Abstract
Glioblastoma multiforme is the most malignant tumor of the brain and is challenging to treat due to its highly invasive nature and heterogeneity. Malignant brain tumor displays high metabolic activity which perturbs its redox environment and in turn translates to high oxidative stress. Thus, pushing the oxidative stress level to achieve the maximum tolerable threshold that induces cell death is a potential strategy for cancer therapy. Previously, we have shown that gap junction inhibitor, carbenoxolone (CBX), is capable of enhancing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in glioma cells. Since CBX is known to induce oxidative stress, we hypothesized that the addition of another potent mediator of oxidative stress, powerful SOD mimic MnTnBuOE-2-PyP5+ (MnBuOE), could further enhance TRAIL-driven therapeutic efficacy in glioma cells. Our results showed that combining TRAIL + CBX with MnBuOE significantly enhances cell death of glioma cell lines and this enhancement could be further potentiated by CBX pretreatment. MnBuOE-driven cytotoxicity is due to its ability to take advantage of oxidative stress imposed by CBX + TRAIL system, and enhance it in the presence of endogenous reductants, ascorbate and thiol, thereby producing cytotoxic H2O2, and in turn inducing death of glioma cells but not normal astrocytes. Most importantly, combination treatment significantly reduces viability of TRAIL-resistant Asian patient-derived glioma cells, thus demonstrating the potential clinical use of our therapeutic system. It was reported that H2O2 is involved in membrane depolarization-based sensitization of cancer cells toward TRAIL. MnBuOE is entering Clinical Trials as a normal brain radioprotector in glioma patients at Duke University increasing Clinical relevance of our studies.
Keywords: Carbenoxolone, TRAIL-modified human mesenchymal stem cells, Manganese porphyrin, Glioma, SOD mimic, Ascorbate, NAC
Introduction
Trail
Glioblastoma multiforme (GBM) is the most malignant tumor of the brain. Specific tumor targeting has posed some treatment challenges due to its highly invasive nature and heterogeneity. Human mesenchymal stem cells (MSCs) have been employed as potential carriers of therapeutic agents due to their inherent ability to migrate towards tumor cells [1]. Genetically modified MSCs with interferon-β have been shown to augment antitumor effect and prolong the survival of glioma-bearing animals [2]. Likewise, MSCs derived from the umbilical cord blood, with or without activation by a series of cytokines such as interleukin-2, interleukin-15, granulocyte macrophage colony-stimulating factors, have also been shown to be cytotoxic to human glioma cells but not to normal brain cells [3]. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-expressing MSCs have also been shown to elicit significant anti-tumor effect in models of lung and cervical cancer [4], as well as brain cancer [5]. TRAIL-modified MSCs are capable of clearing lung metastases in the treated animals when compared to non-treated animals [6].
Carbenoxolone, CBX
In our previous study, we have shown that dual arm therapy using TRAIL-modified MSCs in the presence of a gap junction inhibitor CBX could significantly prolong the survival of intracranial glioma bearing mice [7]. One of the underlying mechanisms of CBX is attributed to its capability to upregulate the expression of TRAIL death receptor, DR5 (TRAIL-R2). Increase in DR5 expression has been reported in many studies that explore the use of therapeutic compounds, such as curcumin [8], protease inhibitors MG132 [9] and lipoxygenase inhibitor MK886 [10], to enhance efficacy of TRAIL. It has also been demonstrated that upregulation of DR5 could be mediated through induced oxidative stress which in turn, activated the CCAAT/enhancer-binding protein homologous protein (CHOP) [9, 10]. Similarly, CBX has also been reported to induce oxidative stress through the production of reactive oxygen species in neurons [11], as well as in liver and brain mitochondria [12, 13].
Mn Porphyrins
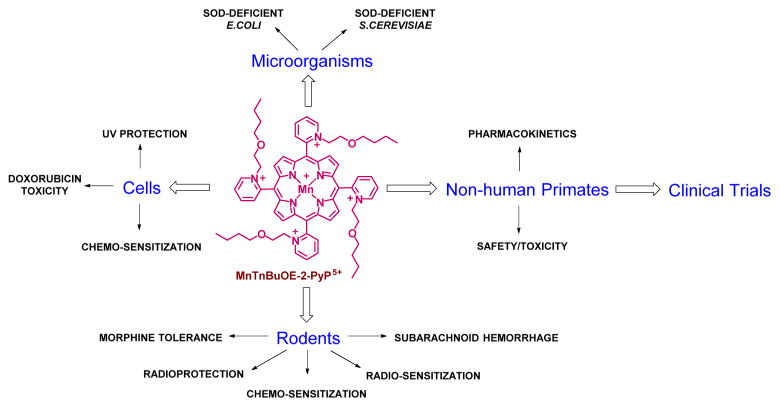
We have developed cationic Mn(III) ortho isomeric N-substituted pyridylporphyrins (MnPs) as SOD mimics [14, 15]. Over years we have shown that they are also the most potent peroxynitrite scavengers [15]. We have further shown that while their actions in vivo may be not limited to SOD mimicking (see below), their SOD-like activity parallels their therapeutic potency for the reasons detailed in recent reviews [14, 15]. In brief, their redox-properties and charge are tuned to be optimized for redox cycling with redox-active components of cellular metabolism. We have also shown that their therapeutic effects are in large part controlled by their bioavailability. Due to pentacationic charge those compounds are hydrophilic. In an effort to increase their lipophilicity and in turn mitochondrial accumulation as well as transport across the blood brain barrier, first a lipophilic MnTnHex-2-PyP5+ was developed. Its structure was subsequently modified with the goal to suppress its micellar properties and in turn its toxicity [14, 16]. The oxygen atoms were introduced into alkyl pyridyl chains of MnTnHex-2-PyP5+. As a result of such synthetic approach, Mn(III) meso-tetrakis(N-n-butoxyethylpyridinium-2-yl) porphyrin, MnTnBuOE-2-PyP5+ (BMX-001, MnBuOE) emerged as less toxic while still a compound lipophilic enough, with nearly identical and preferential mitochondrial over cytosolic accumulation [14, 16]. All aspects of the work done on this compound are summarized in Scheme 1.
Scheme 1.
Major therapeutic effects, as well as the other aspects of development of Mn(III) meso-tetrakis(N-n-butoxyethylpyridinium-2-yl)porphyrin, MnTnBuOE-2-PyP5+ (MnBuOE, BMX-001), that guided its Clinical development [14, 16–24]
MnBuOE has been successfully tested in numerous cellular and animal models. Its heart and brain mitochondrial accumulation, as well as its plasma and organ pharmacokinetic profiles have been established. Its radioprotective properties on salivary glands, mouth mucosa and normal brain have been demonstrated [14, 17, 24]. Moreover, the differential effects of this and analogous cationic Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP5+ (BMX-010, AEOL 10113) and Mn (III) meso-tetrakis (N-n -hexylpyridinium-2-yl)porphyrin, MnTnHex-2-PyP5+ on normal vs cancer tissue have been reported and are based on the differential redox environments of those tissues. Indeed while radioprotection of normal brain was demonstrated, the tumor radiosensitization was shown in a sc mouse xenograft model of human glioma cell line D 245-MG [24]. Further, in a cellular lymphoma model MnBuOE enhanced the cytotoxic effect of dexamethasone [25–27], while, MnTE-2-PyP5+ enhanced the cytotoxic effect of ascorbate in a 4T1 mouse sc flank tumor model [28]. MnBuOE has successfully passed safety/toxicity studies both in mice and in non-human primates [Gad, Spasojevic, Crapo et al., unpublished, [22]]. Due to such dual role of MnPs and the therapeutic effects demonstrated in rodent models, NIH funding has been secured for Phase I/II Clinical trials on radioprotection of salivary glands and mouth mucosa with head and neck cancer patients and on radioprotection of normal brain tissue with glioma patients (first patients are expected beginning January 2016).
