Abstract
The aim of this study was to investigate if chemically produced nanotopography on titanium (Ti) surface induces osteoblast differentiation of cultured human bone marrow mesenchymal stem cells (hMSCs) by regulating the expression of microRNAs (miRs). It was demonstrated that Ti with nanotopography induces osteoblast differentiation of hMSCs as evidenced by upregulation of osteoblast specific markers compared with untreated (control) Ti at day 4. At this time-point, miR-sequencing analysis revealed that 20 miRs were upregulated (>2 fold) while 20 miRs were downregulated (>3 fold) in hMSCs grown on Ti with nanotopography compared with control Ti. Three miRs, namely miR-4448, -4708 and -4773, which were significantly downregulated (>5 fold) by Ti with nanotopography affect osteoblast differentiation of hMSCs. These miRs that directly target SMAD1 and SMAD4, both key transducers of the bone morphogenetic protein 2 (BMP-2) osteogenic signal, were upregulated by Ti with nanotopography. Overexpression of miR-4448, -4708 and 4773 in MC3T3-E1 pre-osteoblasts noticeably inhibited gene and protein expression of SMAD1 and SMAD4 and therefore repressed the gene expression of key bone markers. Additionally, it was observed that the treatment with BMP-2 displayed a higher osteogenic effect on MC3T3-E1 cells grown on Ti with nanotopography compared with control Ti, suggesting that the BMP-2 signaling pathway was more effective on this surface. Taken together, these results indicate that a complex regulatory network involving a miR-SMAD-BMP-2 circuit governs the osteoblast differentiation induced by Ti with nanotopography.
Keywords: Mesenchymal stem cells, microRNA, Nanotopography, Osteoblast, SMAD, Titanium
Introduction
One of the challenges of bone/implant interface research is to achieve improved and high quality physiological osseointegration, mostly in critical bone sites. In this scenario, nanotechnology arises as a powerful tool, which may regulate the osseointegration phenomenon by affecting the osteoblast activity on titanium (Ti) implant surfaces (Zuo et al., 2013; Wazen et al., 2013; Ballo et al., 2011; Bueno et al., 2011). Several studies have shown that nanostructured surfaces produced by different treatments and exhibiting distinct patterns of topography may modulate osteoblast responses from cell attachment to extracellular matrix mineralization (Hori et al., 2011; Oh et al., 2009; Vetrone et al., 2011; de Oliveira et al., 2007).
A controlled chemical oxidation of Ti surfaces using a mixture of H2SO4/H2O2 creates a well-characterized nanotopography in terms of physical structure and surface chemistry (Variola et al., 2008; Yi et al., 2006). Compared with an untreated Ti surface, this nanotopography exhibits a three-fold increase in the surface roughness due to the presence of nanopits with an average size of 22 nm, an increase in the TiO2 layer thickness, and low rates of contaminants such as N and Si (Yi et al., 2006). Studies from our research group revealed that the Ti with nanotopography enhances the osteoblast differentiation of cells grown under both osteogenic and non-osteogenic conditions (Rosa et al., 2014; de Oliveira et al., 2007; de Oliveira and Nanci, 2004). Considering these results, it is of relevance to investigate the intracellular mechanisms involved in the osteogenic potential of this surface. Recently, we have shown the striking role of α1β1 integrin signaling pathway in the osteoinductive effect of Ti with nanotopography (Rosa et al., 2014). As osteogenesis is regulated by a plethora of signals, other pathways may participate in the Ti with nanotopography-mediated osseointegration.
Among the pathways involved in the osteogenesis modulation, microRNAs (miRs) have recently emerged as one of the most important post-transcriptional mechanisms (Eguchi et al., 2013; Lian et al., 2012; Hassan et al., 2010). miRs represent a class of small functional noncoding RNAs that regulate the process of protein translation in higher organisms (Duchaine et al., 2006; Bartel, 2004). Some studies have shown the modulation of both osteoblast-related gene expression and skeletogenesis by miR-mediated mechanisms (Eguchi et al., 2013; Shi et al., 2013; Wei et al., 2012; Li et al., 2008). It has been demonstrated that the miR cluster 23a~27a~24-2 inhibits, while miR-30d and -218 stimulate osteoblast differentiation (Eguchi et al., 2013; Hassan et al., 2012). Also, miR-140 contributes to endochondral ossification and transgenic mice expressing miR-206 in osteoblasts exhibit low bone mass (Nakamura et al., 2011; Inose et al., 2009). As several miRs are involved in the control of bone formation and homeostasis (Lian et al., 2012), we hypothesized that they also regulate the process of Ti osseointegration.
Few studies have indeed evaluated miR expression in osteoblasts grown on Ti surfaces subjected to distinct treatments such as sand-blasted, large grit, acid etching and anatase coating (Chakravorty et al., 2012; Palmieri et al., 2008a; Palmieri et al., 2008b; Palmieri et al., 2007). Thus, going deep into the investigation that first showed the involvement of α1β1 integrin signaling pathway in the osteogenic potential of Ti with nanotopography (Rosa et al., 2014), here, we have demonstrated that this surface stimulates osteoblast differentiation by down-regulating the expression of miR-4448, -4708 and -4773, which inhibit SMAD1 and SMAD4, both transducers of bone morphogenetic protein 2 (BMP-2) osteogenic signal (Beederman et al., 2013; Ryoo et al., 2006).
