Summary
Circulating liver enzymes such as alanine transaminase are often used as markers of hepatocellular damage. Ischaemia/reperfusion (I/R) injury is an inevitable consequence of prolonged liver ischaemia. The aim of this study was to examine the correlation between liver enzymes and volume of liver cell necrosis after ischaemia/reperfusion injuries, using design‐unbiased stereological methods. Forty‐seven male Wistar rats were subjected to 1 h of partial liver ischaemia, followed by either 4 or 24 h of reperfusion. Within each group, one‐third of animals were subjected to ischaemic preconditioning and one‐third to ischaemic postconditioning. At the end of reperfusion, blood and liver samples were collected for analysis. The volume of necrotic liver tissue was subsequently correlated to circulating markers of I/R injury. Correlation between histological findings and circulating markers was performed using Pearson's correlation coefficient. Alanine transferase peaked after 4 h of reperfusion; however, at this time‐point, only mild necrosis was observed, with a Pearson's correlation coefficient of 0.663 (P = 0.001). After 24 h of reperfusion, alanine aminotransferase was found to be highly correlated to the degree of hepatocellular necrosis R = 0.836 (P = 0.000). Furthermore, alkaline phosphatase (R = 0.806) and α‐2‐macroglobulin (R = 0.655) levels were also correlated with the degree of necrosis. We show for the first time that there is a close correlation between the volume of hepatocellular necrosis and alanine aminotransferase levels in a model of I/R injury. This is especially apparent after 24 h of reperfusion. Similarly, increased levels of alkaline phosphatase and α‐2‐macroglobulin are correlated to the volume of liver necrosis.
Keywords: experimental animal study, hepatic necrosis, HPB surgery, ischaemia/reperfusion injuries, liver
Hepatocyte necrosis may result from a wide range of injuries, including alcohol and other toxins, hepatitis, genetic defects, autoimmune disorders and as a consequence of ischaemia/reperfusion (I/R). Liver transaminases, especially alanine amino transferase (ALT), are widely used as surrogate markers of liver cell damage (Schmidt & Schmidt 1993). ALT is synthesized in liver cells, and serum levels are usually low. However, when hepatocytes are damaged (by whatever cause), their membranes become more permeable, or may even be destroyed, allowing their enzymes to leak into the blood (Scheig 1996). In daily clinical practice, it is widely accepted that the serum levels of ALT and other transaminases are correlated to the extent of hepatocyte damage in the liver and, thus, may be used as quantitative markers (Scheig 1996; FDA, 2009). Histopathological evaluation is the reference against which surrogate markers should be compared. However, this has been made difficult by the lack of studies providing quantitative unbiased data. Thus, most published works investigating liver cell damage have used standard pathological techniques with, at best, semi‐quantitative methods of evaluation (Suzuki et al. 1991, 1993; Franco‐Gou et al. 2004; Tapuria, et al., 2009). These methods are highly investigator‐dependent and include no component of randomization. Furthermore, they do not produce quantitative data, making it impossible to perform statistical comparisons. These potential errors can be avoided by applying stereological methods to sample and quantify histological sections in a design‐unbiased randomized manner (Nyengaard 1999; Muhlfeld et al. 2010).
The aim of this study was to use design‐unbiased stereological methods in a rat model and to correlate potential surrogate circulating markers of hepatocyte damage with the volume of liver cell necrosis following I/R injury.
Methods
Experimental design
Liver I/R injuries were induced by a standard rat model of 70% liver ischaemia (Knudsen et al. 2013). All animals were subjected to 60 min of continuous inflow occlusion. I/R injuries were evaluated after 4 and 24 h of reperfusion as we previously have found ALT to peak within this time interval (Knudsen et al. 2013). To induce varying degrees of I/R injury, some of the rats were subjected to ischaemic conditioning by either IPC (preconditioning) or IPO (postconditioning), as described below. We have previously shown that these methods reduce liver cell necrosis following I/R (Knudsen et al. 2012, 2013).
Ethical approval
The surgical and experimental protocols were approved by the Danish Research Animal Committee, Copenhagen, Denmark, according to license number 2009/561‐1644, and followed the rules of Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health.