The Goal of This Study – Exploring Oxidative Stress-Driven Mn Porphyrin Mechanism(s) of Action(s)
We have demonstrated that actions of MnPs are redox-based [14]. The concentrations of MnPs and the reactive species they encounter in vivo, as well as their co-localization control the type and magnitude of MnPs reactions. MnPs readily cycle with endogenous reductants, ascorbate and thiols - simple thiols and thiols of signaling proteins such as NF-κB. Such cycling is critically involved in the mechanism(s) of MnPs actions [15, 29, 30]. Aqueous chemistry and in vivo reactions of MnPs are summarized in recent review in Redox Biology [15], while therapeutic effects of analogous compounds are summarized in several manuscripts of the Forum Issue on “SOD therapeutics” of Antioxid Redox Signal, 2014 [Antioxidant & Redox Signaling 2014 Forum Issues on “SOD therapeutics” (vol.20/15)]. In cancer cells MnP can produce H2O2: (i) in its own right (whereby using the available cellular reductants in a re-oxidation step), or (ii) in combination with exogenous drugs such as steroids (or other chemo-agents), or (iii) with radiation therapy. It can subsequently utilize the H2O2 produced for the catalysis of H2O2-driven oxidation of critical thiol-bearing proteins such as NF-κB and complexes I and III of mitochondrial respiration [25, 26]. The oxidative modifications of protein thiols led to their concomitant inactivation. For such reasons and based on its potential in treatment of glioma patients, we have chosen to see if MnP will enhance the cytotoxicity of TRAIL + CBX and if this will not been suppressed, but rather enhanced, with major endogenous cellular reductans, ascorbate and thiols. Thiols were exemplified herein with N-acetylcysteine, NAC. NAC is frequently used as a mechanistic tool in in vitro and in vivo studies. Depending upon the cellular redox environment, NAC can act as either pro-or antioxidant [31–36]; both aspects are also demonstrated in this study.
Clinical Relevance of our Study
This study has a strong clinical relevance. A number of compounds, including H2O2, sensitize TRAIL-resistant gliomas [37]. We therefore aim here to show that our treatment (having production of H2O2 in its essence) will overcome resistance to TRAIL frequently seen with Asian gliomas. Ascorbate has already been in Phase I Clinical Trials in its own right [38, 39]. Its oxidation, catalyzed by endogenous metalloproteins resulting in cytotoxic H2O2 production, was proposed as its mode of action. However, we have shown that cationic Mn(III) ortho N-substituted pyridylporphyrins, one of which will be used herein, are superior to endogenous metalloproteins as they are optimized as catalysts for ascorbate oxidation [30, 40]. Thus, such MnPs are prospective for clinical development as enhancers of ascorbate/H2O2 driven cancer cell killing. CBX is clinically approved for the treatment of esophageal ulceration [41], and different natural compounds as sulfur donors, such as garlic components [42], disulfiram [43], or even the simpliest thiol, H2S, bear therapeutic potential [44]. MnBuOE, which we used herein, has excellent safety profile, and shows excellent radioprotection in preclinical studies [14, 15, 24, 45]. It is therefore entering Clinical Trials at Duke Cancer Institute on glioma and head & neck cancer patients as a radioprotector of normal tissue (normal brain, salivery glands and mouth mucosa) while radiosensitizing tumors. Meanwhile, we have shown that ascorbate when combined with MnBuOE and radiation enhances significantly tumor suppression (in a mouse sc flank model of 4T1 mammary carcinoma) when compared with MnBuOE + radiation treatment [46]. We thus expect that introduction of ascorbate into Clinical Trials to be straightforward due to the successful testing of ascorbate alone in Phase I Clinical Trials on pancreatic cancer [39]. Based on the data obtained herein, we believe that successful therapeutic modalities studied herein (CBX + TRIAL + MnBuOE + ascorbate) bear potential to be translated into Clinical Trials also.
Materials and Methods
Cell Lines and Reagents
This study has been approved by the SingHealth Centralized Institutional Review Board, Singapore. Human glioma cells Gli36 expressing a constitutively active variant of EGFRvIII (denoted as ΔGli36 cells are kindly provided by Esteves MS, University of Massachusetts) were cultured as previously described [47]. Human glioblastoma cell lines Gli36 (kind gift from A.T. Campagnoni, UCLA School of Medicine, Los Angeles), U87MG (American Type Culture Collection, Rockville, MD, USA) were cultured in DMEM supplemented with 10 % fetal bovine serum (FBS) (Hyclone Laboratories), 100 U/ml penicillin/streptomycin (Invitrogen Life Technologies), 2 mM L-glutamine (Sigma-Aldrich). Immortalized normal human astrocytes (iNHA) that overexpress E6, E7, and human telomerase reverse transcriptase (hTERT) were kindly provided by R.O. Pieper (University of California, San Francisco, CA) and was cultured in DMEM supplemented with 10 % FBS, 0.5 μg/ml puromycin (Invivogen, San Diego, CA), 25 μg/ml blasticidin and 1.25 μg/ml fungizone (Life Technologies, Grand Island, NY). The identity of the cells was authenticated by short tandem repeats profiling. Asian primary GBM lines, GBM8401, GBM8901 and G5T/VGH were purchased from Food Industry Research and Development Institute, Bioresource Collection and Research Center (Hsinchu, Taiwan), and were cultured as previously described [7].
Isolation of primary glioma cells NNI23 (Age: 60, Sex: F) from local GBM patients were performed, after informed consent, as follows. In brief, brain tumor specimens, from patients undergoing tumor resection surgery, were cut into smaller pieces and washed thoroughly with phosphate-buffered saline (PBS) prior to be digested with 0.25 % Trypsin at 37 °C for 30 min with constant stirring. Equal volumes of Astrocyte Growth Medium (AGM; Lonza, Basel, Switzerland) were then added to the suspension. Tumor pieces were allowed to settle prior to collecting the supernatant and filtering through a 70-μm membrane filter (BD Biosciences, Franklin Lakes, NJ). Filtered supernatant was centrifuged at 1000 rpm for 5 min at room temperature (r. t). The cell pellet was then resuspended in fresh media and plated as usual.
Sodium L-ascorbate, CBX, glycyrrhizic acid (GZA), and N-acetyl-cysteine (NAC) was purchased from Sigma-Aldrich (St. Louis, MO). GZA is structurally similar to CBX without its ability to block gap junction communication; GZA serves as the inactive analogue control for the CBX. MnTnBuOE-2-PyP5+ was synthesized by us [16].
TRAIL Conditioned Media (TRAIL-CM) Collection
TRAIL-CM was obtained from HGCX-TRAIL-transduced MSCs [7]. MSCs were transduced at multiplicity of infection (MOI) of 1, and after 24 h, CM from transduced MSC was collected and subjected to centrifugation at 1000 rpm for 5 min. TRAIL-CM was quantified using the human TRAIL/TNFSF10 Immunoassay Kit (R & D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Absorbance reading at 450 nm was obtained by the TECAN plate reader (Männedorf, Switzerland).
Cell Counting Kit 8 (CCK-8) Cell Viability Assay
Cell viability assay was determined using Cell Counting Kit-8 (CCK-8) solution (Dojindo Molecular Technologies Pte. Ltd., Japan), which is a colorimetric assay that measures dehydrogenase activities of living cells. At pre-determined assay time point, 10 % (v/v) CCK-8 solution was added into each well of 96-well plate and incubated for 1 h. Absorbance at 450 nm was obtained by Victor 3 V microplate reader (PerkinElmer, Waltham, MA).
Immunoblotting
Cells were harvested and pelleted prior to lysis with lysis buffer containing 50 mM Tris-Cl, 150 mM NaCl, 0.5 % SDS, 1 % Triton X-100 with protease inhibitor cocktail (Roche). 50 μg of total protein lysates were resolved on 12 % SDS polyacrylamide gel (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Darmstadt, Germany), and probed with the following primary antibodies: caspase 3, Bcl-2, Bax, Bid (1:1000; all from Cell Signaling Technology Inc., Danvers, MA) and pan-actin antibody (1:10,000; NeoMarkers, Fremont, CA). Goat anti-mouse HRP-conjugated secondary antibody (1:10,000, Dako) or goat anti-rabbit HRP-conjugated secondary antibody (1:10,000, Dako) were used. All antibodies were diluted in blocking buffer [5 % bovine serum albumin (Sigma-Aldrich), 10 mM Tris-HCl pH 7.4, 100 mM NaCl, 0.1 % Tween-20 (Merck)]. Protein bands were visualized using chemiluminesence by adding ECL substrate solution containing luminol in peroxide buffer (Thermo Scientific) to the membrane for 30s. Protein expression was quantified using MetaVue software (Molecular Devices, Sunnyvale, CA), normalized against actin levels.
Cell Death Assay
Cell death was determined using trypan blue dye exclusion assay. In brief, cells were harvested by trypsinization and re-suspended in 1:1 ratio of PBS and 0.4 % Trypan blue dye (Sigma-Aldrich). Cells were incubated for 1 min prior to cell count. Cell death was expressed as percentage of blue cells over total cells.
