Abstract
Introduction
Anti-integrin therapy for the treatment of patients with Crohn’s disease is rapidly evolving. Two agents, natalizumab and vedolizumab, are approved by the United States Food and Drug Administration for the treatment of Crohn’s disease, with vedolizumab the primary anti-integrin used due to a more favorable safety profile. Several other anti-integrins are in various stages of development.
Areas Covered
This review discusses the current state of anti-integrin therapy as well as suggestions for positioning of these agents in clinical practice. Emerging anti-integrin therapies, their underlying mechanisms of action, and available safety and clinical data are also reviewed.
Expert Opinion
Anti-integrins are effective for the treatment of Crohn’s disease, even in patients refractory to other therapies. Their use should be considered in patients with Crohn’s disease who do not respond to, develop non-response to, or have contraindications to anti-TNF therapy. Anti-integrin therapies can be offered as a first biologic therapy, in particular for older patients, patients with concurrent multiple sclerosis (natalizumab only), and in patients with contraindications to anti-TNF therapy. In patients with more severe symptoms, providers should consider co-induction with corticosteroids if possible to hasten remission.
Keywords: Crohn’s disease, ulcerative colitis, inflammatory bowel disease, leukocyte trafficking, natalizumab, vedolizumab, etrolizumab, AJM300, AMG 181, PF-00547659, alicaforsen
1. Introduction
Inflammatory bowel disease (IBD) consists of two primary subtypes: Crohn’s disease (CD) and ulcerative colitis (UC). Both CD and UC are chronic inflammatory disorders of the gastrointestinal (GI) tract. Although CD and UC differ with respect to the segments of the GI tract that can be affected, the depth of inflammation (transmural vs. mucosal) that occurs, and propensity for developing serious complications, therapeutic options for the treatment of moderate to severe CD and UC are similar. Medical therapies commonly utilized in these patients include corticosteroids, immune suppressants, and/or biologic agents. Surgery is typically offered to patients with disease nonresponsive to medical therapy or to patients who develop complications of disease including strictures, fistulas, or intra-abdominal abscesses.
Anti-integrin agents, like tumor necrosis factor (TNF)-α inhibitors, are biologic agents designed to target molecules that contribute to the development of intestinal inflammation. Unlike TNF-α inhibitors, which are antibodies directed against the pro-inflammatory cytokine TNF-α, anti-integrin therapy modulates inflammation by binding to integrins that contribute to leukocyte trafficking, thereby preventing leukocyte migration into GI mucosa and the development of inflammation within these tissues. Potential advantages associated with targeting integrins are blockade of an inflammatory pathway alternate to the TNF-α pathway, possibly reducing inflammation in patients who would otherwise be deemed refractory to medical therapy, and the possibility of decreased side effects compared to a drug that acts systemically, particularly if the integrin targeted is expressed only in GI tissue. The following review discusses anti-integrin agents that are currently available, novel anti-integrin therapies under development, and how anti-integrin therapy may impact the future treatment of patients with CD.
2. Background
2.1. Epidemiology
Both CD and UC are becoming increasingly common, particularly in industrialized nations.[1] In the United States (US) and Europe, approximately 3.6 million individuals have IBD with approximately 30,000 new cases of IBD diagnosed in the US each year.[2] In North America, the prevalence of CD is 26–199 cases per 100,000 persons with an incidence of 3.1–14.6 cases per 100,000 person-years.[3] Most individuals with IBD are diagnosed during the second and third decades of life, although a second smaller peak in diagnoses occurs after the age of 40 years.[2,4] Data from epidemiologic studies suggests a genetic contribution as first-degree relatives of patients with IBD have a five-fold risk of developing either CD or UC.[4] In patients with CD, up to 35% of patients have at least one relative with IBD.[5] Furthermore, the economic impact of CD is significant with a systematic review finding the total economic burden due to CD to be between $10.9 and $15.5 billion in the US. Direct costs per patient in the US are approximately $18,500 per patient per year.[6]
Article highlights.
CD is a chronic inflammatory disorder of the GI tract. Medical management of CD is generally favored, except for severe cases that may require surgical resection.
Traditional biologic agents target the pro-inflammatory cytokine TNF-α. Anti-integrins block efflux of immune cells from the vascular compartment into GI mucosal tissues.
In the United States, two anti-integrin agents, natalizumab and vedolizumab, are approved for the treatment of CD. Several additional anti-integrin molecules are in various stages of development.
Natalizumab is linked to the development of PML, an often fatal neurologic disease.
Vedolizumab gained regulatory approval in 2014 and is not linked to PML.
Anti-integrin molecules may be used to induce and maintain remission in patients with CD.
Anti-integrins can be used for maintenance therapy in patients who undergo induction therapy with corticosteroids. They may also be used in patients who do not respond to, who lose response to, or in those with contraindications to anti-TNF therapy.
Additional data demonstrating efficacy of novel anti-integrin agents for the treatment of CD will be required prior to their introduction to the market.
Continued development and eventual regulatory approval of additional anti-integrins for the treatment of UC is expected.
This box summarizes key points contained in the article.
2.2. Pathophysiology
Multiple factors contribute to the development of IBD, including environmental exposures, aberrant host immune responses, genetic predisposition, and the composition of luminal microbes.[7,8] Environmental exposures linked to the development of CD include smoking, diet, oral contraceptives, infections, vaccinations, and childhood factors [7]; however, only smoking has been linked definitively to CD pathogenesis. [9] With regard to genetics, a total of 163 loci have been linked to IBD, 140 of which are linked to both CD and UC or to CD alone.[10] Specific genes implicated in the development of CD include NOD2, IL23R, TNFSF15, ATG16L1, and TLR4.[7] In CD a variety of leukocytes including macrophages, neutrophils, T lymphocytes, and dendritic cells, contribute to the inflammatory milieu.[11] In CD, innate immune cells such as macrophages and dendritic cells, which are typically conditioned to be noninflammatory and induce tolerance, display an activated phenotype and enhanced production of pro-inflammatory cytokines.[7] In addition, antibody production by B cells is increased along with T-cell production of Th1 and Th17 cytokines.[7]
2.3. Treatment options
A variety of treatment options exist for patients with CD, including antibiotics, 5-aminosalicylates, corticosteroids, immune suppressants, and/or biologic therapy. Medical therapy is typically selected based on the severity of symptoms, likelihood of recurrent symptoms developing, and whether remission is being induced or maintained. Surgery remains the treatment of choice in patients who develop complicated CD such as strictures, fistulas, or intra-abdominal abscesses. Complications associated with CD are common, with up to 80% of patients undergoing surgery during their lifetime.[12] Biologic therapy has previously been used after failure or intolerance of conventional therapy. More recently, however, anti-TNF therapy has been used earlier in a patient’s disease course or even at the time of diagnosis, particularly in patients at increased risk for the development of complicated CD. Unfortunately, up to 40% of CD patients do not respond to induction therapy with TNF-α inhibitors with an additional 40% losing response over time.[13] Strategies such as dose escalation or narrowing of dosing interval can recapture response in the majority of patients.[14] Changing therapy to a second TNF-α inhibitor can also be effective although this effect typically wanes over time.[14] In addition to TNF-α inhibitors, anti-integrin therapies have also been developed for the treatment of patients with CD and UC. At present, natalizumab and vedolizumab are the only two anti-integrin agents approved by the US Food and Drug Administration (FDA) for use in CD.