Materials and Methods
Ti surface preparation
Discs of commercially pure grade 2 Ti (12 mm in diameter and 1.5 mm thick) were polished using 320 and 600 grit silicon carbide, cleaned by sonication and rinsed with toluene. Samples were treated with a blend of 10 N H2SO4 and 30% aqueous H2O2 (1:1 v/v) for 4 h at room temperature under continuous agitation to produce the surface nanotopography (Yi et al., 2006). Treated and untreated (control) Ti discs were rinsed with deionized H2O several times, sterilized and air-dried. The surfaces were examined using a field emission scanning electron microscope (Inspect S50, FEI, Hillsboro, OR, USA) operated at 5 kV.
Cell culture
Human mesenchymal stem cells (hMSCs) were obtained from bone marrow of four donors (two female – 56- and 62-year-old and two male – 54- and 60-year-old) following the research protocols approved by the Committee of Ethics in Research from the University of São Paulo. Cells were cultured in growth medium (α-MEM - alpha-minimum essential medium, Invitrogen-Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal calf serum (Gibco-Life Technologies), 50 μg/ml gentamicin (Gibco-Life Technologies) and 0.3 μg/ml fungisone (Gibco-Life Technologies) until subconfluence. First passage cells were cultured in 24-well culture plates on Ti with nanotopography and control Ti discs at a cell density of 2×104 cells per disc in osteogenic medium, which was growth medium supplemented with 5 μg/ml ascorbic acid (Gibco-Life Technologies), 7 mM β-glycerophosphate (Sigma-Aldrich, Saint Louis, MO, USA) and 10−7 M dexamethasone (Sigma-Aldrich) for periods of up to 21 days. Cultures were kept at 37°C in a humidified atmosphere of 5% CO2 and 95% air; the medium was changed 3 times a week.
Gene expression of the key bone markers
Quantitative real-time PCR was carried out at day 4 to evaluate the gene expression of the osteoblast markers runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), osteocalcin (OC) and osteopontin (OPN) in cells grown on Ti with nanotopography and control Ti discs. The total RNA was extracted with Trizol reagent (Invitrogen-Life Technologies) according to the manufacturer’s instructions. The concentration and purity of RNA samples were determined by optical density at 260 and 280 nm, respectively and only samples presenting 260:280 ratios higher than 1.8 were analyzed. Complementary DNA (cDNA) was synthesized using 1 μg of the RNA through a reverse transcription reaction (Kit High Capacity, Invitrogen-Life Technologies). Real-time PCR was carried out in a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Philadelphia, PA, USA) using SybrGreen PCR Master-Mix (Applied Biosystems), specific primers (Invitrogen-Life Technologies - Table S1) designed with Primer Express 2.0 (Applied Biosystems, Foster City, CA, USA) and 12.5 ng of cDNA. The relative gene expression was calculated by ΔCT method and normalized to β-actin to show values of mRNAs, which were plotted relative to control Ti surface.
ALP protein detection
Western blots were carried out at 17 day. Cells grown on Ti with nanotopography and control Ti discs were lysed in 500 μl of lysis buffer containing 1X protease inhibitor mixture (Roche Applied Science, Indianapolis, IN, USA), and 25 μM MG132 proteasome inhibitor (Roche Applied Science) and boiled for 5 min. Equal amount of total protein (20 μg) for each sample was subjected to electrophoresis in a denaturing 8.5% polyacrylamide gel and transferred to a Hybond C-Extra membrane (GE Healthcare Life Science, Piscataway, NJ, USA) using a semidry transfer apparatus (Bio-Rad Laboratories). Membrane was blocked for 1 h in Tris-buffered saline, 0.1% Tween 20 (TBS-T, Sigma-Aldrich) containing 5% nonfat powdered milk (Bio-Rad Laboratories). ALP protein was detected by incubating the membrane with rabbit polyclonal anti-ALP antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by goat anti-rabbit IgG HRP secondary antibody (Santa Cruz Biotechnology). Both primary and secondary antibodies were diluted 1:2000 in TBS-T containing 2.5% nonfat powdered milk (Bio-Rad Laboratories). Mouse monoclonal anti-α-tubulin (1:8000, Sigma-Aldrich) was used as a control followed by goat anti-mouse IgG HRP secondary antibody (1:2000, Santa Cruz Biotechnology). Secondary antibodies were detected using western lightning chemiluminescence reagent (Perkin Elmer Life Sciences, Waltham, MA, USA) and the images were acquired using G-Box gel imaging (Syngene, Cambridge, UK). The ALP expression was normalized by α-tubulin.
