Summary
Most patients with epithelial ovarian cancer (EOC) are diagnosed at an advanced stage, and therapeutic options for these patients are limited. The identification of suitable biomarkers could be helpful for patients with EOC, who might benefit from targeted therapies even in advanced stages of the disease. Trophoblast cell surface antigen 2 (TROP2) is highly expressed in various human malignant tumours; however, this has not been demonstrated clearly in EOC. In this study, we further evaluated whether TROP2 is a promising marker for EOC, and thus also a potential target for EOC immunotherapy. Quantitative real‐time polymerase chain reaction (qPCR) and fluorescence‐activated cell sorting (FACS) analysis were employed to determine TROP2 mRNA and protein expression in both human EOC and normal ovarian cell lines. Additionally, TROP2 protein expression was measured by immunohistochemistry in 128 EOC tissue samples, 21 normal ovarian tissues and 18 normal fallopian tubes. The correlations between TROP2 protein expression and patients' clinicopathological features were investigated, and survival outcomes were analysed. TROP2 mRNA and protein levels were upregulated significantly in EOC cell lines compared with normal cell lines. The protein of TROP2 was expressed in the majority of EOC tissue samples (90.6%) and overexpressed in 75 (58.6%) of the 128 tumour samples. TROP2 overexpression was correlated with relevant clinicopathological characteristics and was associated with significantly shortened overall survival and disease‐free survival. Furthermore, TROP2 was an independent prognostic marker for EOCs as analysed by Cox regression. TROP2 was a potential biomarker for targeted therapy in patients with TROP2‐overexpressing EOC.
Keywords: biomarker targeted therapy, epithelial ovarian cancer, immunohistochemistry, independent prognostic marker, TROP2
Ovarian cancer is a significant cause of mortality in women worldwide (Siegel et al. 2011). The most common form of ovarian malignancy is epithelial ovarian cancer (EOC), which accounts for up to 90% of all cases and originates from either the normal endosalpinx or ovarian surface epithelium (Auersperg et al. 2001; Carlson et al. 2008; Crum 2009; Ng et al. 2012). Patients are primarily diagnosed with EOC at an advanced stage, when the tumour has already metastasized or disseminated, making therapeutic options limited (Cannistra 2004). Despite advances for early detection and the multimodal approaches for diagnosis, approximately 85% of patients with EOC who achieve complete remission following first‐line therapy (i.e. cytoreductive surgery and platinum‐based chemotherapy) eventually develop recurrent disease (Coleman et al. 2013; Foley et al. 2013). The poor prognosis of EOC is also due to increased resistance to cytostatic chemotherapy, which hampers chemotherapeutic treatment (Materna et al. 2005; Lage & Denkert 2007) and reduces the survival times of patients. Therefore, the development of new therapeutic agents for this disease is an important task, and better biomarkers for predicting disease behaviour are urgently needed. Trophoblast cell surface antigen 2 (TROP2), also termed GA733‐1 or EGP‐1, is a transmembrane glycoprotein, first identified in human trophoblasts and choriocarcinoma cell lines (Lipinski et al. 1981). TROP2 is a member of the tumour‐associated calcium signal transducer gene family, which includes epithelial cell‐adhesion molecule (Cubas et al. 2009). As a cell surface receptor, TROP2 increases intracellular calcium levels by recognizing specific ligands and also plays an important role in regulating the growth of carcinoma cells (Fornaro et al. 1995); however, its functional role has not been fully determined (Ripani et al. 1998; Sukhthankar et al. 2010).
Previous studies have reported that TROP2 is highly expressed in various human malignant tumours including colorectal adenocarcinoma and oral cavity squamous cell carcinoma and is associated with tumour aggressiveness and poor prognosis (Ohmachi et al. 2006; Fong et al. 2008a). A similar correlation has also been shown in pancreatic cancers that overexpress TROP2 (Iacobuzio‐Donahue et al. 2002). Progress in immunotherapy has also been made based on the studies of TROP2 in several carcinomas (Govindan et al. 2004; Varughese et al. 2011; Cardillo et al. 2015; Goldenberg et al. 2015; Starodub et al. 2015); however, the information about its role in ovarian cancer is limited, and only one group led by Bignotti et al. (2010) has evaluated its expression level in patients from Italy. So, it is important to thoroughly elucidate the TROP2 expression pattern in EOC and determine its potential role in the prognosis and immunotherapy of more patients with EOC. To address this, we studied both several ovarian cancer cell lines and large quantities of patient tissue samples to better determine TROP2 expression in EOC and its associations with relevant clinicopathological characteristics and prognosis. These findings will help to further evaluate whether TROP2 is a promising biomarker for immunotherapy in ovarian cancer.
