotypic characteristics. Orphanet J Rare Dis. 2011;6:33. doi: 10.1186/1750-1172-6-33. doi: 10.1186/1750-1172-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Serratosa JM, Minassian BA, Ganesh S. Gene defects in progressive myoclonus epilepsy. Epilepsia. 2010;51:75. doi: 10.1111/j.1528-1167.2010.02861.x. [Google Scholar]
- 104.Rossi M, Perez-Lloret S, Doldan L, et al. Autosomal dominant cerebellar ataxias: a systematic review of clinical features. Eur J Neurol. 2014;21:607–615. doi: 10.1111/ene.12350. doi: 10.1111/ene.12350. [DOI] [PubMed] [Google Scholar]
- 105.Tan NCK, Zhou Y, Tan ASC, Chong SS, Lee WL. Spinocerebellar ataxia type 2 with focal epilepsy – an unusual association. Ann Acad Med Singap. 2004;33:103–106. [PubMed] [Google Scholar]
- 106.Choubtum L, Witoonpanich P, Hanchaiphiboolkul S, et al. Analysis of SCA8, SCA10, SCA12, SCA17 and SCA19 in patients with unknown spinocerebellar ataxia: a Thai multicentre study. BMC Neurol. 2015;15:166. doi: 10.1186/s12883-015-0425-y. doi: 10.1186/s12883-015-0425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manto M-U. The wide spectrum of spinocerebellar ataxias (SCAs) Cerebellum. 2005;4:2–6. doi: 10.1080/14734220510007914. doi: 10.1080/14734220510007914. [DOI] [PubMed] [Google Scholar]
- 108.Pulst SM. Spinocerebellar ataxia type 13. GeneReviews(®) [Internet] 1993 [cited 2016 Mar 30] Available from: http://www.ncbi.nlm.nih.gov/books/NBK1225/
- 109.Park H, Kim H-J, Jeon BS. Parkinsonism in Spinocerebellar ataxia. BioMed Res Int. 2015;2015:1–11. doi: 10.1155/2015/125273. doi: 10.1155/2015/125273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedreich ataxia [Internet] Genetics Home Reference. 2016 [cited 2016 Mar 30] doi: https://ghr.nlm.nih.gov/condition/friedreich-ataxia. [Google Scholar]
- 111.Gatti R. Ataxia-telangiectasia. GeneReviews. 1993 [Internet] [cited 2016 Mar 30] Available from: http://www.ncbi.nlm.nih.gov/books/NBK26468/