Extracellular matrix mineralization
At day 21, cells grown on Ti with nanotopography and control Ti discs were fixed in 10% formalin for 2 h at room temperature, dehydrated and stained with 2% Alizarin Red S (Sigma-Aldrich), pH 4.2, for 10 min. The calcium content was detected using a colorimetric method. Briefly, 280 μl of 10% acetic acid were added to each well and the plate was incubated at room temperature for 30 min under shaking. This solution was vortexed for 1 min, heated to 85°C for 10 min, and transferred to ice for 5 min. The slurry was centrifuged at 13,000 g for 15 min and 100 μl of the supernatant was mixed with 40 μl of 10% ammonium hydroxide and this solution was spectrophotometrically read at 405 nm in the plate reader μQuant (Biotek) and the data were expressed as absorbance.
miR next-generation sequencing on Illumina platforms
Cells grown on Ti with nanotopography and control Ti discs for 4 days were submitted to miR-sequencing performed in the Illumina HiSeq2000 (Illumina, San Diego, CA, USA) using the latest versions of the sequencing reagents and flow cells providing up to 300 Gb of sequence information per flow cell. Briefly, the quality of the total RNA was assessed using the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) followed by a conversion to cDNA. The TruSeq library generation kits were used following the manufacturer’s instructions (Illumina). Library construction consists of random fragmentation of the miR followed by cDNA production using random primers. The ends of the cDNA were repaired, A-tailed and adaptors ligated for indexing (up to 12 different barcodes per lane) during the sequencing runs. The cDNA libraries were quantified using qPCR in a Roche LightCycler 480 with the Kapa Biosystems kit (Kapa Biosystems, Woburn, MA, USA) prior to cluster generation. Clusters were generated to yield approximately 725K-825K clusters/mm2. Cluster density and quality were determined during the run after the parameters of first base addition were assessed. It was run paired end 2×50 bp sequencing runs to align the cDNA sequences to the reference human genome. Before alignment, the data were converted to the FASTQ Sanger format using FASTQ Groomer. TopHat was used to align RNA-Seq reads to the reference genome using the short read aligner Bowtie and to analyze the mapping results to identify splice junctions between exons. Cufflinks used the aligned reads from TopHat to assemble transcripts, to estimate their abundances and to test for differential expression and regulation. Cuffcompare, which is part of Cufflinks, compared the assembled transcripts to a reference annotation and tracked Cufflinks transcripts across multiple experiments. Finally, Cuffdiff indicated significant changes in miR transcript expression. The BAM file generated by TopHat was filtered and the read was paired and mapped. A pileup from this filtered file with calling the consensus according to the MAQ model was created. This pileup was then filtered to report variants as well as to convert coordinates to intervals that were covered by a specified number of reads with bases above a set quality threshold. Gene targets that induce osteoblast differentiation were selected by bioinformatics from the topmost potential targets listed in the Diana microT, TargetScan or MIRANDA. The predicted targets include SMAD1 (miR-4448 and -4708) and SMAD4 (miR-4708 and -4773) (Fig. S1), which were selected for further investigation. To confirm the results of miR-sequencing analysis, the expression of miR-4448, -4708 and -4773 was evaluated using QuantimiR-RT kit (Systems Biosciences-SBI, Mountain View, CA, USA) according to manufacturer’s instructions. The relative expression were calculated by ΔCT method and normalized to U6 to show values of miRs, which were plotted relative to control Ti surface. Also, their putative targets SMAD1 and SMAD4 were evaluated by real-time PCR as described above. Primer sequences (Invitrogen-Life Technologies) are listed in Table S1.
miR transfection
MC3T3-E1 pre-osteoblasts cultured in growth medium, as described above, until 30% to 50% confluence were transfected with 100 nM miR-4448, -4708 and -4773 (Table S2), or non-silencing (NS) miR (Sigma-Aldrich) using RNAiMax (Invitrogen-Life Technologies) following the manufacturer’s instructions and harvested after 48 h for gene and protein expression analyses. The gene expression of SMAD1 and SMAD4 and of the key bone markers, Runx2 and OC, was evaluated as described above. Primer sequences (Invitrogen-Life Technologies) are listed in Table S1. Western blot was carried out using antibodies to detect SMAD1 (1:2000, Santa Cruz Biotechnology) and SMAD4 (1:2000, Santa Cruz Biotechnology) proteins as described above.
BMP-2 treatment
MC3T3-E1 pre-osteoblasts cultured in growth medium on Ti with nanotopography and control Ti discs, as described above, until 30% to 50% confluence, were treated with either vehicle (growth medium) or 100 ng of BMP-2 and harvested after 4 days for gene expression analyses. The gene expression of the key bone markers, Runx2 and OC, was evaluated as described above. Primer sequences (Invitrogen-Life Technologies) are listed in Table S1.
Statistical analysis
The data presented in this work are the representative results of four independent experiments using four sets of hMSC cultures established from four different donors. For each experiment, gene expression was carried out in triplicate (n=3) and extracellular matrix mineralization assay, in quintuplicate (n=5). The data obtained were analyzed by Mann-Whitney U-test, to compare either Ti with nanotopography with control Ti, each miR with NS miR in transfected MC3T3-E1 pre-osteoblasts or BMP-2- with vehicle-treated MC3T3-E1 cells. For all experiments the level of significance was established at p≤0.05.