Animals and surgical procedures
Forty‐seven male Wistar rats, weighing mean 299 g (range 256–345 g; M&B Taconic, Eiby, Denmark), were used for the experiment. Animals were double housed in standard animal laboratories with the temperature maintained at 23°C and artificial 12‐h light–dark cycles, fed standard food (Altromin) with free access to water. Inhalation anaesthesia was used during the surgical procedures. Induction was performed with animals in an airtight glass cage through which a mixture of oxygen (2.0 l/min) and N2O (0.5 l/min) containing 4% isoflurane (Forene, Abbott Laboratories, Maidenhead, UK) was blown. During surgery, anaesthesia was maintained with 2% isoflurane in oxygen and N2O as above, given through a mask covering the nose of the rat. Rats were placed in a supine position on temperature‐controlled heated pads, maintaining core temperature at 37°C. A model of partial hepatic ischaemia was used to avoid mesenteric congestion by allowing blood flow through the right liver lobe (Hart et al. 2008).
The surgical procedure was performed as follows. After a midline laparotomy, resection of all hepatic ligaments was performed to ensure the abruption of collateral blood supply. Portal triad clamping was performed under a dissecting microscope. Briefly, the bifurcation of the right lobe was identified, after which a microvascular clamp was put in place, interrupting portal triad flow through the median and left lobes. Discoloration of the liver was used as a positive marker for hepatic ischaemia. During the 60 min of ischaemia, the abdomen was temporary closed to avoid major fluid loss. Furthermore, all animals were injected with 1.5 ml isotonic saline and 1 mg Carprofen (Rimadyl, Pfizer Animal Health, Exton, PA, USA). Reperfusion was ascertained by the return of the normal reddish colour of the liver. The abdomen was closed in two layers, single knots. After surgery, rats were allowed to recover for either 4 (n = 23) or 24 h (n = 24). At the end of the reperfusion period, rats were re‐anesthetized as described above. The abdomen and the thoracic cavity were opened, and a cardiac cannulation was performed. This was used to collect blood samples, after which an intravenous pentobarbital overdose was given to euthanize the animals. Rats were then perfused for 10 min with phosphate‐buffered 4% formaldehyde via a cardiac cannula to ensure rapid fixation of the liver. The left part of the median lobe was then resected and fixed for a further 24 h, after which the lobe was paraffin embedded.
Ischaemic conditioning protocol
IPC consisted of 10 min of liver ischaemia followed by 10 min of reperfusion, before a final period of 60 min of prolonged liver ischaemia (n = 16). IPO consisted of three cycles of 30 s of reperfusion and 30 s of ischaemia and applied immediately after the 60 min of total liver ischaemia (n = 16) (Knudsen et al. 2013). The remaining 17 animals were not subjected to any form of conditioning.
Blood samples
ALT, alkaline phosphatase (AP) and bilirubin measurements were performed on the Vitros 5.1 (Ortho Clinical Diagnostics, Johnson and Johnson, Birkerød, Denmark) for routine clinical biochemical measurement, using the dry slide technology.
The rat acute phase protein α‐2‐macroglobulin was evaluated using a specific ELISA kit (Immunology Consultants Laboratory, Newberg, OR, USA). Samples were assayed in duplicate. All assays exhibited intra‐ and interassay coefficients of variance below 5% and 10% respectively.
Stereological sampling
From the paraffin embedded left median lobe a set of systematic, uniformly random sampling (SURS) sections were prepared (Nyengaard 1999). Briefly, the paraffin embedded median left liver lobe was exhaustively sectioned into 2‐μm parallel sections generating approximately 3000 sections. Every 300th section was saved, creating a set of approximately 10 sections per liver. The first section to be saved was decided by choosing a random number between one and 300.
Stereological volume assessment and quantification
The SURS sections were stained with haematoxylin and eosin (H&E) and analysed using a microscope (Olympus BH‐50) modified for stereology with a motorized stage and a digital camera connected to a PC with newCAST 3.6.5.0 software (Visiopharm, Horsholm, Denmark). Volume estimations were performed using the Cavalieri estimator (Gundersen et al. 1988). A point grid was placed randomly over the SURS sections by the software and was used to estimate the area of the entire section and the area of necrotic tissue in the section. Approximately 100 randomly selected non‐overlapping fields of view were used per liver. For a detailed description of the counting rules, see Figure 1. The following formula was used to calculate the volume:
Figure 1.