2.4. Leukocyte trafficking and IBD
An influx of leukocytes, including T cells, into gut mucosa occurs in patients with active IBD. T cells are implicated in the development of chronic inflammatory states and, when activated, contribute to the pro-inflammatory cytokine profiles observed in gut mucosa from patients with active CD and UC. Although leukocyte infiltration and derangements in intestinal barrier function are both observed, the inciting factor that leads to these events occurring has not been definitively identified. Nonetheless, leukocytic infiltration of affected intestinal mucosa contributes to an environment of pro-inflammatory cytokines ultimately resulting in clinical disease.
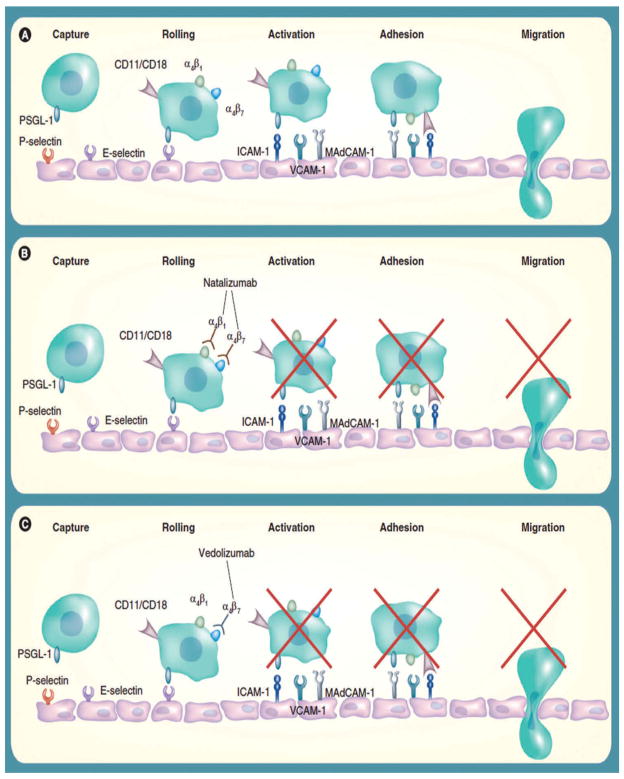
Leukocyte extravasation from the vascular compartment into intestinal mucosa is a highly coordinated and complex process involving interactions between surface receptors on leukocytes and their ligands on vascular endothelial cells. Several steps – tethering/rolling, activation, adhesion, and extravasation/migration – occur allowing immune cells to enter stromal tissues (Figure 1). Initially, transient interactions develop between leukocytes and endothelial cells, decreasing the speed of leukocytes relative to the endothelial surface. When sufficiently slowed, leukocytes ‘roll’ along the endothelium permitting integrins expressed on leukocytes to interact with their ligands on endothelial cells. During this process, leukocytes are exposed to pro-inflammatory cytokines, which further enhance binding between integrins and their ligands readying them to cross the endothelial surface and ultimately enter GI mucosa.[15–17]
Figure 1.
Leukocyte extravasation into gut mucosa.[17] (A) Leukocyte recruitment into gut mucosa occurs in stages and depends on interactions between receptors and ligands expressed on leukocytes and the endothelial surface. (B) Natalizumab prevents leukocyte extravasation by binding α4, causing pharmacologic inhibition of the α4β1 and α4β7 integrins. (C) Vedolizumab is more selective as binding is directed against the α4β7 integrin, inhibiting leukocyte migration into gut mucosa without off-target effects from binding α4β1. Adapted from [17] with permission
Integrins, which play a critical role in leukocyte trafficking and extravasation from the vascular compartment into target tissues, are heterodimers and consist of an α and β subunit. In total, 18 α subunits and 8 β subunits have been identified. The integrins α4β1, α4β7, αEβ7, and αLβ2 have been implicated as receptors that contribute to leukocyte trafficking. Consequently, they have been identified as potential pharmacologic targets with efforts being made to manipulate or block these integrins, their subunits, and/or their ligands in order to prevent or reduce leukocyte influx into target tissues.[18] The following manuscript discusses the current state of anti-integrin therapy for the treatment of patients with CD as well as emerging therapies currently undergoing clinical trials.
3. Available agents
3.1. Natalizumab
The first anti-integrin agent approved for use in patients with CD was natalizumab, a monoclonal antibody directed against the α4-integrin chain. By targeting α4, natalizumab blocks both the α4β1 and α4β7 integrins. The International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) study demonstrated that natalizumab was effective for inducing clinical response and remission in 509 patients with moderate to severely active CD with objective evidence of inflammation (Table 1).[19] Patients were randomized to natalizumab 300 mg or placebo administered intravenously at weeks 0, 4, and 8 with a primary end point of clinical response, defined as ≥70 point decrease in the Crohn’s Disease Activity Index (CDAI) at week 8 that was sustained through week 12. Clinical response was achieved in 48% of patients receiving natalizumab compared to 32% of those receiving placebo (p < 0.001) with sustained remission observed in 26% of patients receiving natalizumab compared to 16% of patients receiving placebo (p = 0.002).[19]
Table 1.
Summary of natalizumab trials.
| Study | Sample size | Treatment arms | Clinical remission (%) | Clinical response (%) | Serious adverse events | Follow-up period | Conclusion | Ref |
|---|---|---|---|---|---|---|---|---|
| ENCORE | 509 Patients with moderate to severe CDa | Natalizumab 300 mg | 26%b | 48%b | 5%c | 12 weeks | Natalizumab demonstrated early and sustained efficacy for induction therapy in active CD | [19] |
| Placebo | 16% | 32% | 10% | |||||
| ENACT-2 | 339 Patients who responded to natalizumab initially | Natalizumab 300 mg q 4 weeks | 44%d | 61%d | 8%e | 60 weeks | Patients who responded to natalizumab induction had increased rates of sustained response and remission with natalizumab every 4 weeks | [20] |
| Placebo | 26% | 28% | 10% |
ENCORE: Efficacy of Natalizumab in Crohn’s Disease Response and Remission; ENACT-2: Evaluation of Natalizumab as Continuous Therapy.
C-reactive protein > 2.87 mg/L.
Week 8 sustained through week 12.
No progressive multifocal leukoencephalopathy (PML).
Week 36.
One death from PML during an open-label extension study.