Materials and methods
Cell lines and reagents
The human EOC cell lines, SKOV3 (Karlan et al. 1994) and HO8910 (Tang et al. 2015) derived from ovarian adenocarcinoma, and the immortalized ovarian epithelial cell line IOSE386 (Maines‐Bandiera et al. 1992), preserved in our laboratory, were used in this study. Another human EOC cell line A2780 (Barretina et al. 2012) established from tumour tissue from an untreated patient was purchased from KeyGEN BioTECH (Nanjing, China). Cells were maintained in Dulbecco's modified Eagle's medium (Gibco, Waltham, MA, USA) containing 10% foetal bovine serum.
Quantitative real‐time polymerase chain reaction (qPCR)
qPCR was performed to quantify messenger RNA expression of TROP2 using SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA, USA) and the ABI PRISM 7300 real‐time PCR system (Applied Biosystems). RNA was collected from cultured EOC and control cells using Trizol (Invitrogen, Waltham, MA, USA). Total RNA was reverse‐transcribed in a 20 μl reaction mixture (Invitrogen). A housekeeping gene, glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), was used to normalize the quantity of cDNA in the PCR. The primers used were as follows: for TROP2, 5′‐TAT TAC CTG GAC GAG ATT CCCC‐3′ (forward) and 5′‐CCC CGA CTT TCT CCG GTTG‐3′ (reverse); for GAPDH, 5′‐GGG AAG GTG AAG GTC GGA GT‐3′ (forward) and 5′‐TTG AGG TCA ATG AAG GGG TCA‐3′ (reverse).
To observe TROP2 mRNA expression in EOC cell lines (SKOV3, HO8910, A2780) and in normal ovarian epithelial cells (IOSE386), the comparative cycle threshold (Ct) method was employed. The normal cell line IOSE386 was used as control to compare TROP2 expression in EOC cells. Each assay was performed in triplicate, and the results are expressed as the mean ± standard deviation.
Fluorescence‐activated cell sorting (FACS) analysis
IOSE386, SKOV3, HO8910 and A2780 cells (1 × 106 each) were harvested and blocked in 5% milk. Cells were incubated with anti‐human TROP2 polyclonal goat IgG (AF650; R&D Systems, Minneapolis, MN, USA) at a final concentration of 2.5 μg/106 cells and then stained using diluted fluorescein isothiocyanate (FITC)‐labelled anti‐goat IgG (Sigma, St. Louis, MO, USA). The cells were washed twice and suspended. Fluorescence intensity was analysed using a flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA). Cells incubated with secondary antibody only were analysed as controls.
Patients and tissue samples
This study was conducted according to the regulations of the local ethics committee. A total of 128 formalin‐fixed, paraffin‐embedded EOC tissue samples, 21 normal ovarian tissue samples and 18 normal fallopian tube samples were collected from the Department of Pathology of the first affiliated hospital with Nanjing Medical University, China. All samples were obtained from patients diagnosed between June 2009 and June 2011 prior to the administration of neoadjuvant therapy, who underwent primary cytoreductive surgery with neoplastic cytological evaluation of ascites or peritoneal washing.
Patients' medical records were reviewed to collect data, including age, surgical procedure, tumour histological type, tumour grade, tumour stage, the presence of ascites, omental metastases, lymph node involvement, survival time and survival status. All tumour specimens were reclassified by observing haematoxylin‐and‐eosin‐stained (HE) slides, and the histological type was reassessed by two pathologists according to the World Health Organization (WHO) classification. Tumour staging was determined in accordance with the International Federation of Gynecology and Obstetrics (FIGO) criteria by the physicians in charge.
Overall survival (OS) and disease‐free survival (DFS) were calculated for survival analysis. OS was defined as the period of time from initial diagnosis to death or last contact, which was the date of the last follow‐up visit. DFS was defined as the time between the date of surgery and the date of identification of disease recurrence. In this study, 105 cases who received the same post‐operative platinum‐based chemotherapy were followed up until July 2014 and selected for survival analysis, whereas 23 patients were lost to follow up.
Immunohistochemistry (IHC) of formalin‐fixed tissues
The same purified goat polyclonal antibody used for FACS analysis to detect the human TROP2 extracellular domain was applied at a dilution of 1:40 to determine TROP2 expression by IHC, as described previously (Ohmachi et al. 2006). All EOC and normal tissue samples were evaluated. The results were determined using a double‐blind method by two independent pathologists. Discordant cases were re‐evaluated using a double‐headed microscope to achieve a consensus.
TROP2 protein expression was defined as the presence of specific membranous tumour cell staining, and the expression levels were determined in terms of both incidence of positive cells and staining intensity. Incidence was weighed by the percentage of positive cells and was scored as 0 (0%), 1 (1–9%), 2 (10–49%), 3 (50–79%) or 4(>80%), and staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The total score obtained by multiplying the two components ranged from 0 to 12. TROP2 overexpression was defined as a total score of more than 4, as described previously (Fong et al. 2008a).