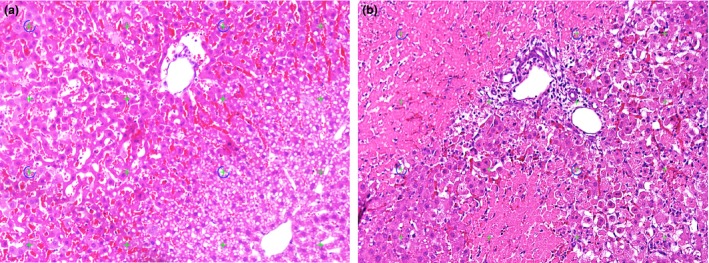
Light microscopic images of haematoxylin and eosin stained liver sections. Necrotic tissue was defined as a lighter stained area with loss of nuclei. (a) 4 h of reperfusion, only a few areas of necrotic tissue are seen, whereas larger areas seem to be at risk. (b) 24 h of reperfusion, large coagulative necrotic areas are observed. Inflammatory cell Infiltration is seen at the border between necrotic and viable liver tissue.
t was the distance between each sampled section (2 μm × 300), was the area covered by a test point, and Pi was the number of points counted. To calculate the volume of the necrotic part of the liver in per cent of total liver volume, the formula below was used. We have previously used NVR (Necrotic volume ratio) to describe the degree of liver necrosis Knudsen et al. (2013). NVR is used in the further analysis below and calculated as:
Statistical analysis
All statistical analyses were performed using SigmaPlot for Windows Version 11.0 (Systat Software Inc., San Jose, CA, USA). Data were tested for a normal distribution. All Group values are given as mean ± CI of the mean. The strength of the correlation between circulating markers and the histological data was tested using Pearson's correlation coefficient. P‐values <0.05 were considered significant.
Results
The histopathological evaluation of liver specimens revealed a low degree of necrosis at 4 h of reperfusion, with a mean NVR of 7%. After 24 h, the level of necrosis had increased markedly to a NVR of 45%, see Figure 1.
Correlations between NVR and surrogate markers of liver ischaemia at both 4 and 24 h of reperfusion are illustrated in Figures 2, 3, 4.
Figure 2.
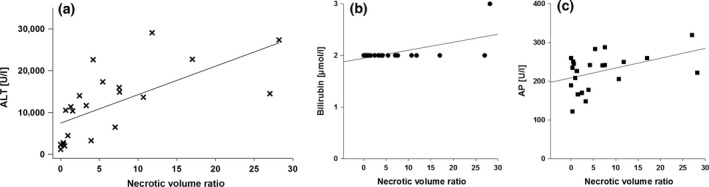
XY plots of surrogate markers of liver cell damage, ALT, AP and bilirubin, and histological findings of liver cell necrosis after 4 h of reperfusion. (a) Pearson's correlation coefficient is r 2 = 0.44 (P = 0.00); (b) the coefficient is r 2 = 0.35; and (c) the coefficient is r2 = 0.19.
Figure 3.
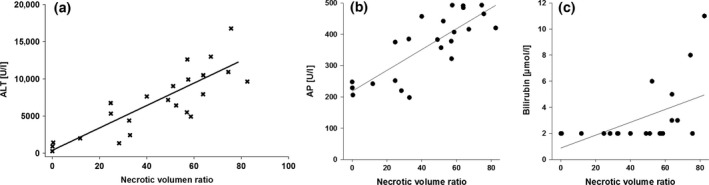
XY plots of surrogate markers of liver cell damage, ALT, AP and bilirubin, and histological findings of liver cell necrosis after 24 h of reperfusion. (a) Pearson's correlation coefficient is r 2 = 0.70 (P = 0.00); (b) the coefficient is r 2 = 0.28; and (c) the coefficient is r 2 = 0.65.
Figure 4.