Statistical Analysis
All statistical analysis was performed by student’s t-test using Prism 3.0 (Graphpad Software Inc., San Diego, CA). p-value <0.05 was considered statistically significant.
Results
MnBuOE Enhanced CBX-Mediated TRAIL-Induced Cell Death in Glioma but not Immortalized Normal Human Astrocytes
Previously, we showed that concurrent treatment of human glioma cells with CBX and MSC-TRAIL markedly increase TRAIL-induced apoptosis in vitro and prolonged survival in intracranial-glioma bearing mice [7]. The enhanced efficacy of TRAIL correlated with upregulation of DR5 expression, blockage of the gap junction intercellular communication and downregulation of connexins 43 expression. Extended studies confirmed that the death signals from DR5 resulted in activation of caspase-3 (Supplementary Figure S1A). In addition, a mitochondria-dependent amplification loop by activating Bid was observed (Supplementary Figure S1B). Collectively, CBX but not its inactive analogue GZA could promote apoptosis in the presence of MSC-TRAIL.
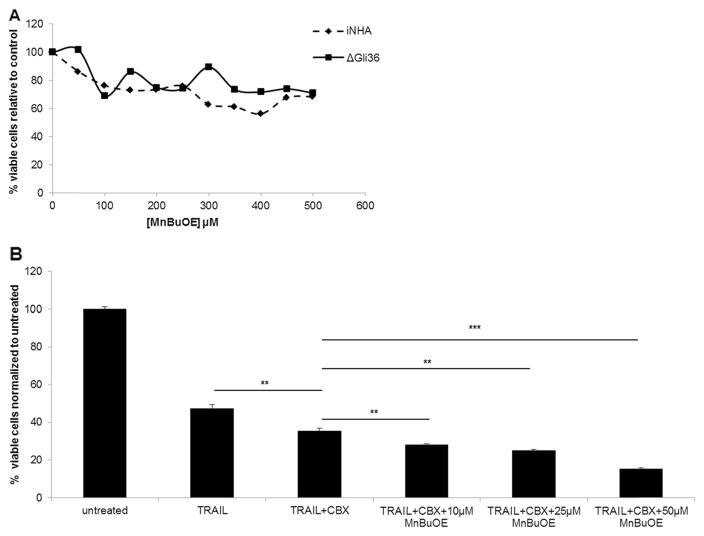
Since CBX is known to induce oxidative stress, we hypothesized that the addition of another potent mediator of oxidative stress, powerful SOD mimic could further enhance TRAIL-driven therapeutic efficacy in glioma cells. The effect of MnBuOE on the viability of ΔGli36 and iNHA cells was first determined by exposing the cells to a range of concentration of MnBuOE (Fig. 1a). MnBuOE did not cause significant toxicity to the cells at lower concentration (≤ 50 μM) but affected iNHA viability at higher concentrations. The effect of MnBuOE in CBX-mediated TRAIL-induced cell death was then evaluated. ΔGli36 cells were exposed to different concentrations of MnBuOE in combination with pre-optimized concentration of TRAIL and 100 μM CBX or GZA. The addition of MnBuOE could further enhance glioma cell death when compared to a single treatment (TRAIL) or double treatment (TRAIL and CBX) (Fig. 1b). Collectively, the results showed that increasing the concentration of MnBuOE could induce glioma cell death in a dose-dependent manner.
Fig. 1.
MnBuOE is non-cytotoxic and enhanced CBX-mediated TRAIL-induced cell death in human glioma. a The effect of various concentrations of MnBuOE on ΔGli36 human glioma cells and immortalized normal human astrocytes (iNHA) was examined at 48 h. At the desired time point, CCK-8 assay was performed to determine the cell viability. b ΔGli36 glioma cells were treated with different dose of MnBuOE in presence of TRAIL and 100 μM of CBX. CCK-8 assay was then performed at 48 h to determine the effect of treatment on the cells’ viability. **, p < 0.01; ***, p < 0.001
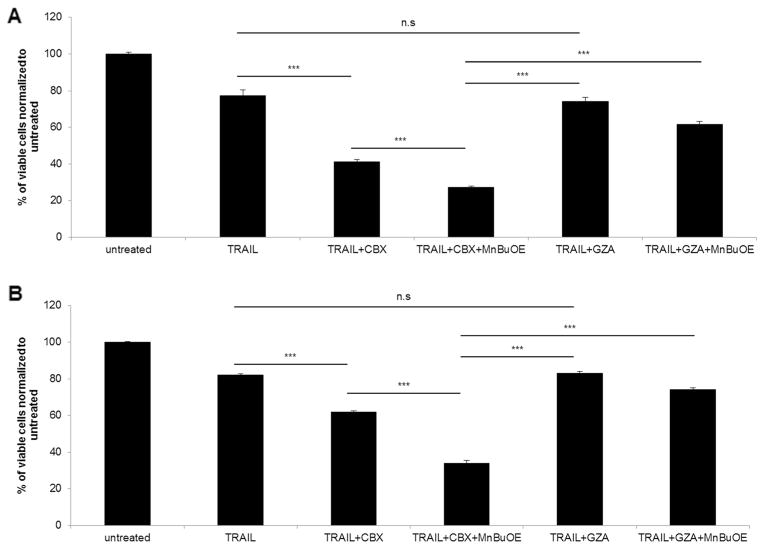
We then evaluated the effect of combination treatment at different time points. ΔGli36 and iNHA cells were exposed to combined treatment of TRAIL, CBX and MnBuOE (50 μM); their viability was then assessed by CCK-8 assay. As confirmed in Fig. 2ai, the triple combination of TRAIL, CBX and MnBuOE significantly enhanced ΔGli36 cell death at all-time points when compared to single (~20–42 % cell death) or double (~8–28 % cell death) treatment. The increase in glioma cell death was not seen when GZA was used instead of CBX (Fig. 2aii). By contrast, the effect of the triple combination in iNHA cells was markedly reduced (~20–30 % cell death; Fig. 2bi) when compared to ~70–90 % in human gliomas Fig.(2ai). Even though the combination effect with 50 μM MnBuOE demonstrated the best efficacy (Fig. 1b), this concentration was cytotoxic to the iNHA as reduced viability was observed in the GZA co mbination treatment (Fig. 2bii). Based on this, 25 μM MnBuOE was used in subsequent combination experiments. Taken together, these results suggested that MnBuOE could further enhance CBX-mediated TRAIL-induced cell death in glioma cells with minimal cytotoxic effect on iNHA.
Fig. 2.
Triple combination of TRAIL, CBX and MnBuOE conferred better efficacy in human gliomas than single or double treatment. a ΔGli36 glioma cells and b Immortalized normal human astrocytes, iNHA cells were subjected to TRAIL, (i) 100 μM CBX or (ii) GZA, as well as 50 μM MnBuOE for 48 h and 72 h. At the desired time points, cell viability was determined with CCK-8 assay. n.s, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
Pre-Conditioning of Glioma Cells with CBX Makes Them More Susceptible to TRAIL + CBX + MnBuOE
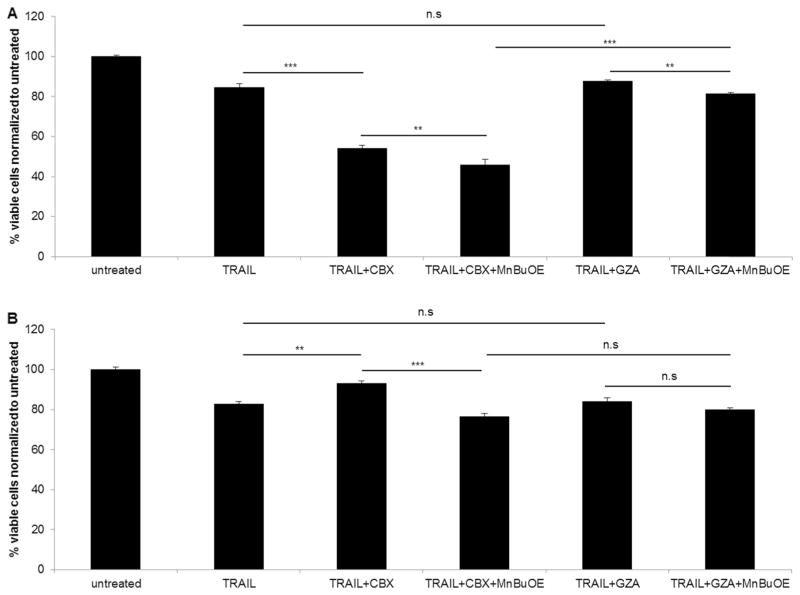
We then asked whether pre-conditioning the cells with CBX could further enhance the efficacy. ΔGli36 cells were pre-incubated with 100 μM CBX for 24 h prior to exposure to combination treatment. The results showed pre-incubation of MSC-TRAIL CM treated cells with CBX further increased cell death by 21 % (Fig. 3a) when compared to cells without pre-incubation (Fig. 3b). Similarly, the pre-incubation of MSC-TRAIL CM treated cells with CBX and MnBuOE also enhanced cell death by 7 % (Fig. 3b) when compared to those without pre-incubation. Thus, subsequent experiments were performed by pre-incubating other MSC-TRAIL CM treated cells with CBX. MSC-TRAIL treated U87MG (84.5 % ± 2) and iNHA (82.5 % ± 1.4) resulted in a similar cell kill efficiency, suggesting that these cells were resistant to TRAIL treatment. Pre-treatment of these cells with CBX and CBX with MnBuOE could sensitize U87MG to enhanced cell death by ~30 % and ~39 % respectively. This was not observed with iNHA. In fact, the iNHA-treated with MSC-TRAIL CM and CBX pre-treatment (93.1 % ± 1.3) showed a higher cell viability compared to MSC-TRAIL CM alone (82.5 % ± 1.4). Taken together, these results indicated that pre-conditioning the cells with CBX could further increase the efficacy of a triple combination of TRAIL, CBX and MnBuOE. Such results might have been anticipated given the ability of CBX to impose oxidative stress and increase in H2O2 which, being more expressed during 24 h, MnBuOE used in its H2O2-driven catalysis of critical signaling proteins (Fig. 4).