Results
Ti with nanotopography upregulates osteoblast differentiation markers of hMSCs
The control Ti showed a smooth surface while the controlled chemical oxidation using a mixture of H2SO4/H2O2 resulted in a Ti surface with nanotopography exhibiting a network of nanopits (Fig. 1A and B). The gene expression of Runx2 (3.0-fold), ALP (1.7-fold), OC (28.8-fold), OPN (3.5-fold) (Fig. 2A) and BMP-2 (2.9-fold) (Fig. S2A) was higher (p<0.001) on Ti with nanotopography compared with control Ti at day 4 (Fig. 2A). Western blot analysis revealed that the amount of ALP protein was higher (2.2-fold) at day 17 on Ti with nanotopography compared with control Ti (Fig. 2B). At day 21, extracellular matrix mineralization was detected on Ti with nanotopography and control Ti but the amount of calcium was not statistically significant between both surfaces (p>0.05) (Fig. 2C).
Fig. 1.
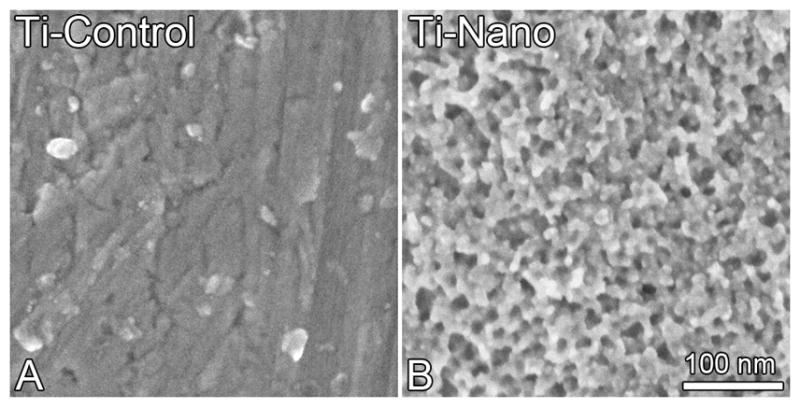
High resolution scanning electron micrographs of control (A) and nanotopography (B) Ti surfaces. Control Ti (A) presents a smooth surface while Ti with nanotopography (B) exhibits a network of nanopits.
Fig. 2.
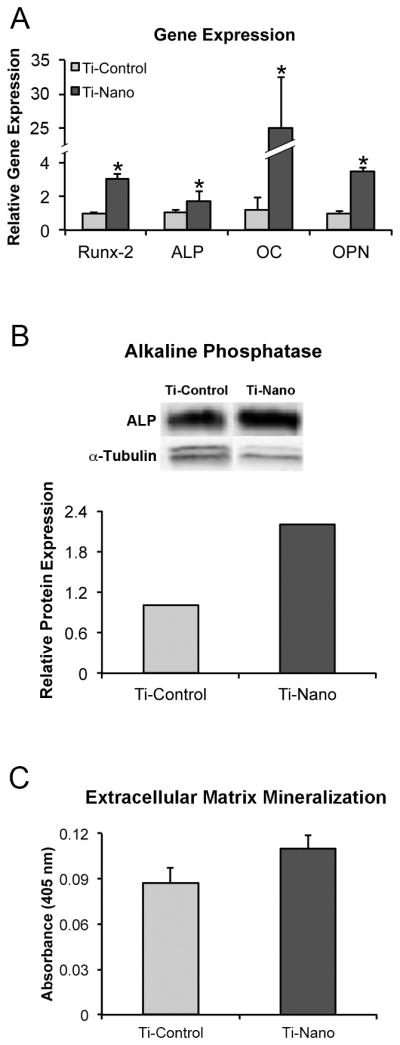
Gene expression of runt-related transcription factor 2, alkaline phosphatase, osteocalcin and osteopontin at day 4 (A), alkaline phosphatase protein expression at day 17 (B), and extracellular matrix mineralization at day 21 (C) of hMSCs differentiated into osteoblasts and cultured on nanotopography and control Ti surfaces. The data are presented as mean ± standard deviation (n=3 for gene expression and n=5 for extracellular matrix mineralization). * Indicates statistically significant difference (p≤0.05).
Ti with nanotopography directs osteoblast differentiation of hMSCs through a miR-SMAD-BMP-2 circuit regulation
In two separate experiments under the same conditions, a total of 40 miRs displayed significant changes in response to Ti with nanotopography compared with control Ti at day 4. Twenty miRs were upregulated (>2 fold) while 20 miRs were downregulated (>3 fold) by Ti with nanotopography (Table S3). Bioinformatic data pointed out three miRs, miR-4448, -4708 and -4773, which were significantly downregulated (>5 fold) by Ti with nanotopography, that affected osteoblast differentiation of hMSCs. These miR downregulations, miR-4448 (1.4-fold), -4708 (1.4-fold) and -4773 (1.8-fold), were confirmed by real-time PCR analysis (p<0.001) (Fig. 3A). Differences in fold change between both methods could be due to the fact that while miR-sequencing analysis quantifies primary miR, pre-miR and mature miR, real-time PCR quantifies only mature miR. The predicted targets of these miRs, SMAD1 (miR-4448 and -4708) and SMAD4 (miR-4708 and -4773), were upregulated (1.5- and 1.7-fold, respectively) by Ti with nanotopography (p<0.001) (Fig. 3B).
Fig. 3.