ENACT-2 examined the ability of natalizumab to act as a maintenance agent in patients with active CD (Table 1). A total of 339 patients who had an initial response to natalizumab received either 300 mg of natalizumab or placebo every 4 weeks for 56 weeks. The primary end point was sustained clinical response (≥70 point decrease in CDAI) through week 36 with clinical remission (CDAI < 150) being a secondary end point. At week 36, clinical response was achieved in 61% of patients receiving natalizumab compared to 28% of those receiving placebo (p < 0.001). Clinical remission was maintained in 44% of patients receiving natalizumab compared to 26% of those receiving placebo (p = 0.003).[20]
Although natalizumab is effective for the induction of clinical response and remission in patients with moderate to severely active CD, its use is associated with the development of progressive multifocal leukoencephalopathy (PML), a rare but often fatal neurologic disease. Two multiple sclerosis and one CD patient receiving natalizumab developed PML in postmarketing surveillance. Because of this, natalizumab was withdrawn from the market by the US FDA and later reintroduced under a special prescribing program for the treatment of multiple sclerosis.[21] In 2008, natalizumab was granted approval for the treatment of CD, but prescribers were required to participate in a monitoring program.[22] Factors contributing to the development of PML include ≥2 years of natalizumab therapy, prior exposure to immune suppressants, and John Cunningham virus seropositivity. The overall incidence of PML in patients exposed to natalziumab is approximately 1.4 cases per 1000 patient years (95% CI: 1.20–1.72).[21] More recently, the use of natalizumab has largely been supplanted by vedolizumab, which has similar efficacy to natalizumab,[23] but has not been linked to PML.
3.2. Vedolizumab
Vedolizumab is a humanized monoclonal IgG1 antibody that blocks the α4β7 integrin heterodimer without binding to the α4β1 integrin. By only binding α4β7, vedolizumab prevents leukocyte extravasation into GI mucosa as mucosal vascular addressin cell adhesion molecular 1 (MAdCAM-1), α4β7’s ligand, is expressed on the endothelial surface of venules and lymphoid tissue within the GI tract.[24] Unlike natalizumab, vedolizumab does not bind α4β1. As a result, the ability of α4β1 to bind its ligand, vascular cell adhesion protein 1 (VCAM-1) is preserved (Figure 1), permitting continued immune surveillance within the central nervous system (CNS) and theoretically eliminating the risk of PML. Preliminary studies indicated that vedolizumab does not affect T-cell recruitment to the cerebrospinal fluid (CSF) nor does it affect immune surveillance of the CNS.[25,26]
The efficacy of vedolizumab for the treatment of CD was demonstrated in the GEMINI studies [27,28] (Table 2). Investigators conducted two randomized, double-blind, placebo-controlled trials of vedolizumab in patients with active CD. One trial was an induction trial and included 368 patients assigned to receive vedolizumab or placebo at weeks 0 and 2 and 747 receiving open-label vedolizumab at weeks 0 and 2. Disease activity was assessed at week 6. Approximately 15% of patients receiving vedolizumab were in clinical remission at week 6 versus 7% of patients receiving placebo (p = 0.02). The second trial examined the effects of vedolizumab maintenance therapy. A total of 461 patients who responded to vedolizumab were randomly assigned to receive placebo or vedolizumab until week 52. In patients receiving vedolizumab every 8 weeks, 39% were in clinical remission at week 52 versus 22% of those receiving placebo (p < 0.001).[27] Vedolizumab was also found to be effective for the treatment of patients with UC, with 47% of UC patients receiving vedolizumab attaining clinical response compared to 26% of those receiving placebo (p < 0.001).[28] Based on the data from the above studies, vedolizumab gained approval from the US FDA for the treatment of moderate to severe CD and UC on 20 May 2014.[29]
Table 2.
Summary of vedolizumab trials.
| Study | Sample size | Treatment arms | Clinical remission (%) | Clinical response (%) | Follow-up period | Conclusion | Ref |
|---|---|---|---|---|---|---|---|
| CD Induction | 368 Patients with active CD (Cohort 1) | Vedolizumab 300 mg Week 0 and 2 | 14.5%a | 31.4%b | 6 weeks | At week 6, patients receiving vedolizumab more likely to attain remission | [27] |
| 747 Patients with active CD (Cohort 2) | Placebo | 6.8% | 25.7% | ||||
| Week 0 and 2 Open-label vedolizumab | 17.7% | 34.4% | |||||
| CD Maintenance | 461 Patients who responded to induction therapy with vedolizumab | Vedolizumab 300 mg every 8 weeks | 39.0%c | 43.5%e | 52 weeks | Patients responding to induction therapy more likely to be in remission at week 52 | [27] |
| Vedolizumab 300 mg every 4 weeks | 36.4%d | 45.5%f | |||||
| Placebo | 21.6% | 30.1% | |||||
| UC Induction | 374 Patients with active UC (Cohort 1) | Vedolizumab 300 mg Week 0 and 2 | 47.1%g | 16.9%h | 6 weeks | Vedolizumab was more effective than placebo for induction therapy for UC | [28] |
| 521 Patients with active UC (Cohort 2) | Placebo | 25.5% | 5.4% | ||||
| Week 0 and 2 | |||||||
| UC Maintenance | 373 Patients who responded to induction therapy with vedolizumab | Vedolizumab 300 mg every 8 weeks. | 41.8%i | 56.6%k | 52 weeks | Vedolizumab was more effective than placebo for maintenance therapy for UC | [28] |
| Vedolizumab 300 mg every 4 weeks | 44.8%j | 52.0%l | |||||
| Placebo | 15.9% | 23.8% |
p = 0.02 vs. placebo.
p = 0.23 vs. placebo.
p < 0.001 vs. placebo.
p = 0.004 vs. placebo.
p = 0.01 vs. placebo.
p = 0.005 vs. placebo.
p < 0.001 vs. placebo.
p = 0.001 vs. placebo.
p < 0.001 vs. placebo.
p < 0.001 vs. placebo.
p < 0.001 vs. placebo.
p < 0.001 vs. placebo.
A subsequent meta-analysis comparing the efficacy of natalizumab and vedolizumab found that both agents have similar efficacy in inducing remission and response in anti-TNF-naïve and anti-TNF-exposed patients with similar safety profiles and an absence of PML in patients treated with vedolizumab.[23,30]
4. In development
4.1. Etrolizumab
Etrolizumab (rhuMAb β7) is a humanized IgG1 monoclonal antibody that targets the β7 subunit of the α4β7 and αEβ7 integrins, preventing binding between these integrins and their ligands MAdCAM-1 and E-cadherin, respectively.[31] As described above, leukocyte migration into gut mucosal tissues is regulated in part by interactions between α4β7 on the surface of leukocytes and MAdCAM-1 expressed on endothelial cells. Unlike α4β7, αEβ7 is expressed on mucosal intraepithelial T cells and binds E-cadherin on epithelial cells. It is thought that αEβ7/E-cadherin-1 binding promotes T-cell retention within mucosal tissues.[32–34] Expression of αEβ7 is increased in both active UC and CD [35,36] and blockade of this integrin has been shown to attenuate colitis in animal models.[37] Animal studies have also shown that blockade of the β7 subunit of α4β7 and αEβ7 reduces lymphocyte homing to GI mucosa.[38,39] By targeting both α4β7 and αEβ7, it is thought that inflammation can be modulated by decreasing leukocyte recruitment into GI mucosa as well as by decreasing leukocyte retention within the gut.[31]
A phase I study examined the safety and pharmacology of etrolizumab in patients with moderate to severe UC. No dose-limiting toxicities were noted, nor did patients develop infusion or injection site reactions. Clinical response was observed in 12 (66%) etrolizumab-treated patients compared to 4 (80%) placebo-treated patients. Three (16%) etrolizumab-treated patients were in clinical remission compared to 1 (20%) placebo-treated patient. Anti-etrolizumab antibodies developed in two patients (5%) who received drug [31] (Table 3).