Statistical analysis
Statistical analysis was performed using spss 15.0 software package (IBM, Armonk, NY, USA). The chi‐square test was used to compare TROP2 expression rates between EOC tissue samples and normal tissue samples. The relationship between TROP2 overexpression and clinicopathological parameters was also assessed using the chi‐square test. For OS and DFS calculation, data were censored on the last date of follow‐up. To determine their prognostic significance, factors were evaluated using the Cox proportional hazards model for univariate and multivariate analyses, and survival curves were generated using the Kaplan–Meier method and compared using the log–rank test. In all analyses, differences were considered statistically significant when the P‐value was less than 0.05.
Ethical approval statement
This study was conducted by the department of Pathology. All the implements are under full compliance with all government policies and the Helsinki declaration.
Results
Measurement of TROP2 mRNA expression in cell lines by qPCR
Compared with IOSE386, the average relative gene expression of TROP2 mRNA normalized to GAPDH mRNA was 83.64 ± 7.22 in SKOV3, 640.02 ± 33.58 in HO8910 and 0.18 ± 0.01 in A2780. TROP2 mRNA expression was notably high in SKOV3 and HO8910 when compared to the normal ovarian epithelial cell line, with the latter showing a more significant increase (approximately 640 times the expression level in IOSE386, Figure 1). However, the TROP2 mRNA expression in A2780 was considerably low (approximately one‐sixth the expression level in IOSE386, Figure 1).
Figure 1.
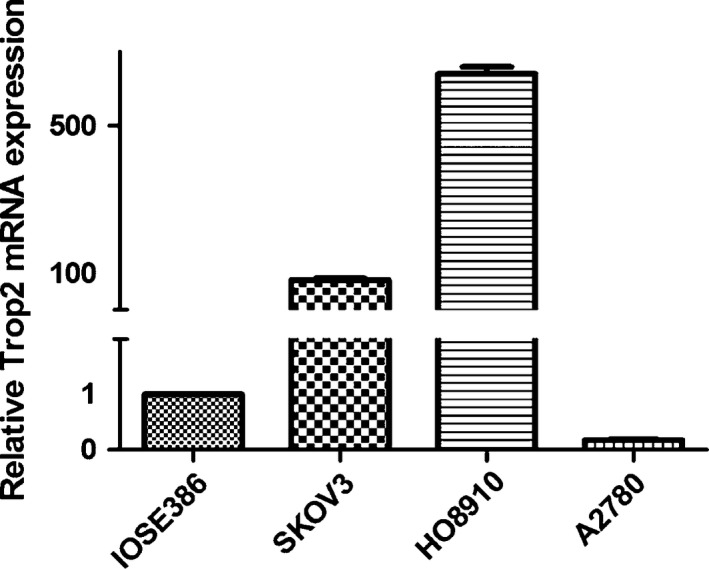
Relative TROP2 mRNA expression in the EOC cell lines SKOV3 (83.64 ± 7.22), HO8910 (640.02 ± 33.58) and A2780 (0.18 ± 0.01) compared with that in the normal ovarian cell line IOSE386.
Evaluation of TROP2 protein expression in cell lines by FACS
We further examined TROP2 protein expression in different cell lines. As expected, TROP2 protein expression was not obvious in the normal cell line IOSE386; the expression levels differed considerably among the EOC cell lines (Figure 2). The HO8910 showed high expression of TROP2, whereas SKOV3 exhibited only moderate TROP2 expression; expression was undetectable in A2780. The TROP2 protein expression pattern in cell lines corresponded with the mRNA expression. Therefore, we assumed that TROP2 protein might be present in several EOCs but that the protein expression levels might vary widely. To confirm this and estimate the potential prognostic and therapeutic value of TROP2 in EOC, large numbers of primary EOC tissues were collected for further assessment.
Figure 2.