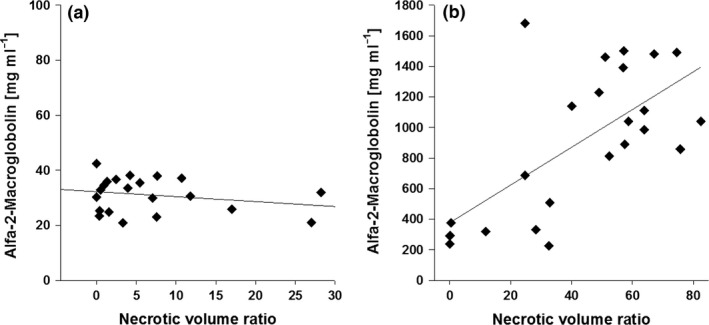
XY plots of inflammatory marker alfa‐2‐macroglobulin and histological findings of liver cell necrosis. (a) Pearson's correlation coefficient is r 2 = 0.06 (P = 0.00); (b) the coefficient is r 2 = 0.43.
ALT and AP were elevated following the ischaemic insult. At 4 h of reperfusion, the mean ALT level was 11.854 U/l (±3720), while AP was 225 U/l (±21). After 24 h, the mean ALT was 6.812 U/l (±1886) and AP was 364 U/l (±44). Mean bilirubin levels were unaffected at both 4 h (2 μM; ±0.1) and 24 h (3 μM; ±1). Acute phase protein α‐2‐macroglobulin levels were slightly elevated at 4 h of reperfusion (31 mg/ml; ±3) and were highly elevated at 24 h (917 mg/ml; ±203), as shown in Figure 4.
Discussion
In this study, on rats, we used unbiased stereological methods for the first time to show that there is a close correlation between the absolute volume of hepatocellular necrosis and the ALT level after I/R injury. This relationship is especially clear after 24 h of reperfusion. Seventy per cent of the variations found in ALT could be explained by necrotic tissue. We also demonstrated that AP and α‐2‐macroglobulin could be used in a similar way as markers of liver necrosis after 24 h of reperfusion.
Damaged or necrotic hepatocytes release their contents, including aminotransferases, into the extracellular space, from where they ultimately enter into the circulation. We found a moderately close correlation comparing ALT levels and the volume of necrotic hepatic tissue at 4 h of reperfusion (correlation coefficient 0.44). After 24 h of reperfusion, we found an even closer correlation between the volume of necrotic hepatic tissue and the serum ALT (correlation coefficient 0.70). As described in the Results section, ALT peaked at 4 h even though only minor volumes of necrotic tissue were observed by histological evaluation. This can be explained by leakage of ALT through the cell membrane of the damaged hepatocytes, before clear morphological evidence of cell damage or death can be observed. As the half‐life of circulating ALT is around 47 h (Scheig 1996), serum levels will decline rapidly once there ceases to be further cellular damage.
Our main result, that is ALT increases with the extent of liver cell necrosis, is in accordance with an earlier study on mice by Fahrner et al. (2014). They investigated the impact of the expression level of natural killer cell tumour necrosis factor‐related apoptosis‐inducing ligand (TRAIL) on hepatic I/R injury after 60 min of left‐sided liver ischaemia. Although not the primary focus of the study, this group's data show that ALT was significantly higher in mice after both six and 24 h of reperfusion in animals with a higher degree of liver cell necrosis, as judged by a semi‐quantitative histological evaluation method.
Hepatocyte necrosis may also result from other causes than I/R injury, including infections, toxins and manipulation during surgery. A positive correlation between HIV viral load and ALT has previously been demonstrated (Mata‐Marin et al. 2009). In that study, the authors did not correlate transaminase levels to histopathology, however, the results were attributed to apoptosis and necrosis induced by viral proteins. Zechini et al. (2004) demonstrated a statistical significant correlation of aminotransferases values with the histological activity index in patients with chronic hepatitis. van den Broek et al. (2013) assessed liver damage in humans after manipulation during surgery and found that the degree of hepatocellular damage judged by a semi‐quantitative histological scoring system was correlated to plasma aminotransferase concentrations. Although the three above‐mentioned studies used indirect or semi‐quantitative techniques to correlate hepatic damage with aminotransferase levels, their findings are in agreement with the present study in which we applied unbiased evaluation methods.
The type of cell death following I/R injuries of the liver has been vigorously studied and debated (Gao et al. 1998; Gujral et al. 2001; Jaeschke & Lemasters 2003). From our previous experience, we have observed that apoptotic cell death following I/R injuries of the liver constitutes only a minor fraction of dying hepatocytes (Knudsen et al. 2013). However, apoptotic cells leaking ALT can explain some of the circulating ALT.