Fig. 3.
Pre-incubation with CBX enhanced the efficacy of the triple combination in human glioma. ΔGli36 human glioma cells were subjected to either a 24 h pre-incubation or b no pre-incubation with 100 μM CBX or GZA prior to be treated in combination with TRAIL and 25 μM of MnBuOE. Following 48 h of combination treatment, viability of the cells was determined with CCK-8 assay. n.s, p > 0.05; **, p < 0.01; ***, p < 0.001
Fig. 4.
Enhanced efficacy of triple combination of TRAIL, CBX and MnBuOE was also seen in U87MG human glioma cells but not iNHA. a U87MG glioma cells and b iNHA cells were pre-incubated with 100 μM of CBX or GZA for 24 h. Cells were then subjected to combined treatment of TRAIL, CBX and MnBuOE for 48 h, followed by CCK-8 cell viability assay. n.s, p > 0.05; **, p < 0.01; ***, p < 0.001
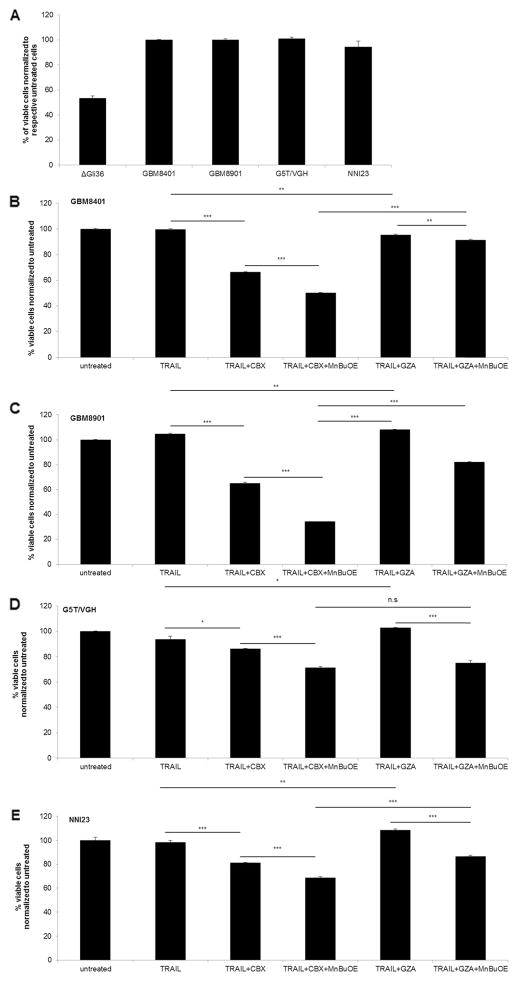
Combination Treatment Significantly Enhanced Cell Death in TRAIL-Resistant Asian Primary Gliomas
In Singapore and Asian countries, the molecular signature of gliomas is neither well-defined nor documented. The latest information available in PubMed search is a study performed almost a decade ago by a local neurosurgeon in the “Journal of Neuro-Oncology”, where he reported that the genetic profiles of Asian glioma patients did not appear to follow the conventional molecular pathways [48]. Thus, we firstly determined the TRAIL-responsiveness of three Asian glioma cell lines derived from Chinese GBM patients from Taiwan (GBM8401; GBM8901; G5T/VGH) and freshly isolated short-term GBM cultures derived from Singaporean patient (NNI23). As observed in Fig. 5a, all of the Asian primary glioma cell lines tested were TRAIL-resistant. By contrast, the glioma cell line derived from a Caucasian patient, ΔGli36, was TRAIL-sensitive. Double combination of TRAIL and CBX could improve their TRAIL responsiveness to different extent in GBM8401 (Fig. 5b; ~33 %), GBM8901 (Fig. 5c; ~40 %), G5T/VGH (Fig. 5d; ~7 %) and NNI23 (Fig. 5e; ~17 %). Furthermore, the presence of MnBuOE could significantly increase TRAIL-induced cell death of these TRAIL-resistant primary gliomas (Fig. 5b). Taken together, these results demonstrated that TRAIL, CBX and MnBuOE could potentially be used to target TRAIL-resistant glioma cells.
Fig. 5.
Triple combination could enhance TRAIL-induced apoptosis in resistant Asian primary GBM. a Short-term culture of Asian primary glioma cells was exposed to TRAIL for 72 h. Cell death was determined with trypan blue dye exclusion assay. Asian primary glioma cells, b GBM8401, c GBM8901, d G5T/VGH, and e NNI23 were pre-incubated with 100 μM of CBX or GZA for 24 h. Cells were then treated with combination treatment of TRAIL, CBX and MnBuOE for 48 h, followed by CCK-8 cell viability assay. n.s, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
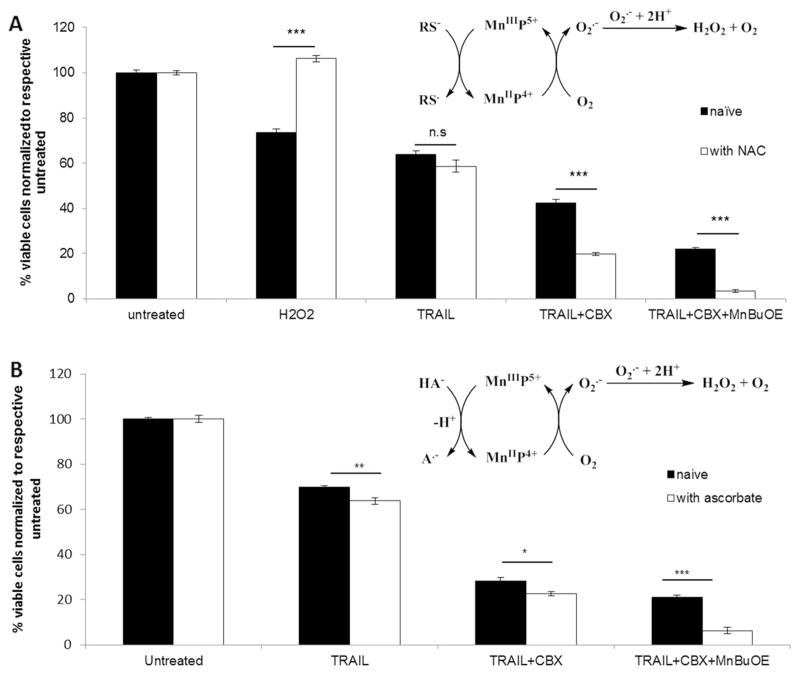
Presence of Cellular Reductants Ascorbate and Thiols, Exemplified with NAC, Further Enhanced Cell Death
Ascorbate and thiols, most so glutathione, are major cellular reductants and are present intracellularly at high mM levels. MnPs are known to readily couple with those reductants as soon as they enter cell. Such coupling plays major role in their actions [31–36]. We thus explored here what would happen with TRAIL + CBX + MnBuOE once it is applied in animals or humans. Our experiment confirmed that the enhancement of CBX-mediated TRAIL-induced cell death by MnBuOE is indeed ROS dependent, as it has been potentiated by the redox-active MnP. The cytotoxicity was further enhanced by the additional source of reactive species – either ascorbate or NAC. ΔGli36 cells were subjected to 1 h pre-incubation with 10 mM NAC prior to the addition of combined treatment. NAC is commonly regarded as an antioxidant, but depending upon the cellular redox environment, it can act as a pro-oxidant also [31–36]. As seen in Fig. 6a, NAC in its own right was able to rescue the cells from.