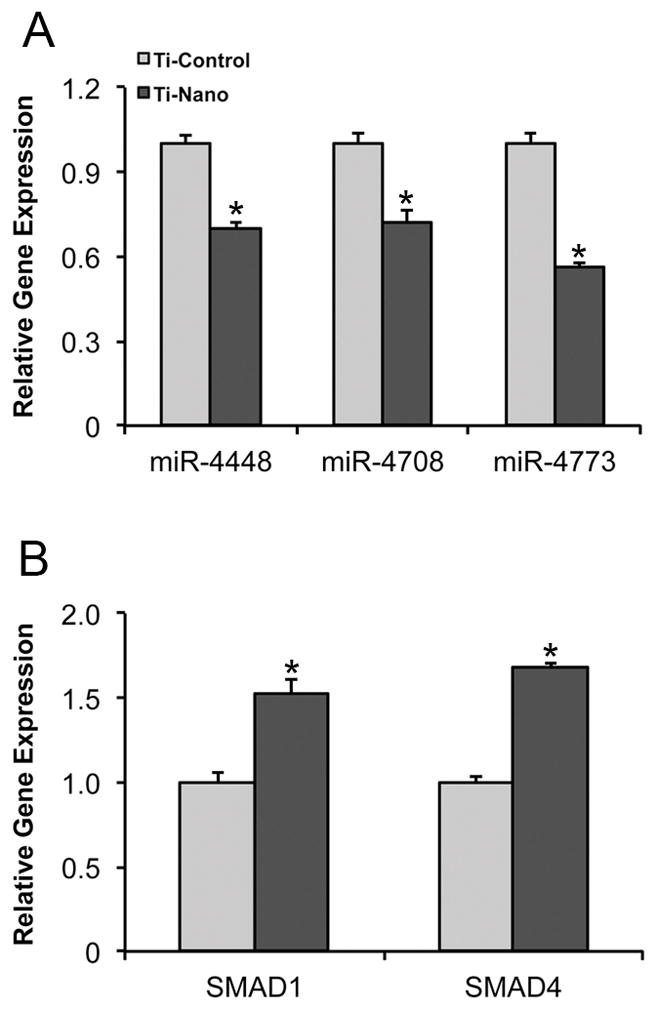
Gene expression of miR-4448, -4708 and -4773 (A), and SMAD1 and SMAD4 (B) of hMSCs differentiated into osteoblasts and cultured on nanotopography and control Ti surfaces at day 4. The data are presented as mean ± standard deviation (n=3). * Indicates statistically significant difference (p≤0.05).
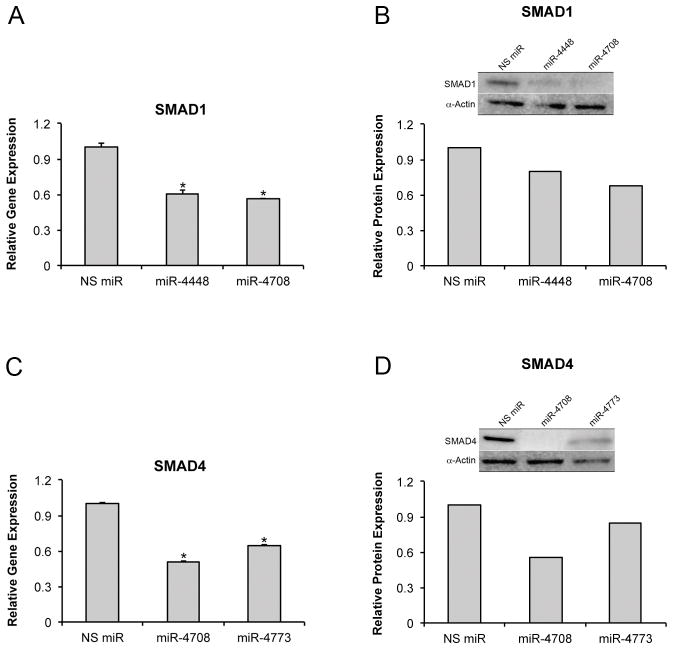
Overexpression of miR-4448 and -4708 inhibited gene (1.7- and 1.8-fold, respectively; p<0.001) and protein (1.3- and 1.4-fold, respectively) expression of SMAD1 (Fig. 4A and B), and miR-4708 and -4773 inhibited gene (2.0- and 1.6-fold, respectively; p<0.001) and protein (1.8- and 1.3-fold, respectively) expression of SMAD4 (Fig. 4C and D) in MC3T3-E1 pre-osteoblasts. By targeting SMAD1 and SMAD4, miR-4448, -4708 and -4773 downregulated the gene expression of the key bone markers Runx2 (1.6-, 1.2- and 1.2-fold, respectively; p<0.001) (Fig. 5A), OC (3.2-, 1.6- and 1.7-fold, respectively; p<0.001) (Fig. 5B) and BMP-2 (1.4-, 2.0- and 1.6-fold, respectively; p<0.001) (Fig. S2B).
Fig. 4.
Gene and protein expression of SMAD1 (A, B) and SMAD4 (C, D) of MC3T3-E1 pre-osteoblasts transfected with either miR-4448, -4708 or NS miR (A, B) and with either miR-4708, -4773 or NS miR (C, D) 48 h post-transfection. The data of gene expression are presented as mean ± standard deviation (n=3). * Indicates statistically significant difference (p≤0.05). NS miR: non-silencing miR.
Fig. 5.