Table 3.
Anti-integrin drugs currently or previously in development.
| Name | Molecule | Target | Clinical remission CD | Clinical response CD | Clinical remission UC | Clinical response UC | Conclusions | References |
|---|---|---|---|---|---|---|---|---|
| Etrolizumab (anti-β7, rhuMAb-β7) | Humanized monoclonal IgG1 antibody against β7-integrin | β7 (α4β7, αEβ7) | NDa | NDa | Phase I: 3 of 18 etrolizumabb 1 of 5 Placebo Phase II: 21% etrolizumab 100 mgc,d 10% Etrolizumab 300 mge 0% Placebo |
Phase I: 12 of 18 etrolizumabb 4 of 5 Placebo Phase II: 33% etrolizumab 100 mg 31% Etrolizumab 300 mg 29% Placebo |
Well tolerated with no dose-limiting toxicities or reactions At week 10, etrolizumab more likely to induce clinical remission than placebo | [31] [40] |
| AJM300 AJM300 |
Orally active α4 integrin inhibitor | α4 | No difference between placebo and AJM300 groupsf | Phase II: 24% AJM300 900 mgd,g 3.9% Placebo |
Phase II: 63% AJM300 960 mgd 26% Placebo |
AJM300 is safe and effective. AJM300 decreases CDAI in patients with active CD AJM300 safe and more effective than placebo for inducing clinical response, clinical remission, and mucosal healing in moderate UC |
[41] [42] |
|
| AMG 181 | Subcutaneously administered humanized monoclonal IgG2 antibody against α4β7 | α4β7 | NDa | NDa | Phase I: 2 of 4 AMG 181 0 of 1 Placebo |
Phase I: 1 of 4 AMG 181 0 of 1 Placebo |
Safety profile of AMG 181 is favorable in patients with IBD | [43] |
| PF-00547659 | Subcutaneously administered humanized IgG2 monoclonal antibody against MAdCAM | MAdCAM | Phase I: 13% of PF-00547659 11% of Placeboh,i 22% of PF-00547659h,j 0% of Placebo |
Phase I: 52% of PF-00547659h,i 32% of Placebo 42% of PF-00547659j 21% of Placeboh |
PF-00547659 has a favorable safety profile and preliminary efficacy in UC patients | [44] | ||
| PF-00547659 | Phase II: 29% of PF-00547659 225 mgf,j 28% of PF-00547659 75 mg 37% of PF-00547659 22.5 mg 23% of Placebo |
Phase II: 58% of PF-00547659 225 mgj 65% of PF-00547659 75 mg 62% of PF-00547659 22.5 mg 59% of Placebo |
PF-00547659 is pharmacologically active. Subjects with a high CRP (>18 mg/L) may respond to PF-00547659 | [45] | ||||
| Alicaforsen | Rectally administered phosphorothioate antisense ICAM-1 inhibitor | ICAM-1 | 34% Alicaforsenh,j 34% Placebo |
78% Alicaforsenh,j 76% Placebo |
NDk | NDk | Alicaforsen did not meet any of its primary outcome measures | [46] |
Table adapted with permission from [15].
CRP: C-reactive protein; ICAM-1: intercellular adhesion molecular 1; ND: no data.
Studies underway.
Multiple dose cohort (n = 8). Varying etrolizumab doses used—0.5 mg/kg SC (n = 4), 1.5 mg/kg SC (n = 5), 3.0 mg/kg SC (n = 4), 4.0 mg/kg intravenous (n = 5), or placebo (n = 5).
The results are from week 10.
p < 0.01 vs. placebo.
p < 0.05 vs. placebo.
Only published in abstract form.
The results are from week 8.
p > 0.05 vs. placebo.
The results are from week 4.
The results are from week 12.
Studies underway in pouchitis.
The results from a double-blind, placebo-controlled, randomized, phase II study examining the ability of etrolizumab to induce remission in patients with moderate to severely active UC were recently published.[40] Patients with a Mayo Clinic Score (MCS) of ≥5 (≥6 in the US) and disease extending ≥25 cm from the anal verge were randomized to one of two doses of etrolizumab or to placebo. The primary end point was clinical remission which was defined as a MCS of ≤2. A total of 124 patients were randomized with 119 patients ultimately enrolled. No patients in the placebo group were in clinical remission at week 10. Eight (21%) patients in the etrolizumab 100 mg group (p = 0.004) and 4 (10%) in the 300 mg group (p = 0.048) achieved clinical remission at week 10. Clinical response did not differ between treatment arms with 39 (33%) patients in the etrolizumab 100 mg group, 39 (31%) patients in the 300 mg group, and 41 (29%) patients in the placebo group achieving clinical response at week 10. Serious adverse events occurred in five (12%) etrolizumab 100 mg subjects, two (5%) etrolizumab 300 mg subjects, and five (12%) placebo subjects [40] (Table 3).
A phase III study examining the safety and efficacy of etrolizumab in patients with moderately to severe active CD is currently recruiting patients.[47] Phase III studies examining the safety and efficacy of etrolizumab in UC patients are also currently underway [48] as are studies examining its efficacy in patients naïve to TNF-α inhibitors [49–52] and in patients refractory or intolerant of TNF-α inhibitors.[53] At present, etrolizumab holds promise as an alternative agent for the treatment of patients with moderate to severe UC; however, its efficacy in patients with CD remains unclear.
4.2. AJM300
AJM300 is an orally administered humanized anti-α4 integrin antagonist, preventing α4β1 from binding VCAM-1 and α4β7 from binding MAdCAM-1. It is effective in attenuating murine models of colitis.[54] A randomized, double-blind, placebo-controlled multicenter trial was performed to evaluate the safety, efficacy, and dose response of AJM300 in patients with CD. Seventy-one patients with active CD were randomized to receive AJM300 40 mg, AJM300 120 mg, AJM300 240 mg, or placebo three times daily for 8 weeks (Table 3). Patients had a CDAI of ≥150 and an abnormal C-reactive protein (CRP). The primary end point was a decrease in CDAI from baseline to week 4 or later. Secondary end points were clinical response defined as a ≥70 point decrease in CDAI. CDAI decreases were greater in all three AJM300 groups when compared to placebo; however, no significant difference in clinical response was observed in patients receiving AJM300 when compared to placebo. The safety profile of AJM300 was favorable.[41] A full manuscript describing the safety and efficacy of AJM300 in patients with CD has not been published.