Fig. 6.
Enhancement of TRAIL-induced apoptosis by triple combination treatment in presence of cellular reductants. ΔGli36 human glioma cells were pre-incubated with 100 μM of CBX for 24 h. Cells were then incubated with a 10 mM NAC for 1 h followed by combination treatment of TRAIL, 100 μM of CBX and 25 μM of MnBuOE or b combination treatment in presence of 0.5 mM ascorbate. Viability was assessed at 48 h post-treatment. Schematic diagrams summarize involvement of redox cycling between MnBuOE and a thiols (RS−) and b ascorbate (HA−). n.s, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001
H2O2-based effects. Yet, presence of NAC did not rescue cells from the effect of triple combination treatment, involving MnBuOE, but further augmented glioma cell death by ~80%. When MnBuOE was added into medium, it cycled with NAC, thereby increasing the levels of peroxides and in turn glioma cell death (Fig. 6a diagram). We have reported such one-electron redox cycling of MnIIIP with different thiols (glutathione, cysteine and NAC) and provided evidence that it led to the generation of thiyl radical and reduced MnIIP [29]. MnIIP will in turn be re-oxidized with either oxygen or superoxide producing superoxide or peroxide, respectively [49]. We further confirmed this observation by utilizing another cellular reductant, ascorbate. The increase in cell death by ~60% was observed with ascorbate addition as compared to TRAIL+CBX+ MnBuOE, which is due to the redox cycling of MnBuOE with ascorbate in a fashion similar to NAC (Fig. 6b diagram).
Discussion
Malignant brain tumor tissues display high metabolic activity through the increased uptake of glucose compared to normal brain tissues [50]. The increase in metabolism, along with perturbed balance between reactive species (oxidants) and endogenous antioxidative defenses (such as SODs, GPx, catalases, peroxiredoxins), translate into high oxidative stress. Such imbalance makes cancer cell more susceptible to any further increase in oxidative stress. Thus, modulation of intracellular ROS level to achieve threshold that induces cell death has been explored as potential strategy for cancer therapy. In this study, we demonstrated that redox-active compound, MnBuOE, could be used to enhance TRAIL-induced glioma cell death in the presence of a gap junction inhibitor. The combination therapy was effective against many of the patient-derived glioma cells which are TRAIL-resistant while only minimal cytotoxicity was seen in immortalized normal human astrocytes and will be further enhanced by endogenous reductants, ascorbate and thiols.
Our results showed that the combination treatment of TRAIL, CBX and MnBuOE promoted glioma cell death when compared to either single treatment of TRAIL or double treatment of TRAIL and CBX at 48 h and 72 h post-treatment (Fig. 2ai). In comparison to the double treatment of TRAIL and CBX group, the addition of MnBuOE exhibited a greater inhibitory effect on cell viability at 72 h compared to 48 h. It is noted, however, that there was an overall increase in cell viability in all treated groups at 72 h, suggesting that a continuous replenishment of the combination agents may be required to maintain or enhance glioma cell death. By contrast, the addition of MnBuOE did not promote further the cell death in those cells treated with TRAIL and GZA, the inactive analogue of CBX (Fig. 2aii). Our results also showed that pre-conditioning the cells with CBX (Fig. 3a and 4a), allowing for the amplification of oxidative stress, further enhanced TRAIL-induced cell death compared to TRAIL alone group or the group without the pre-conditioning step. Statistical analysis showed that enhanced cell death could be observed in ΔGli36 cells pre-treated with CBX (i.e. TRAIL+ CBX in Fig. 3a and 3b has a p value less than 0.001, paired TTEST), and in U87MG cells pretreated with CBX (i.e. the TRAIL + CBX group in Fig. 4a and 4b has a p value of less than 0.001, paired TTEST). CBX has recently been shown to reduce neuroinflammation and protect the mitochondrial functions in the rotenone model of Parkinson’s disease [51]. Specifically, the expression levels of various inflammatory mediators such as COX-2, iNOS, and NF-κB were reduced in the presence of CBX.
Previously, we have reported that treatment of glioma cells with CBX alone induces expression of DR5, while the DR4 expression was not affected [7]. This was not observed with the control GZA. Recent analysis showed that CBX-treated U87MG and ΔGli36 cells could induce cell cycle arrest compared to untreated or GZA-treated control cells (data not shown). Moreover, such impact is differential and expressed on human glioma but not on immortalized human astrocytes. It is highly likely, given the ability of both CBX and MnBuOE to inhibit NF-κB, that this major transcription factor plays a role in our TRAIL + CBX + MnBuOE system. The synergistic/additive effect of TRAIL has also been reported with chemotherapeutic drugs, such as temozolomide and histone deacetylase inhibitors, in glioma cells [52, 53].
Immunoblotting analysis on proteins involved in apoptotic pathways (Supplementary Figure S1) demonstrated no significant change in the expression of Bax protein in TRAIL alone, TRAIL + CBX, or TRAIL + GZA group. However, there was a significant downregulation of Bcl-2 expression brought about by the TRAIL + CBX group (Bax/Bcl-2 ratio = 1.82) which was not observed in the TRAIL alone or the TRAIL + GZA group (Bax/Bcl-2 ratio = 1.36 and 1.23 respectively), suggesting that CBX promotes TRAIL-induced apoptosis via down-regulation of anti-apoptotic proteins as one of its key mechanisms of action (Supplementary Figure S1). Further, the ratio of cleaved caspase 3 to pro-caspase 3 was 0.69 in cells treated with TRAIL and CBX, while the ratio was only 0.33 when the inactive analogue GZA was used instead. The ratio of caspase activities with TRAIL alone, CBX alone and GZA alone were 0.38; 0.38 and 0.35 respectively. This finding, together with findings published by us [7] and other groups [13, 54, 55], reconfirms that CBX modulates proteins involved in apoptotic pathways. The upregulation of pro-apoptotic caspase 3 as well as downregulation of anti-apoptotic Bcl-2 by the action of MnPs in the presence of a exogenous source of H2O2 has been reported [14, 26]. Such effect of MnP (in addition to the joint effect of TRAIL and CBX) contributed to the effects observed in this work.