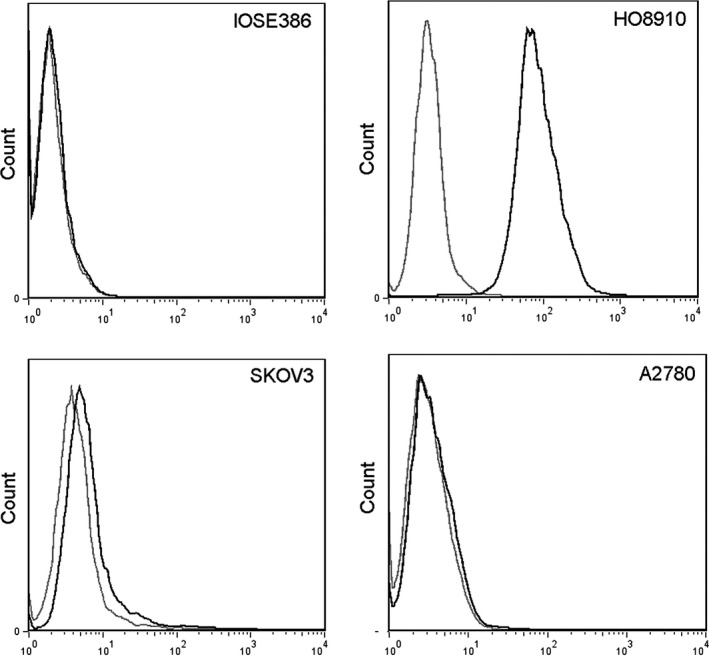
Fluorescence intensity showing significant TROP2 expression in HO8910 cells, moderate expression in SKOV3 cells, and no expression in A2780 and IOSE386 cells. Black line: cells treated with TROP2 polyAb and anti‐goat IgG‐fluorescein isothiocyanate; grey line: cells treated with anti‐goat IgG‐fluorescein isothiocyanate only.
Clinicopathological characteristics of patients with EOC
Tumour samples from 128 patients with primary EOC of different histological types, 21 normal ovaries and 18 normal fallopian tubes were selected and stained by IHC. The patients' median age at diagnosis was 52.6 years (range, 25–82 years). Clinicopathological characteristics are shown in Table 1. One hundred and two cases were followed up until July 2014 for survival analysis. At the time of the last clinical follow‐up, 66 patients had died. The median OS and DFS were 27 and 22 months respectively.
Table 1.
Patients' characteristics and correlation of patients' clinicopathological parameters with TROP2's protein expression
| Total patients (n) | TROP2 overexpression | P‐value* | ||
|---|---|---|---|---|
| No n (%) | Yes n (%) | |||
| Age at diagnosis(year) | ||||
| <50 | 50 | 19 (38) | 31 (62) | 0.53 |
| ≥50 | 78 | 34 (44) | 44 (56) | |
| Histological type | ||||
| Serous | 69 | 17 (25) | 52 (75) | <0.01 |
| Mucinous | 24 | 18 (75) | 6 (25) | |
| Endometrioid | 17 | 4 (24) | 13 (76) | |
| Clear cell | 9 | 8 (89) | 1 (11) | |
| Undifferentiated | 9 | 6 (67) | 3 (33) | |
| FIGO stage | ||||
| <IIIC | 45 | 22 (49) | 23 (51) | 0.21 |
| ≥IIIC | 83 | 31 (37) | 52 (63) | |
| Histological grade | ||||
| G1 | 15 | 10 (67) | 5 (33) | 0.04 |
| G2 | 113 | 43 (38) | 70 (62) | |
| Omental metastases | ||||
| No | 61 | 30 (49) | 31 (51) | 0.09 |
| Yes | 67 | 23 (34) | 44 (66) | |
| Lymph node involvement | ||||
| No | 79 | 40 (51) | 39 (49) | 0.03 |
| Yes | 40 | 12 (30) | 28 (70) | |
| Missing | 9 | |||
| Presence of ascites | ||||
| No | 53 | 27 (51) | 26 (49) | 0.07 |
| Yes | 75 | 26 (35) | 49 (65) | |
G1: Low‐grade serous, low‐grade mucinous, both grades I and II endometrioid EOCs; G2: High‐grade serous, high‐grade mucinous, clear cell, grade III endometrioid and undifferentiated EOCs. *P values were calculated by chi‐square test.
Immunohistochemical TROP2 protein expression in human tissues
TROP2 protein staining was primarily localized to the membrane of neoplastic cells, whereas staining was mostly negative in tumour stromal cells. Representative immunohistochemical staining patterns are shown in Figure 3. The majority of primary serous and endometrioid EOC showed strong TROP2 expression (Table 1, Figure 3c and f), whereas the mucinous and undifferentiated types showed moderate staining (Table 1, Figure 3d and e). However, most clear cell EOCs showed weak or negative staining (Table 1, Figure not shown). Positive staining was detected in 116 (90.6%) of 128 EOC specimens, and according to the previously defined criteria, TROP2 was overexpressed in 75 (58.6%) cases (total score greater than 4) (Table 1). However, only 5 (23.8%) of 21 normal ovarian tissue samples and 2 (11.1%) of 18 normal fallopian tubes showed very weak expression of TROP2, whereas the others were negative (Figure 3a and b). The TROP2 expression rate differed significantly between EOC tissues and normal tissues, including the ovaries and fallopian tubes (P < 0.01).
Figure 3.