Data from a phase II study examining the efficacy of AJM300 for treating moderately active UC in 102 patients who had an inadequate response or intolerance to 5-amino-salicylic acid or corticosteroids was recently published (Table 3). Patients were randomized to receive AJM300 960 mg or placebo three times daily for 8 weeks. The primary end point was clinical response at week 8. Patients receiving AJM300 were significantly more likely to achieve a clinical response (63% vs. 26%) and clinical remission (24% vs. 3.9%) compared to patients receiving placebo. Mucosal healing rates were also significantly greater in the AJM300 groups (59% vs. 29%). No serious adverse events were detected.[42]
4.3. AMG 181
AMG 181 is an IgG2 humanized monoclonal antibody directed against the α4β7 integrin. The in vitro pharmacology of AMG 181 was studied in cynomolgus monkeys for up to 13 weeks and favorable pharmacokinetic, pharmacodynamic, and safety profiles were observed,[18] prompting further study in humans. A phase I clinical trial enrolled 68 healthy male subjects who received a single dose of AMG 181 at varying doses or placebo. In addition, three UC patients received AMG 181 and one UC patient received placebo. At day 43, two UC patients receiving AMG 181 were in remission and one UC patient had achieved clinical response. All three UC patients had mucosal healing. The patient who received placebo did not achieve a clinical response, clinical remission, or mucosal healing. No serious adverse events were reported [43] (Table 3).
A randomized, double-blind, placebo controlled, multiple dose study to evaluate the efficacy of AMG 181 in patients with moderate to severe CD is underway. The primary end point of the study is clinical remission, defined as CDAI < 150, at week 8. Up to 80% of patients recruited for this study may have had prior anti-TNF exposure. Patients must have had an inadequate response or loss of response or intolerance to immune suppressants and/or anti-TNF agents or corticosteroids. After completing the double-blind portion of the trial, subjects may enter an open-label extension during which AMG 181 will be administered at a single dose. Patients who do not achieve improvement or who develop worsening disease will be eligible to enter the open-label phase of the study early.[55] This study is no longer recruiting participants, but results have not yet been made available.
4.4. PF-00547659
PF-00547659 is a fully human IgG2 monoclonal antibody directed against MAdCAM-1, blocking its ability to act as a ligand to α4β7. A phase I study enrolled 80 patients with active UC who received single or multiple doses of PF-00547659 or placebo (Table 3). At week 4, 52% of patients receiving PF-00547659 achieved a clinical response compared to 32% of those receiving placebo (p = 0.102). At week 12, 42% of patients receiving PF-00547659 achieved a clinical response compared to 21% of those receiving placebo (p = 0.156). There was no difference in clinical remission rates at week 4 between patients receiving PF-00547659 and placebo (13% vs. 11%, p = 0.551), but there was a trend toward significance at week 12 with 22% of PF-00547659 patients achieving clinical remission compared to none of those receiving placebo (p = 0.056). Although there was a trend toward endoscopic response and remission in the PF-00547659 group at week 4, this was lost by week 12. Adverse event rates were similar between patients receiving placebo and those receiving PF-00547659. In addition, a trend toward an increase in circulating α4β7 lymphocytes was observed in patients receiving PF-00547659.[44]
Preliminary results from a randomized, multicenter, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of PF-00547659 in patients with CD were presented at Digestive Disease Week (DDW) 2015 (Table 3). This phase II study enrolled 267 patients. The primary end point was a ≥70 point decrease in CDAI at week 8 or week 12. Secondary end points included remission, ≥100 point decrease in CDAI, and safety. Subjects must have failed or been intolerant of TNF-α inhibitors and/or immune suppressants, have a CRP > 3 mg/L, and ulcerations on colonoscopy. No statistically significant difference in CDAI-70 response was observed between subjects receiving PF-00547659 (58–62%) and those receiving placebo (59%); however, remission rates at week 12 in subjects with a baseline CRP > 18 mg/L were higher in PF-00547659 treated subjects when compared to those receiving placebo. Molecular analysis demonstrated that soluble MAdCAM levels were lower in subjects receiving PF-00547659. Similarly, a dose-related increase in β7+ lymphocytes was observed in those receiving PF-00547659, indicating that PF-00547659 is pharmacologically active. The authors argued that no difference in primary end point was observed due to a high placebo response rate.[45,56]
4.5. Alicaforsen
Alicaforsen (ISIS 2302) is an antisense oligodeoxynucleotide that inhibits expression of intercellular adhesion molecular 1 (ICAM-1). ICAM-1 is a member of the immunoglobulin superfamily and is expressed on vascular endothelial cells and leukocytes. ICAM-1 binds β2 integrins, among other molecules, and facilitates leukocyte migration from the vascular space.[57] ICAM-1 also signals T cells during antigen presentation [58] and facilitates cytotoxic T cell, natural killer cell, and neutrophil damage of target cells.[59] Preventing ICAM-1 expression was considered a potential therapeutic target in CD as ICAM-1 expression is upregulated in patients with IBD and also increased in the setting of increased TNF-α production.[46]
Two double-blind, placebo-controlled studies examined the ability of alicaforsen or placebo to induce remission in 331 patients with active CD. Alicaforsen was administered three times weekly for 4 weeks. This study failed to demonstrate a difference in primary end point (Table 3), which was clinical remission by week 12 (alicaforsen 34% vs. placebo 34%). Alicaforsen was well tolerated although infusion reactions occurred more frequently in the alicaforsen group. The authors posited several explanations for the lack of efficacy including onerous dosing schedule, inclusion of refractory CD patients, and/or inclusion of patients with noninflammatory causes of symptoms as only 3% of patients had a CRP ≥ 10 mg/L.[46] We are unaware of any further studies exploring the efficacy of alicaforsen in either patients with CD or UC, although a study examining topical alicaforsen in antibiotic refractory pouchitis is planned.[60]
5. Conclusions
Continued investigation of agents that modulate inflammation within the GI tract, including, but not limited to, anti-integrin therapies is clearly warranted. In addition to anti-integrin therapies, drugs targeting cytokines continue to be developed for use in CD. Examples include ustekinumab (anti-p40 [IL-12/IL-23]), brodalumab (anti-IL-17), AMG 139 (anti-p19), BE-8 (anti-IL-6), and tocilizumab (anti-IL-6 receptor).[61] In addition to targeting pro-inflammatory cytokines directly, anti-inflammatory cytokine, anti-T-cell therapy, and hormone therapy are also being studied for use in CD.[61] Orally active molecules represent promising new therapies as they will be easier to administer compared to intravenous infusions or subcutaneous injections. Tofactinib is a JAK1/JAK3 inhibitor that mechanistically decreases the downstream generation of pro-inflammatory cytokines. It is currently being studied for the treatment of both CD and UC patients, as are other JAK inhibitors. Mongersen is an orally administered antisense oligonucleotide that is being studied in patients with active CD. Mongersen inhibits SMAD7, which itself inhibits transforming growth factor β1, an anti-inflammatory cytokine.
New therapies continue to be approved and developed for the treatment of patients with IBD. Anti-integrins are the newest class of biologic approved for the treatment of CD and UC. These agents have an important role in the treatment of patients with an inadequate response, loss of response, or intolerance of anti-TNF biologics. Despite being available for use, many gastroenterologists are unfamiliar with administering these agents. It is likely that these agents will be used more frequently as a first biologic if vedolizumab’s long-term safety profile continues to be excellent, as expected. In addition, providers await retrospective and prospective studies detailing the use of anti-integrin agents in clinical practice. In particular, it will be interesting to see if pharmacodynamic data emerges to help providers optimize treatment based on drug levels. It is also critical that novel diagnostics be developed to assist providers in identifying which patients are more likely to benefit from specific therapies (personalized medicine). These diagnostics will allow providers to move away from a one-size-fits-all approach to treatment. At present, a significant number of patients have refractory or aggressive CD and are subjected to multiple surgical interventions and long-term complications. Identifying these patients early in the disease course and offering more aggressive treatment with biologic agents (like anti-integrins) is expected to improve outcomes.