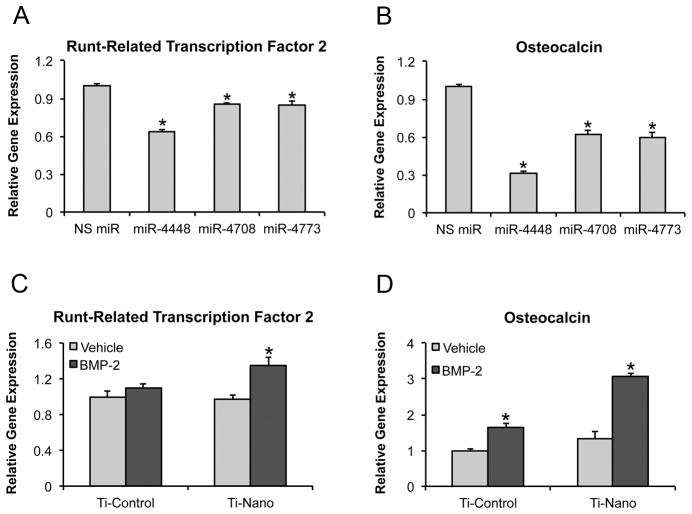
Gene expression of runt-related transcription factor 2 (A) and osteocalcin (B) of MC3T3-E1 pre-osteoblasts transfected with either miR-4448, -4708, -4773 or NS miR 48 h post-transfection. Gene expression of runt-related transcription factor 2 (C) and osteocalcin (D) of MC3T3-E1 pre-osteoblasts treated with either 100 ng of bone morphogenetic protein 2 or vehicle and cultured on nanotopography and control Ti surfaces at day 4. The data are presented as mean ± standard deviation (n=3). * Indicates statistically significant difference (p≤0.05). NS miR: non-silencing miR.
Treatment of MC3T3-E1 pre-osteoblasts with BMP-2 upregulated the gene expression of Runx2 (Fig. 5C) and OC (Fig. 5D) on Ti with nanotopography (p<0.001) and OC (p<0.001) on control Ti (Fig. 5D). While BMP-2 treatment increased 1.1- and1.6-fold the gene expression of Runx2 and OC on control Ti, the same treatment increased 1.4- and 2.3-fold the gene expression of Runx2 and OC on Ti with nanotopography (Fig. 5C and D).
Discussion
Our results have shown that Ti with nanopotography favors osteoblast differentiation of hMSCs as evidenced by an increased gene and protein expression of key bone markers at a specific time-point. It was also noted the differential expression of several miRs in cells grown on both surfaces. Specifically, Ti with nanotopography significantly inhibited the expression of 3 miRs, miR-4448, -4708 and -4773, that directly inhibit the expression of SMAD1 and SMAD4, two key transducers of the BMP-2 signaling pathway. Thus, we propose that one mechanism by which Ti with nanotopography induces osteoblast differentiation involves a regulatory network mediated by miR-4448, -4708, and -4773 downregulation that attenuates SMAD1 and SMAD4 degradation and consequently intensifies BMP-2 signal transduction, which was confirmed by cell responses to BMP-2 treatment.
In order to track the culture progression through the osteoblast differentiation the gene expression of some key bone markers (Runx2, ALP, OC and OPN), ALP protein expression, and the extracellular matrix mineralization were assayed (Beck Jr et al., 1998; Stein et al., 1996). The results showed that all osteoblast marker genes were upregulated in cells grown on Ti with nanotopography. Corroborating this finding, a nanotopography produced by aluminium oxide nanocoating also increases the gene expression of a panel representative of the osteoblast differentiation in hMSCs (Mendonça et al., 2009). In addition, the high ALP protein expression did not lead to a higher extracellular matrix mineralization on the Ti with nanotopography, matching our previous study using rat MSCs cells grown on the same substrate (Rosa et al., 2014).
During osteoblast differentiation of hMSCs, the miR sequencing analysis revealed that 20 miRs were upregulated while 20 miRs were downregulated by Ti with nanotopography. Agreeing with these findings, it has been shown that Ti surfaces modulate miR expression (Palmieri et al., 2008a; Palmieri et al., 2008b; Palmieri et al., 2007). However, those studies did not explore the mechanisms by which miRs regulate the osteogenic potential of Ti surfaces. Recently, it was demonstrated that large-grit acid-etched Ti surfaces downregulate miRs (e.g.: miR-146a and -155), which are predicted to target TGF-β/BMP signaling transducers, but these targets were not experimentally confirmed (Chakravorty et al., 2012). Thus, up to now, the involvement of miRs in the cell-Ti surface interactions is poorly understood. In this context, we are presenting novel data on 3 miRs, miR-4448, -4708 and -4773, which were significantly downregulated (>5 fold) by Ti with nanotopography and target SMAD1 and SMAD4 proteins, two key transducers of the BMP-2 signaling pathway (Beederman et al., 2013; Ryoo et al., 2006). We have shown that the overexpression of these miRs in MC3T3-E1 pre-osteoblasts noticeably inhibited gene and protein expression of SMAD1 and SMAD4, which attenuates BMP-2 signal transduction, leading to an inhibition of the gene expression of the key bone markers Runx2 and OC. In addition, MC3T3-E1 cells cultured on Ti with nanotopography displayed an enhanced response to BMP-2 treatment compared with control Ti, suggesting that the BMP-2 signaling pathway was more effective on this surface.