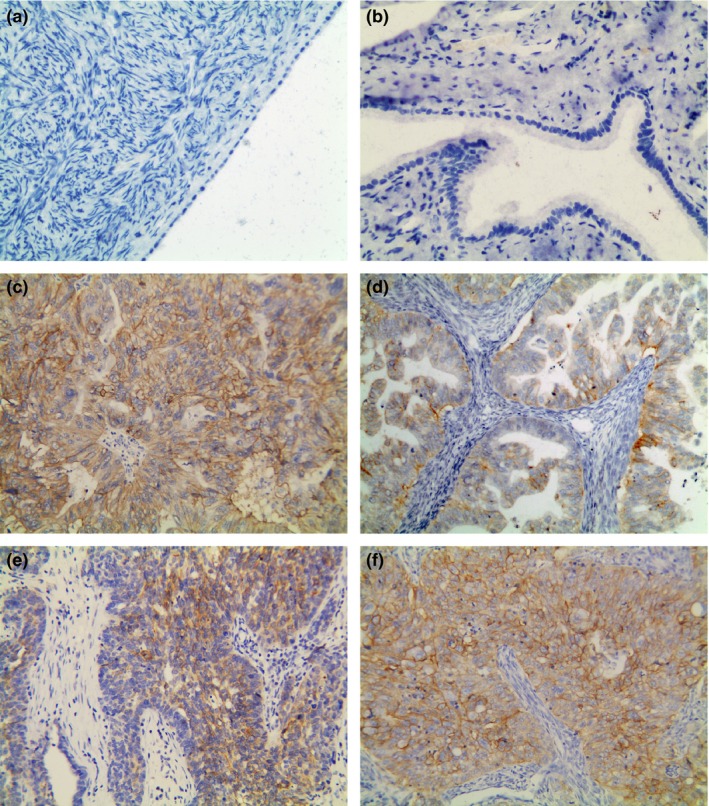
Representative immunohistochemical staining of TROP2 in tissues. (a) Normal ovarian tissue (score 0). (b) Normal fallopian tube (score 0). (c–f) Serous (c, score 12), mucinous (d, score 6), undifferentiated (e, score 8) and endometrioid (f, score 12) EOC tissues. Original magnification ×200.
Correlation between TROP2 expression and clinicopathological parameters
The relationship between TROP2 overexpression and clinicopathological parameters is shown in Table 1. Statistical analysis showed that TROP2 overexpression was significantly associated with EOC histological type, lymph node involvement and histological grade, but not with age, FIGO stage, the presence of ascites or omental metastases.
Survival analysis
To assess the impact of clinicopathological features and TROP2 overexpression on survival, we used Cox proportional hazard models for survival analysis. Univariate analysis showed that TROP2 overexpression was significantly correlated with poor OS and DFS (P < 0.01, Table 2). Kaplan–Meier curves also revealed that patients with TROP2 overexpression had shorter OS and DFS (Figure 4a and b). The median OS time with or without TROP2 overexpression was 20 or 56 months, respectively, as OS declines with increasing TROP2 expression scores. Additionally, serous histological type, advanced FIGO stage, omental metastases and lymph node involvement were significantly associated with unfavourable OS and DFS, as shown in Table 2.
Table 2.
Univariate analyses (Cox regression model) of OS and DFS in relation to various prognostic parameters and TROP2's expression
| Variables | OS P‐value | HR | 95% CI | DFS P‐value | HR | 95% CI |
|---|---|---|---|---|---|---|
|
TROP2 IHC Low/overexpression |
0.00 | 2.84 | 1.65–4.88 | 0.00 | 3.00 | 1.74–5.17 |
|
Age <50/≥50 |
0.16 | 0.70 | 0.42–1.15 | 0.14 | 0.69 | 0.42–1.14 |
|
Histological type Non‐serous/serous |
0.00 | 2.13 | 1.28–3.53 | 0.00 | 2.23 | 1.34–3.70 |
|
FIGO stage <IIIC/≥IIIC |
0.00 | 5.46 | 2.67–11.16 | 0.00 | 5.29 | 2.60–10.78 |
|
Histological grade G1/G2 |
0.23 | 1.76 | 0.70–4.39 | 0.19 | 1.84 | 0.74–4.59 |
|
Omental metastases No/yes |
0.00 | 2.60 | 1.55–4.36 | 0.00 | 2.54 | 1.52–4.24 |
|
Lymph node involvement No/yes |
0.00 | 2.52 | 1.52–4.18 | 0.00 | 2.57 | 1.55–4.26 |
|
Presence of ascites No/yes |
0.83 | 1.06 | 0.64–1.75 | 0.66 | 1.12 | 0.68–1.85 |
TROP2 IHC Low expression: total score 0–4; TROP2 IHC Overexpression: total score 6–12.
OS, the time between the date of initial diagnosis and death or last contact; DFS, the time between the date of surgery and identification of disease recurrence; CI, confidence interval; HR: hazard ratio.