Anti-integrins, such as vedolizumab, are effective for the induction and maintenance of response/remission in patients with CD and UC. Because of a slower onset of action, it may take up to 10 weeks to attain a response. Providers can consider co-induction with corticosteroids in patients with greater disease activity. Vedolizumab has largely supplanted natalizumab given its improved safety profile. Vedolizumab is appropriate for patients with an inadequate response, loss of response, or intolerance to anti-TNF agents. Earlier use of vedolizumab will likely occur with expanded use of the drug and as information emerges regarding long-term safety. Vedolizumab may be a preferred biologic in subsets of patients eligible for biologic therapy such as patients with a contraindication to anti-TNF biologics and in older patients with IBD.
We expect that anti-integrins will continue to be developed for the treatment of patients with IBD. As additional data emerges and experience is gained, these agents will be used more commonly and effectively, and may, in fact, offer an enhanced safety profile compared to TNF-α inhibitors.
6. Expert opinion
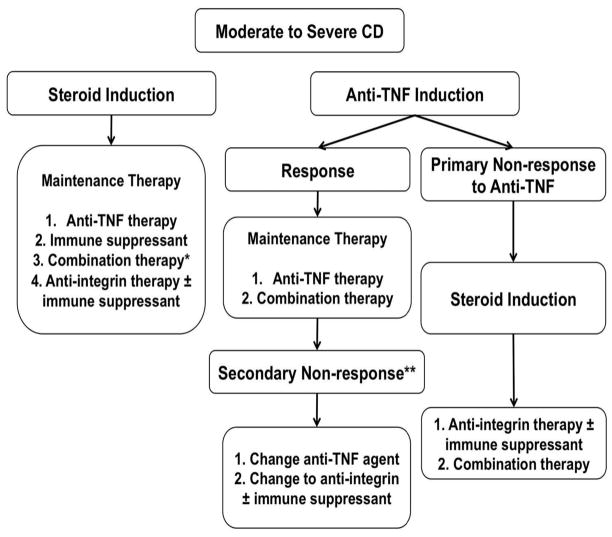
Although a variety of medications and biologic agents exist for the treatment of patients with CD, a significant number of patients do not respond to or lose response to anti-TNF therapy. Anti-integrin therapy offers a different mechanistic target than anti-TNF therapy, affording the opportunity to modulate inflammation in patients who may suffer from ‘non-TNF-α’ mediated inflammation. By targeting integrins, it is possible that symptoms can be improved and inflammation reduced in patients who are primary nonresponders to anti-TNF therapy as well as those who lose response to anti-TNF therapy over time (Figure 2).
Figure 2.
Proposed algorithm for treatment of moderate to severe Crohn’s disease. Remission may be induced either with corticosteroid therapy (intravenous or oral) with transition to maintenance therapy using anti-TNF agents, immune suppressants, combination therapy (anti-TNF therapy with immune suppressant), or anti-integrin therapy with or without immune suppressant. In patients in whom remission is induced with anti-TNF agents, that agent is continued for maintenance therapy if the patient responds to induction therapy. In cases of primary nonresponse to anti-TNF induction, remission may be induced with corticosteroid therapy. In that case, either an immune suppressant or anti-integrin agents with or without an immune suppressant may be used for maintenance therapy. * Anti-TNF and immune suppressant (azathioprine, mercaptopurine, methotrexate). ** Consider addition of immune suppressant, rather than switching within or outside of drug class, in patients with low titer antibodies. NB: In symptomatic patients, active disease should be confirmed prior to therapeutic changes. In addition, therapeutic drug monitoring should be performed, if available (infliximab, adalimumab, azathioprine, mercaptopurine) to ensure dose optimization.
Vedolizumab, which has largely supplanted natalizumab because it does not cross into the CSF and to date has not been linked to the development of PML, is more effective than placebo for inducing and maintaining remission in patients with active CD and has similar efficacy to natalizumab for the treatment of CD.[23] Despite this, over half of patients with CD experience either no response or an incomplete response to vedolizumab therapy. A greater proportion of vedolizumab-treated patients were in remission at week 10 when compared to patients receiving placebo (26.6% vs. 12.1%, p = 0.001).[30] This study, among others, suggests that examining response to vedolizumab at week 6 may be premature and matches our anecdotal experience that response to vedolizumab is typically not observed until at least week 8–10. This observation is further supported by an abstract presented at DDW 2014 which showed that a proportion of week 6 nonresponders to vedolizumab attained a CDAI-100 response after week 6.[62]
Despite the improved safety profile of vedolizumab compared to natalizumab, its efficacy, particularly for the treatment of patients with CD, does not appear to be superior to anti-TNF biologics. Perhaps its greatest limitation is that the onset of action is slow, limiting its use in patients with severely active disease. These patients require co-induction with corticosteroids, with vedolizumab used to maintain a steroid-induced remission. Even so, from a mechanistic perspective, targeting integrins that facilitate leukocyte extravasation into GI mucosa appears to be a sound strategy for treating inflammation in patients with IBD. Consequently, several other biologic agents targeting integrins continue to be developed.
Of the anti-integrin agents currently in development, only alicaforsen has data published in manuscript form regarding its efficacy for the treatment of patients with CD. Etrolizumab, AJM300, AMG 181, and PF-00547659 are in varying stages of premarketing studies for the treatment of UC. Clinical trials studying the efficacy of AJM 300 and AMG 181 for the treatment of patients with CD are either underway or were recently completed, but these data have not been presented. Preliminary data regarding the efficacy of PF-00547659 was presented at DDW 2015,[45] but has not yet been presented in manuscript form. At present, etrolizumab is closest to gaining regulatory approval as it is in phase III trials, although this would be for the treatment of patients with UC as no published data exists regarding its efficacy for the treatment of patients with CD.
In four of five anti-integrin agents currently being developed, clinical trials have focused on the ability of these agents to treat patients with UC, not patients with CD. The reason for the lack of development of these agents in CD is not clear. Studies may have been conducted only in subjects with UC, but the possibility exists that further studies are not being planned due to lack of demonstrated efficacy in this population. If so, this would run counter to the initial anti-integrin experience with efficacy of both natalizumab and vedolizumab demonstrated in patients with CD. Regardless, the lack of data presented in CD for anti-integrins currently being developed is a cause for concern and may reflect a therapeutic limitation in newer agents that have not yet come to market. Natalizumab and vedolizumab both, however, prove that targeting integrins is an effective mechanism for the treatment of CD and, as such, continued development of anti-integrins is warranted. As with anti-TNF therapy, it has been shown that antibodies can develop to vedolizumab. Having other agents available within this drug class will provide patients with options for continued integrin blockade if they lose response to their initial anti-integrin agent.