Cholangiocytes constitute about 3% of the cells in the rodent liver (Si‐Tayeb et al. 2010); AP is an enzyme, which in the liver is localized in the biliary tract mucosa (Poupon 2015). After 24 h, we found AP levels to be highly correlated (coefficient 0.65) to the degree of hepatic necrosis. This finding is consistent with release of AP from damaged cholangiocytes. On the other hand, we found no correlation of total bilirubin, which is a breakdown product of haemoglobin, with the degree of hepatic necrosis, whether this was examined after 4 or after 24 h of reperfusion.
α‐2‐macroglobulin is a widely used and well‐described acute phase protein in rats. We found a moderate correlation (coefficient 0.43) between the degree of necrosis and α‐2‐macroglobulin levels after 24 h. As I/R injuries initiate a strong inflammatory response, which results in a major increase in liver synthesis and release of acute phase proteins, this is probably part of the explanation for our findings (Milland et al. 1990; McCurry et al. 1993; Gabay & Kushner 1999). Another source of α‐2‐macroglobulin could be a reaction to the laparotomy performed.
Necrosis of hepatocytes may also result from other causes than I/R injury and manipulation during surgery, for example alcohol, hepatitis and other infections, toxins, genetic defects and autoimmune disorders. Optimal biomarkers of hepatocellular damage should show strong specificity for liver, well‐documented, strong correlation with histopathological findings and should be independent of the cause of injury (Ozer et al. 2010). As discussed, previous histopathological studies evaluating hepatocellular injury have used standard pathological techniques combined with semi‐quantitative scoring systems (Suzuki et al. 1993; Franco‐Gou et al. 2004; Tapuria, et al., 2009). Routine histopathological examination of a limited arbitrary tissue sample has several drawbacks. Firstly, it is prone to subjectivity, being highly dependent on the observer and has no component of unbiased randomization. Secondly, traditional methods of analysis produce at best only semi‐quantitative data, making it more difficult to make statistical comparisons. In addition, semi‐quantitative methods do not allow for the reference area or volume to be precisely estimated and as a result, run the risk of a highly biased conclusion. These pitfalls were avoided in the present study by applying unbiased stereological methods. Using design‐unbiased stereology, it is possible to evaluate other subcategories of liver damage in a quantitative manner, for example estimation of the total number of apoptotic cells, estimation of liver cell regeneration, estimation of microvessel length, estimation of biliary tree length and several other aspects of liver cell damage. Our study could have been improved by including more groups of rats at different time‐points, to get an even better estimate of the correlation between the different liver parameters and the volume of liver cell necrosis.
In conclusion, we used unbiased stereological methods for the first time to demonstrate a strong correlation between the absolute volume of hepatocellular necrosis and serum ALT levels after I/R injury.
Funding
The Danish Cancer Society and the Health Research Fund of Central Denmark Region supported the work. The authors have no conflict of interest.
Author contributions
All listed authors meet ICMJE authorship criteria and that nobody who qualifies for authorship has been excluded.
Acknowledgement
The authors wish to thank laboratory assistant Rikke Andersen for her assistance with the blood plasma analysis. Special thanks goes to laboratory assistant Svetlana Teplaia for her persistent efforts in preparing the histological sections.
References
- van den Broek M.A., Shiri‐Sverdlov R., Schreurs J.J. et al (2013) Liver manipulation during liver surgery in humans is associated with hepatocellular damage and hepatic inflammation. Liver Int. 33, 633–641. [DOI] [PubMed] [Google Scholar]
- Fahrner R., Trochsler M., Corazza N. et al (2014) Tumor necrosis factor‐related apoptosis‐inducing ligand on NK cells protects from hepatic ischemia‐reperfusion injury. Transplantation 97, 1102–1109. [DOI] [PubMed] [Google Scholar]
- FDA (2009) Guidance for Industry: Drug‐Induced Liver Injury: Premarketing Clinical Evaluation.