Figure 4.
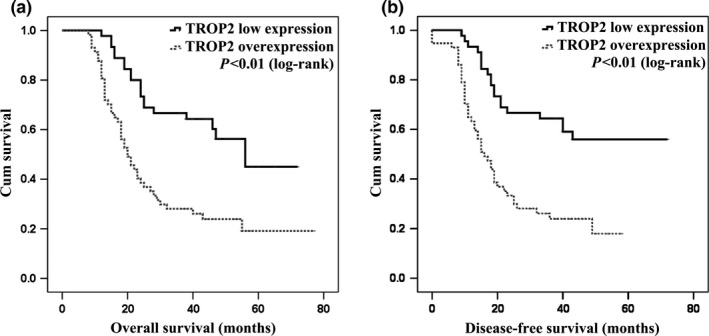
Prognostic significance of TROP2 in patients with EOC as determined using Kaplan–Meier analysis. Patients with TROP2 overexpression (grey‐dash line) had poorer OS and DFS than patients with TROP2 low expression (black line), as measured using the log–rank test (P < 0.01).
In multivariate analysis, TROP2 overexpression with an advanced FIGO stage was identified as an independent prognostic marker for poor OS. Lymph node involvement with an advanced FIGO stage and TROP2 overexpression were independently associated with poor DFS (Table 3).
Table 3.
: Multivariate analyses (Cox regression model) of OS and DFS in relation to various prognostic parameters and TROP2's expression
| Variables | OS P‐value | HR | 95% CI | DFS P‐value | HR | 95% CI |
|---|---|---|---|---|---|---|
|
FIGO stage <IIIC/≥IIIC |
0.00 | 3.94 | 1.66–9.35 | 0.00 | 3.72 | 1.56–8.90 |
|
Histological type Non‐serous/serous |
0.10 | 1.56 | 0.93–2.63 | 0.06 | 1.66 | 1.00–2.79 |
|
Omental metastases No/yes |
0.99 | 1.00 | 0.55–1.82 | 0.97 | 1.01 | 0.56–1.85 |
|
Lymph node involvement No/yes |
0.05 | 1.70 | 0.99–2.91 | 0.04 | 1.76 | 1.03–3.01 |
|
TROP2 IHC Low/overexpression |
0.00 | 2.65 | 1.51–4.65 | 0.00 | 2.77 | 1.58–4.87 |
TROP2 IHC Low expression: total score 0–4; TROP2 IHC Overexpression: total score 6–12.
OS, the time between the date of initial diagnosis and death or last contact; DFS, the time between the date of surgery and identification of disease recurrence; CI, confidence interval; HR: hazard ratio.
Discussion
Ovarian cancer is associated with a high mortality, and limited therapeutic methods are available. The development of targeted therapies in the last several years has led to encouraging results in clinical trials (Vincenzi et al. 2006), particularly for cancers that responded poorly to traditional treatments, such as ovarian cancer. However, despite ongoing efforts for many years, little progress has been made for EOC due to a lack of suitable target molecules. In this study, we described the TROP2 protein expression pattern in EOCs in China and evaluated its value as a biomarker for targeted therapy. Large numbers of tissues were collected, including EOC and normal ovarian tissue samples. TROP2 was detectable in the majority of EOCs (90.6%), with 58.6% of samples showing overexpression; however, TROP2 protein expression was rarely detected in normal tissues. The sensitivity and specificity of TROP2 expression for identifying EOC were satisfactory. Moreover, as displayed in Figure 3, the wide distribution and selective expression of TROP2 in epithelial tumour cells, especially its membrane localization as a cell surface receptor, further render it an attractive target for antibody‐based treatments. Furthermore, the association between TROP2 overexpression in EOC cases and several relevant clinicopathological characteristics suggests that it might play a significant role as an oncogene in EOC, consistent with previous reports (Fong et al. 2008b; Wang et al. 2008; Fang et al. 2009; Lin et al. 2012; Wu et al. 2013). TROP2 overexpression was also associated with a significantly decreased OS and DFS, and could be an independent prognostic marker, as determined by Cox regression analysis. Our results are consistent with the study of Bignotti et al. (2010). However, we collected more EOC tissues and corresponding patients' clinicopathological characteristics, reporting TROP2's expression pattern in Chinese patients with EOC, which would help more patients benefit from future therapy targeting.
Notably, not all EOCs have high TROP2 protein expression levels. In this study, strong signals were detected in the majority of serous and endometrioid EOCs, whereas only very weak signals were detected in clear cell‐type EOCs. TROP2 overexpression rates differed among histological types, as shown in Table 1. Therefore, TROP2 may not be a suitable therapeutic marker for all EOCs. Patients need to be selected on the basis of the histological classification and protein expression level. Additionally, one marker is not enough. More promising therapeutic biomarkers need to be identified to fit the spectrum of ovarian cancer types, which will increase the efficacy of individualized treatment.