It is clear that the anti-integrin field will continue to develop over the coming years. Even if more promising data does not emerge regarding the efficacy of agents in development for the treatment of CD, data does exist suggesting a role for their continued development for the treatment of patients with UC. It is very likely that additional anti-integrins will come to market and gain approval at least for use in patients with UC.
Regarding vedolizumab, whether it is equally effective in CD and UC is currently being investigated. The phase III GEMINI studies, which demonstrated the ability of vedolizumab to maintain remission in UC and CD, examined response rates when compared to placebo at week 6. In CD, 31.4% of subjects responded to vedolizumab compared to 25.7% of those receiving placebo (p = 0.23).[27] This difference did not reach statistical significance with a delta value of only 5.7%. In UC, 47.1% of subjects responded to vedolizumab compared to 25.5% of those receiving placebo (p < 0.001). [28] This difference was statistically significant with a delta value of 21.6%. It has been suggested that these data indicate that vedolizumab is more effective for treating UC than CD; however, it is not clear that week 6, the time point used in the GEMINI studies, is the most appropriate to assess vedolizumab’s efficacy. It is also difficult to compare response to treatment across different studies due to differences in the disease states and populations. CD is a much more heterogeneous disease than UC. CD disease course is frequently complicated by stricturing and penetrating complications which are less likely to response to anti-integrin therapy. Although excluded from clinical trials, these patients may be inadvertently included in pivotal trials. In addition, there is often a disconnect between disease activity and subjective symptoms in patients with CD. Other mechanisms for abdominal pain and diarrhea such as postsurgical changes, small intestinal bacterial overgrowth, concurrent psychiatric disease, and superimposed irritable bowel syndrome often increase disease activity scores resulting in lower response rates. In addition, patients treated with vedolizumab as part of the pivotal CD trials were more likely to be treated with an anti-TNF, including treatment with two or more anti-TNF agents. A recent study determined response rates in 172 refractory IBD patients (107 CD, 59 UC, and 6 IBD of undetermined type) treated with vedolizumab at two large academic centers; 49% and 54% of patients with CD and UC responded to treatment, respectively.[63] This difference was not statistically significant. These patients differed significantly from those in the GEMINI studies, with only 35% meeting eligibility criteria for GEMINI. Over 70% had failed at least two anti-TNFs. Of those who were on steroids, over 70% were off of steroids by week 14. The authors argued that these data provide further evidence supporting vedolizumab’s efficacy, even in a highly refractory, real-world population.
At present, vedolizumab is the de facto sole anti-integrin biologic available for the treatment of patients with CD. Since its efficacy is similar to natalizumab and side-effect profile is more favorable, our practice has been to treat patients refractory to or intolerant of anti-TNF therapy with vedolizumab. We also consider vedolizumab as ‘first-line’ biologic therapy in patients with contraindications to anti-TNF therapy and in older patients. We no longer offer natalizumab to patients with CD due to the risk of developing PML unless they have concurrent multiple sclerosis. Patients who were treated with natalizumab prior to vedolizumab becoming available have been transitioned to vedolizumab therapy.
Footnotes
Declaration of interest
This work was supported by AHRQ grant R01 HS-018975 to RK Cross and NIH grant P30 DK0-090868 to LP McLean. In addition, RK Cross has received an educational grant and income from consulting for Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Molodecky NA, Soon IS, Rabi DM, et al. increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):4654, e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 5.Satsangi J, Jewell DP, Rosenberg WM, et al. Genetics of inflammatory bowel disease. Gut. 1994;35(5):696–700. doi: 10.1136/gut.35.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd DN, Langham S, Severac HC, et al. The economic and quality-of-life burden of Crohn’s disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci. 2015;60(2):299–312. doi: 10.1007/s10620-014-3368-z. [DOI] [PubMed] [Google Scholar]
- 7.Schirbel A, Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. 2010;11(5):266–276. doi: 10.1111/j.1751-2980.2010.00449.x. [DOI] [PubMed] [Google Scholar]
- 8.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263(6):597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 9.Birrenbach T, Bocker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10(6):848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 10.McGovern D, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology. 2015;149:11631176, e2. doi: 10.1053/j.gastro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler D, Chapman T, Yang -L-L, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864–875. doi: 10.1124/jpet.109.153973. [DOI] [PubMed] [Google Scholar]
- 12.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87(12):1697–1701. doi: 10.1046/j.1365-2168.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 13.Leung Y, Panaccione R. Anti-adhesion molecule strategies for Crohn disease. BioDrugs. 2008;22(4):259–264. doi: 10.2165/00063030-200822040-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 15.McLean LP, Shea-Donohue T, Cross RK. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy. 2012;4(9):883–898. doi: 10.2217/imt.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreiro O, Sanchez-Madrid F. Molecular basis of leukocyte-endothelium interactions during the inflammatory response. Rev Esp Cardiol. 2009;62(5):552–562. doi: 10.1016/s1885-5857(09)71837-7. [DOI] [PubMed] [Google Scholar]
- 17.Fiorino G, Correale C, Fries W, et al. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6(4):567–572. doi: 10.1586/eci.10.40. [DOI] [PubMed] [Google Scholar]
- 18.Pan WJ, Hsu H, Rees WA, et al. Pharmacology of AMG 181, a human anti-alpha4 beta7 antibody that specifically alters trafficking of gut-homing T cells. Br J Pharmacol. 2013;169(1):51–68. doi: 10.1111/bph.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132(5):1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 20•.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353(18):1912–1925. doi: 10.1056/NEJMoa043335. Randomized, placebo-controlled, multicenter trial demonstrating maintenance of response and remission in CD patients who responded to induction therapy with natalizumab. [DOI] [PubMed] [Google Scholar]
- 21.Hunt D, Giovannoni G. Natalizumab-associated progressive multifocal leucoencephalopathy: a practical approach to risk profiling and monitoring. Pract Neurol. 2012;12(1):25–35. doi: 10.1136/practneurol-2011-000092. [DOI] [PubMed] [Google Scholar]
- 22.Biogen Idec, Inc. TYSABRI(R), prescribing information. Cambridge (MA): Biogen Idec; 2012. [Google Scholar]
- 23•.Chandar AK, Singh S, Murad MH, et al. Efficacy and safety of natalizumab and vedolizumab for the management of Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(7):1695–1708. doi: 10.1097/MIB.0000000000000373. Meta-analysis demonstrating efficacy of natalizumab and vedolizumab for inducing remission and response in patients with CD. [DOI] [PubMed] [Google Scholar]
- 24.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- 25.Milch C, Wyant T, Xu J, et al. Vedolizumab does not reduce the CD4+: CD8+ ratio in the CSF of healthy volunteers. 19th United European Gastroenterology Week; Stockholm. 2011. [Google Scholar]