Some studies have presented evidences of collaboration between integrin and BMP-2 signaling to induce osteoblast differentiation (Mai et al., 2013; Lai and Cheng, 2005). The inhibition of β1 integrin attenuates both synthesis and secretion of BMP-2 as well as repression of BMP-2 dowregulates β1 integrin gene expression in MC3T3-E1 pre-osteoblasts (Mai et al., 2013). In addition, BMP-2 fails to induce osteoblast differentiation of cells derived from human trabecular bone in presence of a function-blocking antibody against αv integrin subunit, suggesting that αv-containing integrins are critical for the BMP-2 osteoinductive effect (Lai and Cheng, 2005). In a recent study, we have demonstrated the crucial role of α1β1 integrin (Rosa et al., 2014) and here it was identified a miR-SMAD-BMP-2 regulatory network, both involved in the Ti with nanotopography-mediated osteoblast differentiation. Taken together, these results suggest that the osteoblast differentiation induced by Ti with nanotopography is governed by a complex mechanism based on a crosstalk between α1β1 integrin and BMP-2 signaling pathways with participation of miRs.
In conclusion, we have shown that the nanotopography generated by Ti surface oxidation using a mixture of H2SO4/H2O2 directs hMSCs differentiation toward the osteoblast lineage. In addition, we have identified 3 miRs that target SMADs and play critical roles in the osteoinductive effect of this Ti with nanotopography on hMSCs. By downregulating miR-4448, -4708, and 4773, Ti with nanotopography attenuates SMAD1 and SMAD4 degradation, intensifying BMP-2 signal transduction, which stimulates osteoblast differentiation. This novel mechanism involving a miR-SMAD-BMP-2 circuit in the Ti with nanotopography-mediated osteoblast differentiation and the potential crosstalk with α1β1 integrin signaling pathway open new windows for developing Ti surface modifications to fine-tune control of the process of osseointegration.
Supplementary Material
Schematic illustration of the 3′UTR of SMAD1 and SMAD4 with target sequences of miR-4448, -4708 and -4773. The putative target sites of SMAD1 (miR-4448 and -4708) and SMAD4 (miR-4708 and -4773) were predicted in the Diana microT, TargetScan or MIRANDA software.
Gene expression of bone morphogenetic protein 2 of hMSCs differentiated into osteoblasts and cultured on nanotopography and control Ti surfaces at day 4 (A) and of MC3T3-E1 pre-osteoblasts transfected with either miR-4448, -4708, -4773 or NS miR 48 h post-transfection (B). The data are presented as mean ± standard deviation (n=3). * Indicates statistically significant difference (p≤0.05). NS miR: non-silencing miR.
Acknowledgments
Contract grant sponsors: State of São Paulo Research Foundation (FAPESP), Brazil and National Council for Scientific and Technological Development (CNPq, Brazil)
Contract grant numbers: FAPESP # 2010/18395-3, 2010/19280-5, 2012/01291-6 and CNPq # 301023/2010-7
Title- Dental Academic Research Training Program
Grant ID- 5T90DE022736
Institute-NIDCR
We would like to thank Roger R. Fernandes and Milla S. Tavares for the assistance they provided during the cell culture experiments and Lubana K. Afreen for critical comments on the manuscript.
Footnotes
Disclosures
The authors declare that they have no conflict of interest.
Literature Cited
- Ballo A, Agheli H, Lausmaa J, Thomsen P, Petronis S. Nanostructured model implants for in vivo studies: influence of well-defined nanotopography on de novo bone formation on titanium implants. Int J Nanomedicine. 2011;6:3415–3428. doi: 10.2147/IJN.S25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beck GR, Jr, Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J Cell Biochem. 1998;68:269–280. doi: 10.1002/(sici)1097-4644(19980201)68:2<269::aid-jcb13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, Li R, Shui W, Zhang H, Kim S, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers M, Pratt A, Haydon R, Luu H, Angeles J, Shi L, He T. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6:32–52. doi: 10.4236/jbise.2013.68A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno RB, Adachi P, Castro-Raucci LM, Rosa AL, Nanci A, de Oliveira PT. Oxidative nanopatterning of titanium surfaces promotes production and extracellular accumulation of osteopontin. Braz Dent J. 2011;22:179–184. doi: 10.1590/s0103-64402011000300001. [DOI] [PubMed] [Google Scholar]
- Chakravorty N, Ivanovski S, Prasadam I, Crawford R, Oloyede A, Xiao Y. The microRNA expression signature on modified titanium implant surfaces influences genetic mechanisms leading to osteogenic differentiation. Acta Biomater. 2012;8:3516–3523. doi: 10.1016/j.actbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A. 2007;80:554–564. doi: 10.1002/jbm.a.30955. [DOI] [PubMed] [Google Scholar]
- de Oliveira PT, Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials. 2004;25:403–413. doi: 10.1016/s0142-9612(03)00539-8. [DOI] [PubMed] [Google Scholar]
- Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Jr, Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, Yates JR, 3rd, Mello CC. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One. 2013;8:e58796. doi: 10.1371/journal.pone.0058796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287:42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ, Gordon JA, Beloti MM, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci USA. 2010;107:19879–19884. doi: 10.1073/pnas.1007698107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori N, Iwasa F, Tsukimura N, Sugita Y, Ueno T, Kojima N, Ogawa T. Effects of UV photofunctionalization on the nanotopography enhanced initial bioactivity of titanium. Acta Biomater. 2011;7:3679–3691. doi: 10.1016/j.actbio.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, Takeda S. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci U S A. 2009;106:20794–20799. doi: 10.1073/pnas.0909311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CF, Cheng SL. Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Miner Res. 2005;20:330–340. doi: 10.1359/JBMR.041013. [DOI] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai Z, Peng Z, Wu S, Zhang J, Chen L, Liang H, Bai D, Yan G, Ai H. Single bout short duration fluid shear stress induces osteogenic differentiation of MC3T3-E1 cells via integrin β1 and BMP2 signaling cross-talk. PLoS One. 2013;8:e61600. doi: 10.1371/journal.pone.0061600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça G, Mendonça DB, Simões LG, Araújo AL, Leite ER, Duarte WR, Aragão FJ, Cooper LF. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials. 2009;30:4053–4062. doi: 10.1016/j.biomaterials.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Inloes JB, Katagiri T, Kobayashi T. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol Cell Biol. 2011;31:3019–3028. doi: 10.1128/MCB.05178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri A, Pezzetti F, Brunelli G, Lo Muzio L, Scarano A, Scapoli L, Martinelli M, Arlotti M, Guerzoni L, Rubini C, Carinci F. Short-period effects of zirconia and titanium on osteoblast microRNAs. Clin Implant Dent Relat Res. 2008b;10:200–205. doi: 10.1111/j.1708-8208.2007.00078.x. [DOI] [PubMed] [Google Scholar]
- Palmieri A, Pezzetti F, Avantaggiato A, Lo Muzio L, Scarano A, Rubini C, Guerzoni L, Arlotti M, Ventorre D, Carinci F. Titanium acts on osteoblast translational process. J Oral Implantol. 2008a;34:190–195. doi: 10.1563/0.869.1. [DOI] [PubMed] [Google Scholar]
- Palmieri A, Brunelli G, Guerzoni L, Lo Muzio L, Scarano A, Rubini C, Scapoli L, Martinelli M, Pezzetti F, Carinci F. Comparison between titanium and anatase miRNAs regulation. Nanomedicine. 2007;3:138–143. doi: 10.1016/j.nano.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Rosa AL, Kato RB, Castro Raucci LMS, Teixeira LN, de Oliveira FS, Bellesini LS, de Oliveira PT, Hassan MQ, Beloti MM. Nanotopography drives stem cell fate toward osteoblast differentiation through α1β1 integrin signaling pathway. J Cell Biochem. 2014;115:540–548. doi: 10.1002/jcb.24688. [DOI] [PubMed] [Google Scholar]
- Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–57. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B, Li H, Ma C. MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone. 2013;55:487–494. doi: 10.1016/j.bone.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, Van Wijnen AJ, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996;76:593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- Variola F, Yi J-H, Richert L, Wuest JD, Rosei F, Nanci A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials. 2008;29:1285–1298. doi: 10.1016/j.biomaterials.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Vetrone F, Variola F, de Oliveira PT, Zalzal SF, Yi JH, Sam J, Bombonato-Prado KF, Sarkissian A, Perepichka DF, Wuest JD, Rosei F, Nanci A. Nanoscale oxidative patterning of metallic surfaces to modulate cell activity and fate. Nano Lett. 2009;9:659–665. doi: 10.1021/nl803051f. [DOI] [PubMed] [Google Scholar]
- Wazen RM, Kuroda S, Nishio C, Sellin K, Brunski JB, Nanci A. Gene expression profiling and histomorphometric analyses of the early bone healing response around nanotextured implants. Nanomedicine. 2013;8:1385–1395. doi: 10.2217/nnm.12.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Shi Y, Zheng L, Zhou B, Inose H, Wang J, Guo XE, Grosschedl R, Karsenty G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol. 197:509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J-H, Bernard C, Variola F, Zalzal SF, Wuest JD, Rosei F, Nanci A. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surf Sci. 2006;600:4613–4621. [Google Scholar]
- Zuo J, Huang X, Zhong X, Zhu B, Sun Q, Jin C, Quan H, Tang Z, Chen W. A comparative study of the influence of three pure titanium plates with different micro- and nanotopographic surfaces on preosteoblast behaviors. J Biomed Mater Res A. 2013;101:3278–3284. doi: 10.1002/jbm.a.34612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic illustration of the 3′UTR of SMAD1 and SMAD4 with target sequences of miR-4448, -4708 and -4773. The putative target sites of SMAD1 (miR-4448 and -4708) and SMAD4 (miR-4708 and -4773) were predicted in the Diana microT, TargetScan or MIRANDA software.
Gene expression of bone morphogenetic protein 2 of hMSCs differentiated into osteoblasts and cultured on nanotopography and control Ti surfaces at day 4 (A) and of MC3T3-E1 pre-osteoblasts transfected with either miR-4448, -4708, -4773 or NS miR 48 h post-transfection (B). The data are presented as mean ± standard deviation (n=3). * Indicates statistically significant difference (p≤0.05). NS miR: non-silencing miR.