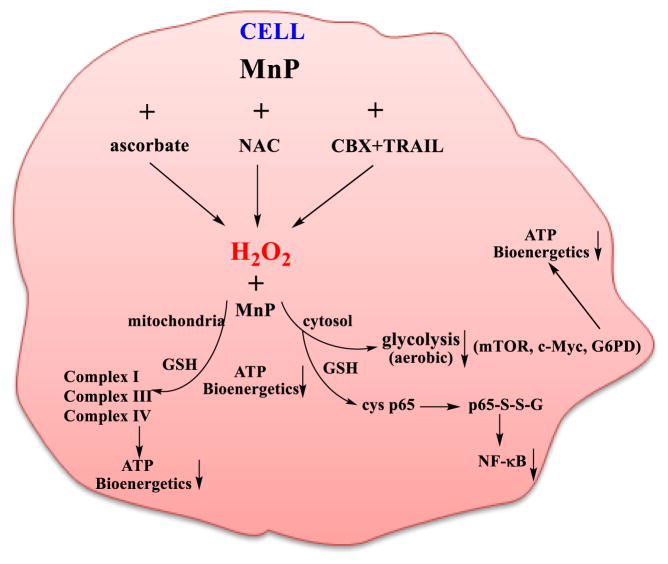
Cancer cells are known to be under constant oxidative stress. While in normal cells H2O2 is readily removed by redundant enzymatic systems, in cancer cells many of those, such as GPx, catalases and peroxiredoxins, are frequently down-regulated; in turn H2O2 accumulates and affects proliferation and metastasis [21, 56]. Cancer cell may use high endogenous H2O2 levels to its own advantage. However, if the levels of H2O2 are excessive (induced by exogenous factors), cancer cell would undergo apoptosis [21]. MnP uses H2O2 to promote apoptosis in cancer yet not in normal tissue as shown here and elsewhere [14]; changes in levels of oxidative stress may render them sensitive towards assaults imposed by TRAIL. It was demonstrated that in vivo MnP readily cycles with different thiols: glutathione, cysteine, protein thiols and NAC [14, 29]. Thiols and ascorbate are major endogenous redox-active species that have protective roles regenerating other endogenous antioxidative defenses such as tocopherol, glutathione peroxidase and peroxiredoxins. However, in the presence of redox-active compounds (metal complexes, metal salts, Mn porphyrins, etc.), which catalyze the oxidation of thiols and ascorbate, the excessive amounts of H2O2 are produced as indicated in Fig. 6. It is critical to understand the behavior of our TRAIL + CBX + MnBuOE system once it reaches cell and encounters different redox-active species/systems. It is in particular important to address the reaction of such system with endogenous antioxidative species (such as GSH, cysteine, ascorbate, protein cysteines) as they are present in vivo at high concentrations and favor reactions with MnP. Due to compromised balance between reactive species and cellular antioxidative defenses, cancer cell can tolerate much smaller increase in oxidative stress burden than normal cell. This is routinely used for therapeutic purposes where oxidative stress burden of a cancer cell is increased with different strategies such as radio- and chemotherapy. In our case such system TRAIL + CBX increased oxidative burden inducing cancer cell death. The cytotoxicity was further enhanced by the addition of MnBuOE. This strongly suggests that further perturbation of cellular redox environment was inflicted by the addition of MnBuOE to TRAIL/CBX. The perturbance in redox environment inflicted by CBX, presumably resulting in increased levels of reactive species was used by MnP in the catalysis of oxidative processes. No impact was observed when CBX-inactive analog GZA was used. The effect is further increased when another redox-active compound, either ascorbate or NAC, was added to TRAIL + CBX+ MnBuOE. Earlier reports demonstrated that H2O2 is produced during cycling of either MnP/ascorbate or MnP/thiol (depicted in Fig. 6 and in Scheme 2). In such scenario MnIIIP gets reduced readily with ascorbate or thiol in a 1st step producing either ascorbyl or thiyl radical, respectively. MnIIP gets subsequently re-oxidized with either oxygen or superoxide in a 2nd step thereby producing eventually H2O2 [Tovmasyan et al., Free Radical Biology and Medicine, In revision]. H2O2 was shown to be subsequently employed by MnP in a catalysis of oxidation/S-glutathionylation of thiols of signaling proteins, such as NF-κB [26]. Such oxidative modification prevents NF-κB activation and promotes cell apoptosis; indeed a large impact on caspases was demonstrated [26]. Additionally, impact of MnP/H2O2 on the inactivation of complexes I and III of mitochondrial respiration, via oxidation of their thiols, was seen in cancer cell but not in normal cell resulting in suppression of mitochondrial function and ATP production [25]. The suppression of glycolysis by MnP/chemotherapy was reported also [25]. Such data are in agreement with earlier data [51] which indicate that CBX alone also suppresses NF-κB signaling and in turn levels of COX2 and iNOS. CBX was also found to suppress mitochondrial respiration [51].
Scheme 2.
The proposed impact of MnP/H2O2-based redox system [TRAIL + CBX + MnBuOE + Ascorbate/Thiol(NAC)] on cellular metabolism
Our data are further in agreement with the impact of mitochondrial dysfunction on suppression of cancer cell resistance to TRAIL. Different natural and synthetic compounds can potentiate TRAIL-induced apoptosis in malignant melanoma cells while sparing normal cells [37]. These compounds include a major garlic organosulfur compound, diallyltrisulfide, H2O2, and mitochondrial metabolic inhibitors such as rotenone, antimycin A, and carbonylcyanide-p-trifluoromethoxyphenylhydrazone, FCCP [37]. All of those induce robust membrane depolarization prior to apoptosis. Based on substantial evidence, provided by us, and those of others as discussed above, all of those effects have likely been implicated in the action of individual components and their combination, TRAIL + CBX + MnP + ascorbate/thiol. Akita et al. also showed that cancer cell may be sensitized to TRAIL if mitochondrial metabolism is perturbed [57].
Based on the established impact of individual components of our system, TRAIL, CBX, MnP and cellular reductants on mitochondrial respiration/energetics and NF-κB, it is highly likely that the combined TRAIL + CBX + MnP + ascorbate/thiol system exhibits cancer cell cytotoxicity and in turn its death via same pathways.
It has been reported that TRAIL + CBX induce oxidative stress via increasing H2O2 levels. Our data (where CBX works and GZA does not) suggest that CBX and TRAIL are involved in MnP-driven perturbation of cellular redox environment resulting in cancer cell death. MnP can increase H2O2 levels in the presence of TRAIL + CBX and/or employ H2O2 originating from the action(s) of TRAIL + CBX, to oxidize thiol-bearing proteins thereby affecting cellular transcription, caspase-based apoptosis, NF-κB activation and mitochondrial respiration. Substantial amount of evidence has been reported on such actions of MnPs [14, 15, 18, 25–28]. All processes will be enhanced with the addition ascorbate and thiols (for further reading see [15, 58].
Another possible variable that might have played a role in our system is the secretome from MSCs. Human MSCs have been shown to constitutively express high levels of glutathione, low levels of intracellular reactive species, as well as other relevant enzymes required to efficiently manage oxidative stress [59]. Conditioned medium derived from MSCs can possess an antioxidant effect as potent as 100 μM of ascorbic acid [60]. Recently, human Wharton’s Jelly-derived stem cells (hWJSCs) were demonstrated to increase intracellular hydrogen peroxide levels with simultaneous suppression of GPx activities in lymphoma cells when exposed to the conditioned media of hWJSCs, resulting in lymphoma cell death [61]. Likewise, it is possible that presence of MSCs secretome in our system may have also contributed to the observed effect. We have tried to measure the total level of intracellular ROS at 48 h post-treatment and found that MnBuOE alone generated exceptionally high levels of intracellular ROS (Mean ΔRFU 5194 ± 122; data not shown). Although in the presence of TRAIL and CBX, the intracellular ROS level was slightly reduced (Mean ΔRFU 4898 ± 45; data not shown), it was still approximately four times higher when compared to TRAIL and CBX double treatment group (Mean ΔRFU 1121 ± 39; data not shown). Despite so, the observed cell death did not seem to be directly associated with the total level of intracellular ROS since cells treated with MnBuOE alone were very much viable. More importantly, as discussed above, the balance between the production of peroxide and its removal may be critical. Thus, better characterization of the superoxide dismutases, catalases, glutathione peroxidases, peroxiredoxins and thioredoxins - their expression and activities - in cells under various treatment conditions requires further investigation.
The role of ROS in glioma stem cells has been recently explored. Sato and colleagues showed that an increase in the intracellular ROS levels in glioma initiating cells resulted in the loss of self-renewal properties and promotion of differentiation [62]. Furthermore, persistent elevation of ROS, in particular O2•−, has been implicated in enhanced resistance in glioma propagating cells. Koh and colleagues demonstrated that the ratio of O2•− to H2O2 dictated the sensitivity of those cells [63]. Depletion of this ratio, i.e. increase in H2O2 levels, rendered these cells sensitive to drug-induced apoptosis [63]. Therefore, our strategy to increase intracellular H2O2 levels by MnBuOE, enabling its cell death-promoting catalytic actions, may be advantageous as it can potentially target glioma stem cells.
Conclusions
We have demonstrated that combined treatment of TRAIL + CBX + MnBuOE exhibited preferential cell death induction in human glioma cells but not in immortalized normal human astrocytes. Our data further demonstrate that in the presence of exogenous cellular reductants (ascorbate and thiol) the effect of TRAIL + CBX + MnBuOE will be further enhanced. MnBuOE uses H2O2, either derived from oxidative stress imposed by CBX+/− TRAIL or via redox cycling with ascorbate and thiols, presumably inhibiting NF-κB, mitochondrial respiration, energy production and glycolysis thereby inducing cancer cell death. Much lower levels of H2O2 in normal cells, maintained by redundant endogenous H2O2-removing enzymes, will prevent such oxidations to occur at high yield. Modest inhibition of NF-κB would moreover suppress excessive inflammation and rescue normal cell from damaging side effects of cancer treatment. Importantly, we demonstrated that this combination therapy was effective against many of the patient-derived glioma cells, which are TRAIL-resistant presumably by sensitizing cancer cell to TRAIL via MnP-based perturbation of its mitochondrial function. The clinical relevance of our studies is strengthened by the fact that MnBuOE is entering Clinical Trials at Duke University on glioma patients as a radioprotector while at the same time being tumor radiosensitizer.