The molecular mechanism of TROP2 in carcinogenesis and tumour progression remains largely unknown. TROP2 upregulation has been shown to increase the expression of cyclic AMP‐responsive‐element binding protein, NF‐κB, STAT1 and other cancer regulatory effectors through the cyclin D and MAPK pathway (Cubas et al. 2010; Ben‐Neriah & Karin 2011). Suppression of TROP2 expression inhibited the invasive tumour phenotype, and blocking experiments with anti‐TROP2 antibodies demonstrated that TROP2 contributed to tumour cell migration (Ripani et al. 1998; Sukhthankar et al. 2010). In our study, three EOC cell lines showed various TROP2 expression levels; especially, the expression of A2780 was downregulated at both the mRNA and protein level compared with the normal epithelial ovarian cell line. The correlation between TROP2 expression and three EOC cells' different biological characteristics and malignant behaviours could only be elucidated by molecular mechanism of TROP2. Besides, whether TROP2 is involved in chemotherapy resistance, another reason responsible for poor prognosis of EOC, needed to be demonstrated. Accordingly, more EOC cell lines, including chemotherapy sensitive and resistant, aggressive and less invasive cell lines, will be collected and further research work is required in the future. Understanding the molecular basis of TROP2 could significantly refine the diagnosis and management of tumours and eventually lead to the development of more specific and effective treatment modalities.
Conclusions
We measured TROP2 expression both in EOC cell lines and tumour tissues and demonstrated that TROP2 was overexpressed specifically in the majority of EOCs and may be a novel prognostic biomarker, making it a potential attractive therapeutic target for patients with TROP2‐overexpressing EOC.
Conflict of interest
All the authors declare that there is no conflict of interest regarding the publication of this article.
Funding source
This work was supported by the Grant of Natural Science Foundation of Jiangsu Province (BK20130895), the Natural Science Research Program for Colleges and Universities in Jiangsu Province (11KJB320006) as well as the National Natural Science Foundation of China (NSFC 81201703); also the project was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801).
Ning Xu and Zhihong Zhang contributed equally to this work.
References
- Auersperg N., Wong A.S., Choi K.C., Kang S.K. & Leung P.C. (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr. Rev. 22, 255–288. [DOI] [PubMed] [Google Scholar]
- Barretina J., Caponigro G., Stransky N. et al (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Neriah Y. & Karin M. (2011) Inflammation meets cancer, with NF‐ κB as the matchmaker. Nat. Immunol. 12, 715–723. [DOI] [PubMed] [Google Scholar]
- Bignotti E., Todeschini P., Calza S. et al (2010) Trop‐2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur. J. Cancer 46, 944–953. [DOI] [PubMed] [Google Scholar]
- Cannistra S.A. (2004) Cancer of the ovary. N. Engl. J. Med. 351, 2519–2529. [DOI] [PubMed] [Google Scholar]
- Cardillo TM, Govindan SV, Sharkey RM et al (2015) Sacituzumab Govitecan (IMMU‐132), an Anti‐Trop‐2/SN‐38 Antibody‐Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjug. Chem. 26, 919–931. [DOI] [PubMed] [Google Scholar]
- Carlson J.W., Miron A., Jarboe E.A. et al (2008) Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 26, 4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R.L., Monk B.J., Sood A.K. & Herzog T.J. (2013) Latest research and treatment of advanced‐stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 10, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C.P. (2009) Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol 3, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas R., Li M., Chen C. & Yao Q. (2009) Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim. Biophys. Acta 1796, 309–314. [DOI] [PubMed] [Google Scholar]
- Cubas R., Zhang S., Li M., Chen C. & Yao Q. (2010) Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer. 9, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.J., Lu Z.H., Wang G.Q. et al (2009) Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int. J. Colorectal Dis. 24, 875–884. [DOI] [PubMed] [Google Scholar]
- Foley O.W., Rauh‐Hain J.A. & del Carmen M.G. (2013) Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park). 27, 288–294 298. [PubMed] [Google Scholar]
- Fong D., Spizzo G., Gostner J.M. et al (2008a) TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod. Pathol. 21, 186–191. [DOI] [PubMed] [Google Scholar]