- 26.Fedyk E, Csizmadia V, Shyu W, et al. The gastrointestinal-selective biologic vedolizumab does not impair immune surveillance of the central nervous system in non-human primates. Inflamm Bowel Dis. 2011;17(Supplement 1):S4–S5. [Google Scholar]
- 27••.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. doi: 10.1056/NEJMoa1215739. Phase III trial demonstrating efficacy of vedolizumab as an induction and maintenance agent in patients with CD. [DOI] [PubMed] [Google Scholar]
- 28.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 29.United States Food and Drug Administration. FDA approves Entyvio to treat ulcerative colitis and Crohn’s disease. Silver Spring (MD): FDA; 2014. [Google Scholar]
- 30•.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618627, e613. doi: 10.1053/j.gastro.2014.05.008. Phase III trial demonstrating efficacy of vedolizumab in inducing remission at week 10 in CD patients who had previously failed anti-TNF therapy. [DOI] [PubMed] [Google Scholar]
- 31.Rutgeerts PJ, Fedorak RN, Hommes DW, et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut. 2013;62(8):1122–1130. doi: 10.1136/gutjnl-2011-301769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cepek KL, Parker CM, Madara JL, et al. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150(8 Pt 1):3459–3470. [PubMed] [Google Scholar]
- 33.Karecla PI, Bowden SJ, Green SJ, et al. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 beta 7 (alpha E beta 7) Eur J Immunol. 1995;25(3):852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 34.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162(11):6641–6649. [PubMed] [Google Scholar]
- 35.Elewaut D, Van Damme N, De Keyser F, et al. Altered expression of alpha E beta 7 integrin on intra-epithelial and lamina propria lymphocytes in patients with Crohn’s disease. Acta Gastroenterol Belg. 1998;61(3):288–294. [PubMed] [Google Scholar]
- 36.Oshitani N, Watanabe K, Maeda K, et al. Differential expression of homing receptor CD103 on lamina propria lymphocytes and association of CD103 with epithelial adhesion molecules in inflammatory bowel disease. Int J Mol Med. 2003;12(5):715–719. [PubMed] [Google Scholar]
- 37.Ludviksson BR, Strober W, Nishikomori R, et al. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2−/− mice. J Immunol. 1999;162(8):4975–4982. [PubMed] [Google Scholar]
- 38.Stefanich EG, Danilenko DM, Wang H, et al. A humanized monoclonal antibody targeting the beta7 integrin selectively blocks intestinal homing of T lymphocytes. Br J Pharmacol. 2011;162(8):1855–1870. doi: 10.1111/j.1476-5381.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danilenko DM, Wang H. The yin and yang of immunomodulatory biologics: assessing the delicate balance between benefit and risk. Toxicol Pathol. 2012;40(2):272–287. doi: 10.1177/0192623311430237. [DOI] [PubMed] [Google Scholar]
- 40.Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384(9940):309–318. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 41.Takazoe M, Watanabe M, Kawaguchi T, et al. Oral alpha-4 integrin inhibitor (AJM300) in patients with active Crohn’s disease — a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;136(5):A-181. [Google Scholar]
- 42.Yoshimura N, Watanabe M, Motoya S, et al. Safety and efficacy of AJM300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology. 2015;149(7):1775–1783. e2. doi: 10.1053/j.gastro.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 43.Pan WJ, Kock K, Rees WA, et al. Clinical pharmacology of AMG 181, a gut-specific human anti-alpha4beta7 monoclonal antibody, for treating inflammatory bowel diseases. Br J Clin Pharmacol. 2014;78(6):1315–1333. doi: 10.1111/bcp.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermeire S, Ghosh S, Panes J, et al. The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut. 2011;60(8):1068–1075. doi: 10.1136/gut.2010.226548. [DOI] [PubMed] [Google Scholar]
- 45.Sandborn WJ, Lee SD, Tarabar D, et al. Anti-MAdCAM-1 antibody (PF-00547659) for active refractory Crohn’s disease: results of the OPERA study. Gastroenterology. 2015;148(4):S-162. [Google Scholar]
- 46.Yacyshyn B, Chey WY, Wedel MK, et al. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active Crohn’s disease. Clin Gastroenterol Hepatol. 2007;5(2):215–220. doi: 10.1016/j.cgh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 47.National Institutes of Health. Open-label extension and safety study for patients with Crohn’s disease previously enrolled in the etrolizumab phase III study GA29144. National Institutes of Health; 2015. [Google Scholar]
- 48.National Institutes of Health. Open-label extension and safety study for patients with ulcerative colitis previously enrolled in etrolizumab phase III studies. National Institutes of Health; 2015. [Google Scholar]
- 49.National Institutes of Health. A study comparing the efficacy and safety of etrolizumab with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients naive to TNF inhibitors (Study #1) National Institutes of Health; 2015. [Google Scholar]
- 50.National Institutes of Health. A study comparing the efficacy and safety of etrolizumab with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients naive to TNF inhibitors (Study #2) National Institutes of Health; 2015. [Google Scholar]
- 51.National Institutes of Health. A study comparing the efficacy and safety of etrolizumab to infliximab in patients with moderate to severe ulcerative colitis who are naive to TNF inhibitors. National Institutes of Health; 2015. [Google Scholar]
- 52.National Institutes of Health. A study of the efficacy and safety of etrolizumab treatment in maintenance of disease remission in ulcerative colitis patients who are naive to TNF inhibitors. National Institutes of Health; 2015. [Google Scholar]
- 53.National Institutes of Health. A study of the efficacy and safety of etrolizumab in ulcerative colitis patients who are refractory to or intolerant of TNF inhibitors. National Institutes of Health; 2015. [Google Scholar]
- 54.Sugiura T, Kageyama S, Andou A, et al. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. J Crohns Colitis. 2013;7(11):e533–e542. doi: 10.1016/j.crohns.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 55.National Institutes of Health. AMG 181 in subjects with moderate to severe Crohn’s disease. National Institutes of Health; 2015. [Google Scholar]
- 56.National Institutes of Health. Study to test whether PF-00547659 is safe and improves disease symptoms in patients with Crohn’s disease (OPERA) National Institutes of Health; 2015. [Google Scholar]
- 57.Oppenheimer-Marks N, Davis LS, Bogue DT, et al. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991;147(9):2913–2921. [PubMed] [Google Scholar]
- 58.Van Seventer GA, Shimizu Y, Horgan KJ, et al. Remote T cell co-stimulation via LFA-1/ICAM-1 and CD2/LFA-3: demonstration with immobilized ligand/mAb and implication in monocyte-mediated co-stimulation. Eur J Immunol. 1991;21(7):1711–1718. doi: 10.1002/eji.1830210719. [DOI] [PubMed] [Google Scholar]
- 59.Makgoba MW, Sanders ME, Ginther Luce GE, et al. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- 60.National Institutes of Health. Randomized study of topical alicaforsen enema in antibiotic refractory pouchitis. National Institutes of Health; 2015. [Google Scholar]
- 61.Bandzar S, Gupta S, Platt MO. Crohn’s disease: a review of treatment options and current research. Cell Immunol. 2013;286(12):45–52. doi: 10.1016/j.cellimm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Sandborn WJ, Feagan BG, Reinisch W, et al. Mo1217 effects of continued vedolizumab therapy for Crohn’s disease in week 6 induction therapy nonresponders. Gastroenterology. 2014;146(5):S-588. [Google Scholar]
- 63.Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21(12):2879–2885. doi: 10.1097/MIB.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]