Supplementary Material
CBX modulates proteins involved in the apoptotic pathway (A) ΔGli36 cells were incubated with CBX (C); GZA (G); MSC-TRAIL-CM + CBX (TC) and MSC-TRAIL-CM + GZA (TG). 24 h post incubation, cells were harvested and their lysates were subjected to immunoblotting against (A) caspase-3; (B) Bid and tBid, Bax and Bcl-2. Densitometry quantification was done for all proteins and normalized to the actin internal loading control. Ratios emphasizing pro-apoptotic pathways were calculated, values listed in (A) and (B) and plotted in (C).
Acknowledgments
We would like to thank the Food Industry Research and Development Institute, Bioresource Collection and Research Center for providing primary GBM lines, GBM8401, GBM8901 and G5T/VGH. This research is supported by institutional fund supports from the National Cancer Centre Research Funds and the National Medical Research Council, Singapore. Dr. Batinic-Haberle and Dr. Tovmasyan are grateful for the support from NIH 1R03-NS082704-01 and BioMimetix JV LLC. Dr. Batinic-Haberle acknowledges Duke University School of Medicine-National University of Singapore travel grant.
Abbreviations
- CBX
carbenoxolone
- GBM
glioblastoma multiforme
- GZA
glyccyrrhizic acid
- INHA
immortalized normal human astrocytes
- SOD
superoxide dismutase
- GPx
glutathione peroxidase
- H2O2
hydrogen peroxide
- MnP
Mn(III) N-substituted pyridylporphyrins
- MSC
mesenchymal stem cells
- NAC
N-acetyl-cysteine
- ROS
reactive oxygen species
- MnTnBuOE-2-PyP5+, (BMX-001, MnBuOE)
Mn(III) meso-tetrakis(N-n-butoxyethylpyridinium-2-yl)porphyrin
- MnTE-2-PyP5+ (BMX-010, AEOL10113)
Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- MnTnHex-2-PyP5+
Mn(III) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin
- NF-κB
nuclear factor-κB
- Sc
subcutaneous
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12015-015-9628-2) contains supplementary material, which is available to authorized users.
Declaration of Interest Dr. Batinic-Haberle is consultant for BioMimetix Pharmaceutical, JVLLC, Duke University and Dr. Batinic-Haberle also has patent rights and have licensed technology to BioMimetix Pharmaceutical, JVLLC related to this technology.
Contributor Information
Ines Batinic-Haberle, Email: ibatinic@duke.edu.
Paula YP Lam, Email: cmrlyp@nccs.com.sg.
References
- 1.Ho IA, Lam PY. Signaling molecules and pathways involved in MSC tumor tropism. Histology and Histopathology. 2013;28:1427–1438. doi: 10.14670/HH-28.1427. [DOI] [PubMed] [Google Scholar]
- 2.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Research. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 3.Kang SG, Jeun SS, Lim JY, Kim SM, Yang YS, Oh WI, Huh PW, Park CK. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Child’s Nervous System. 2008;24:293–302. doi: 10.1007/s00381-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 4.Mohr A, Lyons M, Deedigan L, Harte T, Shaw G, Howard L, Barry F, O’Brien T, Zwacka R. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. Journal of Cellular and Molecular Medicine. 2008;12:2628–2643. doi: 10.1111/j.1582-4934.2008.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park SH, Sung YC, Jeun SS. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Research. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 6.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Research. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yulyana Y, Endaya BB, Ng WH, Guo CM, Hui KM, Lam PY, Ho IA. Carbenoxolone enhances TRAIL-induced apoptosis through the upregulation of death receptor 5 and inhibition of gap junction intercellular communication in human glioma. Stem Cells and Development. 2013;22:1870–1882. doi: 10.1089/scd.2012.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung EM, Park JW, Choi KS, Park JW, Lee HI, Lee KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–2017. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Shiraishi T, Nakata S, Horinaka M, Wakada M, Mizutani Y, Miki T, Sakai T. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Research. 2005;65:5662–5667. doi: 10.1158/0008-5472.CAN-05-0693. [DOI] [PubMed] [Google Scholar]
- 10.Woo JS, Kim SM, Jeong CH, Ryu CH, Jeun SS. Lipoxygenase inhibitor MK886 potentiates TRAIL-induced apoptosis through CHOP- and p38 MAPK-mediated up-regulation of death receptor 5 in malignant glioma. Biochemical and Biophysical Research Communications. 2013;431:354–359. doi: 10.1016/j.bbrc.2012.11.134. [DOI] [PubMed] [Google Scholar]
- 11.Zundorf G, Kahlert S, Reiser G. Gap-junction blocker carbenoxolone differentially enhances NMDA-induced cell death in hippocampal neurons and astrocytes in co-culture. Journal of Neurochemistry. 2007;102:508–521. doi: 10.1111/j.1471-4159.2007.04509.x. [DOI] [PubMed] [Google Scholar]
- 12.Azarashvili T, Baburina Y, Grachev D, Krestinina O, Evtodienko Y, Stricker R, Reiser G. Calcium-induced permeability transition in rat brain mitochondria is promoted by carbenoxolone through targeting connexin43. American Journal of Physiology. Cell Physiology. 2011;300:C707–C720. doi: 10.1152/ajpcell.00061.2010. [DOI] [PubMed] [Google Scholar]
- 13.Salvi M, Fiore C, Battaglia V, Palermo M, Armanini D, Toninello A. Carbenoxolone induces oxidative stress in liver mitochondria, which is responsible for transition pore opening. Endocrinology. 2005;146:2306–2312. doi: 10.1210/en.2004-1128. [DOI] [PubMed] [Google Scholar]
- 14.Batinic-Haberle I, Tovmasyan A, Roberts ER, Vujaskovic Z, Leong KW, Spasojevic I. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxidants & Redox Signaling. 2014;20:2372–2415. doi: 10.1089/ars.2012.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batinic-Haberle I, Tovmasyan A, Spasojevic I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins - from superoxide dismutation to HO-driven pathways. Redox Biology. 2015;5:43–65. doi: 10.1016/j.redox.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajic Z, Tovmasyan A, Spasojevic I, Sheng H, Lu M, Li AM, Gralla EB, Warner DS, Benov L, Batinic-Haberle I. A new SOD mimic, Mn (III) Ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity. Free Radical Biology & Medicine. 2012;52:1828–1834. doi: 10.1016/j.freeradbiomed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashcraft KA, Palmer G, Spasojevic I, Batinic-Haberle I, Dewhirst MW. Radioprotection of the salivary gland and oral mucosa with a novel porphyrin-based antioxidant. 58th Annual Meeting of the Radiation Research Society; 2012. p. 129. [Google Scholar]
- 18.Evans MK, Tovmasyan A, Batinic-Haberle I, Devi GR. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radical Biology & Medicine. 2014;68:302–314. doi: 10.1016/j.freeradbiomed.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holley AK, Xu Y, Noel T, Bakthavatchalu V, Batinic-Haberle I, St Clair DK. Manganese superoxide dismutase-mediated inside-out signaling in HaCaT human keratinocytes and SKH-1 mouse skin. Antioxidants & Redox Signaling. 2014;20:2347–2360. doi: 10.1089/ars.2013.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leu D, Zou Y, Weitner T, Tovmasyan A, Spasojevic I, Batinic-Haberle I, Huang T-T. Radiation protection of hippocampal neurogenesis with Mn-containing porphyrins. The 60th Annual Meeting of the Radiation Research Society; Las Vegas, Nevada, USA. 2014. [Google Scholar]
- 21.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its Mimics. Biochim Biophys Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spasojevic I, Weitner T, Tovmasyan A, Sheng H, Miriyala S, Leu D, Rajic Z, Warner DS, Clair DK, Huang TT, Batinic-Haberle I. Pharmacokinetics, brain hippocampus and cortex, and mitochondrial accumulation of a new generation of lipophilic redox-active therapeutic, Mn(III) Meso tetrakis(N-n-butoxyethylpyridinium-2-yl)porphyrin, MnTnBuOE-2-PyP5+, in comparison with its ethyl and N-hexyl analogs, MnTE-2-PyP5+ and MnTnHex-2-PyP5+ Free Radical Biology & Medicine. 2013;65:S132. [Google Scholar]