- Franco‐Gou R., Peralta C., Massip‐Salcedo M., Xaus C., Serafin A. & Rosello‐Catafau J. (2004) Protection of reduced‐size liver for transplantation. Am. J. Transplant. 4, 1408–1420. [DOI] [PubMed] [Google Scholar]
- Gabay C. & Kushner I. (1999) Acute‐phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454. [DOI] [PubMed] [Google Scholar]
- Gao W., Bentley R.C., Madden J.F. & Clavien P.A. (1998) Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology 27, 1652–1660. [DOI] [PubMed] [Google Scholar]
- Gujral J.S., Bucci T.J., Farhood A. & Jaeschke H. (2001) Mechanism of cell death during warm hepatic ischemia‐reperfusion in rats: apoptosis or necrosis? Hepatology 33, 397–405. [DOI] [PubMed] [Google Scholar]
- Gundersen H.J., Bendtsen T.F., Korbo L. et al (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96, 379–394. [DOI] [PubMed] [Google Scholar]
- Hart M.L., Much C., Kohler D. et al (2008) Use of a hanging‐weight system for liver ischemic preconditioning in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1431–G1440. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. & Lemasters J.J. (2003) Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125, 1246–1257. [DOI] [PubMed] [Google Scholar]
- Knudsen A.R., Kannerup A.S., Dich R. et al (2012) Ischemic pre‐ and postconditioning has pronounced effects on gene expression profiles in the rat liver after ischemia/reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G482–G489. [DOI] [PubMed] [Google Scholar]
- Knudsen A.R., Kannerup A.S., Gronbaek H. et al (2013) Quantitative histological assessment of hepatic ischemia‐reperfusion injuries following ischemic pre‐ and post‐conditioning in the rat liver. J. Surg. Res. 180, e11–e20. [DOI] [PubMed] [Google Scholar]
- Mata‐Marin J.A., Gaytan‐Martinez J., Grados‐Chavarria B.H., Fuentes‐Allen J.L., Arroyo‐Anduiza C.I. & Alfaro‐Mejia A. (2009) Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross‐sectional study. Virol. J. 6, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry K.R., Campbell D.A. Jr, Scales W.E., Warren J.S. & Remick D.G. (1993) Tumor necrosis factor, interleukin 6, and the acute phase response following hepatic ischemia/reperfusion. J. Surg. Res. 55, 49–54. [DOI] [PubMed] [Google Scholar]
- Milland J., Tsykin A., Thomas T., Aldred A.R., Cole T. & Schreiber G. (1990) Gene expression in regenerating and acute‐phase rat liver. Am. J. Physiol. 259, G340–G347. [DOI] [PubMed] [Google Scholar]
- Muhlfeld C., Nyengaard J.R. & Mayhew T.M. (2010) A review of state‐of‐the‐art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc. Pathol. 19, 65–82. [DOI] [PubMed] [Google Scholar]
- Nyengaard J.R. (1999) Stereologic methods and their application in kidney research. J. Am. Soc. Nephrol. 10, 1100–1123. [DOI] [PubMed] [Google Scholar]
- Ozer J.S., Chetty R., Kenna G. et al (2010) Recommendations to qualify biomarker candidates of drug‐induced liver injury. Biomark. Med. 4, 475–483. [DOI] [PubMed] [Google Scholar]
- Poupon R. (2015) Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology 61, 2080–2090. [DOI] [PubMed] [Google Scholar]
- Scheig R. (1996) Evaluation of tests used to screen patients with liver disorders. Prim. Care 23, 551–560. [DOI] [PubMed] [Google Scholar]
- Schmidt E. & Schmidt F.W. (1993) Enzyme diagnosis of liver diseases. Clin. Biochem. 26, 241–251. [DOI] [PubMed] [Google Scholar]
- Si‐Tayeb K., Lemaigre F.P. & Duncan S.A. (2010) Organogenesis and development of the liver. Dev. Cell 18, 175–189. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Nakamura S., Koizumi T. et al (1991) The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation 52, 979–983. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Toledo‐Pereyra L.H., Rodriguez F.J. & Cejalvo D. (1993) Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 55, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Tapuria N., Junnarkar S.P., Dutt N. et al (2009) Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford) 11, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechini B., Pasquazzi C. & Aceti A. (2004) Correlation of serum aminotransferases with HCV RNA levels and histological findings in patients with chronic hepatitis C: the role of serum aspartate transaminase in the evaluation of disease progression. Eur. J. Gastroenterol. Hepatol. 16, 891–896. [DOI] [PubMed] [Google Scholar]