- Fong D., Moser P., Krammel C. et al (2008b) High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br. J. Cancer 99, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M., Dell'Arciprete R., Stella M. et al (1995) Cloning of the gene encoding Trop‐2, a cell‐surface glycoprotein expressed by human carcinomas. Int. J. Cancer 62, 610–618. [DOI] [PubMed] [Google Scholar]
- Goldenberg D.M., Cardillo T.M., Govindan S.V. et al (2015) Trop‐2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU‐132), an antibody‐drug conjugate (ADC). Oncotarget. 6, 22496–22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan S.V., Stein R., Qu Z. et al (2004) Preclinical therapy of breast cancer with a radioiodinated humanized anti‐EGP‐1 monoclonal antibody: advantage of a residualizing iodine radiolabel. Breast Cancer Res. Treat. 84, 173–182. [DOI] [PubMed] [Google Scholar]
- Iacobuzio‐Donahue C.A., Maitra A., Shen‐Ong G.L. et al (2002) Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am. J. Pathol. 160, 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlan B.Y., Jones J., Slamon D.J. et al (1994) Glucocorticoids stabilize HER‐2/neu messenger RNA in human epithelial ovarian carcinoma cells. Gynecol. Oncol. 53, 70–77. [DOI] [PubMed] [Google Scholar]
- Lage H., Denkert C. (2007) Resistance to chemotherapy in ovarian carcinoma: recent results. Cancer Res. 176, 51–60. [DOI] [PubMed] [Google Scholar]
- Lin H., Huang J.F., Qiu J.R. et al (2012) Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp. Mol. Pathol. 94, 73–78. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Parks D.R., Rouse R.V. & Herzenberg L.A. (1981) Human trophoblast cell‐surface antigens defined by monoclonal antibodies. Proc. Natl Acad. Sci. USA 78, 5147–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines‐Bandiera S., Kruk P. & Auersperg N. (1992) Simian virus 40‐ transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am. J. Obstet. Gynecol. 167, 729–735. [DOI] [PubMed] [Google Scholar]
- Materna V., Liedert B., Thomale J., Lage H. (2005) Protection of platinum‐DNA adduct formation and reversal of cisplatin resistance by anti‐MRP2 hammerhead ribozymes in human cancer cells. Int. J. Cancer 115, 393–402. [DOI] [PubMed] [Google Scholar]
- Ng J.S., Low J.J. & Ilancheran A. (2012) Epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 26, 337–345. [DOI] [PubMed] [Google Scholar]
- Ohmachi T., Tanaka F., Mimori K., Inoue H., Yanaga K. & Mori M. (2006) Clinical significance of TROP2 expression in colorectal cancer. Clin. Cancer Res. 12, 3057–3063. [DOI] [PubMed] [Google Scholar]
- Ripani E., Sacchetti A., Corda D. & Alberti S. (1998) Human Trop‐2 is a tumor‐associated calcium signal transducer. Int. J. Cancer 76, 671–676. [DOI] [PubMed] [Google Scholar]
- Siegel R., Ward E., Brawley O. & Jemal A. (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 61, 212–236. [DOI] [PubMed] [Google Scholar]
- Starodub A.N., Ocean A.J., Shah M.A. et al (2015) First‐in‐Human trial of a Novel Anti‐Trop‐2 Antibody‐SN‐38 Conjugate, Sacituzumab Govitecan, for the treatment of diverse metastatic solid tumors. Clin. Cancer Res. 21, 3870–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhthankar M., Alberti S. & Baek S.J. (2010) (‐)‐Epigallocatechin‐3‐gallate (EGCG) post‐transcriptionally and post‐translationally suppresses the cell proliferative protein TROP2 in human colorectal cancer cells. Anticancer Res. 30, 2497–2503. [PubMed] [Google Scholar]
- Tang Z., Zhang N., Di W. et al (2015) Inhibition of microtubule‐associated protein1 light chain 3B via small‐interfering RNA or 3‐methyladenine impairs hypoxia‐induced HO8910PM and HO8910 epithelial ovarian cancer cell migration and invasion and is associated with RhoA and alterations of the actin cytoskeleton. Oncol. Rep. 33, 1411–1417. [DOI] [PubMed] [Google Scholar]
- Varughese J., Cocco E., Bellone S. et al (2011) Uterine serous papillary carcinomas overexpress human trophoblast‐cell‐surface marker (Trop‐2) and are highly sensitive to immunotherapy with hRS7, a humanized anti‐Trop‐2 monoclonal antibody. Cancer 117, 3163–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenzi B., Santini D., Rabitti C. et al (2006) Cetuximab and irinotecan as thirdline therapy in advanced colorectal cancer patients: a single centre phase II trial. Br. J. Cancer 94, 792–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Day R., Dong Y., Weintraub S.J. & Michel L. (2008) Identification of Trop‐2 as an oncogene and an attractive therapeutic target in colon cancers. Mol. Cancer Ther. 7, 280–285. [DOI] [PubMed] [Google Scholar]
- Wu H., Xu H., Zhang S. et al (2013) Potential therapeutic target and independent prognostic marker of TROP2 in laryngeal squamous cell carcinoma. Head Neck 35, 1373–1378. [DOI] [PubMed] [Google Scholar]