- 23.Tovmasyan A, Reboucas JS, Benov L. Simple biological systems for assessing the activity of superoxide dismutase mimics. Antioxidants & Redox Signaling. 2014;20:2416–2436. doi: 10.1089/ars.2013.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weitzel DH, Tovmasyan A, Ashcraft KA, Rajic Z, Weitner T, Liu C, Li W, Buckley AF, Prasad MR, Young KH, Rodriguiz RM, Wetsel WC, Peters KB, Spasojevic I, Herndon JE, 2nd, Batinic-Haberle I, Dewhirst MW. Radioprotection of the brain white matter by Mn(III) N-butoxyethylpyridylporphyrin-based superoxide dismutase mimic MnTnBuOE-2-PyP5 + Molecular Cancer Therapeutics. 2015;14:70–79. doi: 10.1158/1535-7163.MCT-14-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaramillo MC, Briehl MM, Batinic-Haberle I, Tome ME. Manganese (III) meso-tetrakis N-ethylpyridinium-2-yl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Free Radical Biology & Medicine. 2015;83:89–100. doi: 10.1016/j.freeradbiomed.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaramillo MC, Briehl MM, Crapo JD, Batinic-Haberle I, Tome ME. Manganese porphyrin, MnTE-2-PyP5+, Acts as a pro-Oxidant to Potentiate Glucocorticoid-Induced Apoptosis in Lymphoma Cells. Free Radical Biology & Medicine. 2012;52:1272–1284. doi: 10.1016/j.freeradbiomed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaramillo MC, Frye JB, Crapo JD, Briehl MM, Tome ME. Increased manganese superoxide dismutase expression or treatment with manganese porphyrin potentiates dexamethasone-induced apoptosis in lymphoma cells. Cancer Research. 2009;69:5450–5457. doi: 10.1158/0008-5472.CAN-08-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye X, Fels D, Tovmasyan A, Aird KM, Dedeugd C, Allensworth JL, Kos I, Park W, Spasojevic I, Devi GR, Dewhirst MW, Leong KW, Batinic-Haberle I. Cytotoxic effects of Mn(III) N-alkylpyridylporphyrins in the presence of cellular reductant, ascorbate. Free Radical Research. 2011;45:1289–1306. doi: 10.3109/10715762.2011.616199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, St Clair DK, Vujaskovic Z, Dewhirst MW, Piganelli JD. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2012;42:95–113. doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tovmasyan A, Maia CG, Weitner T, Carballal S, Sampaio RS, Lieb D, Ghazaryan R, Ivanovic-Burmazovic I, Ferrer-Sueta G, Radi R, Reboucas JS, Spasojevic I, Benov L, Batinic-Haberle I. A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics. Free Radical Biology & Medicine. 2015;86:308–321. doi: 10.1016/j.freeradbiomed.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn NA, Kemp ML. Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells. Molecular BioSystems. 2012;8:650–662. doi: 10.1039/c1mb05315a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. The Journal of Biological Chemistry. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- 33.Kusano C, Takao S, Noma H, Yoh H, Aikou T, Okumura H, Akiyama S, Kawamura M, Makino M, Baba M. N-acetyl cysteine inhibits cell cycle progression in pancreatic carcinoma cells. Human Cell. 2000;13:213–220. [PubMed] [Google Scholar]
- 34.Monticone M, Taherian R, Stigliani S, Carra E, Monteghirfo S, Longo L, Daga A, Dono M, Zupo S, Giaretti W, Castagnola P. NAC, tiron and trolox impair survival of cell cultures containing glioblastoma tumorigenic initiating cells by inhibition of cell cycle progression. PloS One. 2014;9:e90085. doi: 10.1371/journal.pone.0090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qanungo S, Wang M, Nieminen AL. N-acetyl-L-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. The Journal of Biological Chemistry. 2004;279:50455–50464. doi: 10.1074/jbc.M406749200. [DOI] [PubMed] [Google Scholar]
- 36.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cellular and Molecular Life Sciences. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki-Karasaki Y, Suzuki-Karasaki M, Uchida M, Ochiai T. Depolarization controls TRAIL-sensitization and tumor-selective killing of cancer cells: crosstalk with ROS. Front Oncol. 2014;4:128. doi: 10.3389/fonc.2014.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PloS One. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ, 2nd, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemotherapy and Pharmacology. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tovmasyan A, Carballal S, Ghazaryan R, Melikyan L, Weitner T, Maia CG, Reboucas JS, Radi R, Spasojevic I, Benov L, Batinic-Haberle I. Rational design of super-oxide dismutase (SOD) mimics: the evaluation of the therapeutic potential of new cationic Mn porphyrins with linear and cyclic substituents. Inorganic Chemistry. 2014;53:11467–11483. doi: 10.1021/ic501329p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shearman DJ, Hetzel D. The medical management of peptic ulcer. Annual Review of Medicine. 1979;30:61–79. doi: 10.1146/annurev.me.30.020179.000425. [DOI] [PubMed] [Google Scholar]
- 42.Bhuiyan AI, Papajani VT, Paci M, Melino S. Glutathione-garlic sulfur conjugates: slow hydrogen sulfide releasing agents for therapeutic applications. Molecules. 2015;20:1731–1750. doi: 10.3390/molecules20011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, Nisman B, Peretz T, Peylan-Ramu N. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. The Oncologist. 2015;20:366–367. doi: 10.1634/theoncologist.2014-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin Z, Chan H, Ning J, Lu K, Ma D. The role of hydrogen sulfide in pathologies of the vital organs and its clinical application. Journal of Physiology and Pharmacology. 2015;66:169–179. [PubMed] [Google Scholar]
- 45.Ashcraft KA, Boss M-K, Tovmasyan A, Choudhury KR, Fontanella AN, Young KH, Palmer GM, Birer SR, Landon CD, Park W, Das SK, Weitner T, Sheng H, Warner DS, Brizel DM, Spasojevic I, Batinic-Haberle I, Dewhirst MW. A novel manganese-porphyrin superoxide dismutase-mimetic widens the therapeutic margin in a pre-clinical head and neck cancer model. International Journal of Radiation Oncology*Biology*Physics. 2015 doi: 10.1016/j.ijrobp.2015.07.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tovmasyan A, Bueno-Janice J, Boss M-K, Weitzel DH, Sampaio RS, Spasojevic I, Dewhirst MW, Batinic-Haberle I. Mn porphyrin-based SOD mimic and vitamin C enhance radiation-induced tumor growth inhibition. Free Radical Biology & Medicine. 2015 In revision. [Google Scholar]
- 47.Ho IA, Chan KY, Miao L, Shim WS, Guo CM, Cheang P, Hui KM, Lam PY. HSV-1 amplicon viral vector-mediated gene transfer to human bone marrow-derived mesenchymal stem cells. Cancer Gene Therapy. 2008;15:553–562. doi: 10.1038/cgt.2008.27. [DOI] [PubMed] [Google Scholar]
- 48.Das A, Tan WL, Teo J, Smith DR. Glioblastoma multiforme in an Asian population: evidence for a distinct genetic pathway. Journal of Neuro-Oncology. 2002;60:117–125. doi: 10.1023/a:1020622415786. [DOI] [PubMed] [Google Scholar]
- 49.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 50.Holzer T, Herholz K, Jeske J, Heiss WD. FDG-PET as a prognostic indicator in radiochemotherapy of glioblastoma. Journal of Computer Assisted Tomography. 1993;17:681–687. doi: 10.1097/00004728-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Thakur P, Nehru B. Inhibition of neuroinflammation and mitochondrial dysfunctions by carbenoxolone in the rotenone model of Parkinson’s disease. Molecular Neurobiology. 2015;51:209–219. doi: 10.1007/s12035-014-8769-7. [DOI] [PubMed] [Google Scholar]
- 52.Bangert A, Cristofanon S, Eckhardt I, Abhari BA, Kolodziej S, Hacker S, Vellanki SH, Lausen J, Debatin KM, Fulda S. Histone deacetylase inhibitors sensitize glioblastoma cells to TRAIL-induced apoptosis by c-myc-mediated downregulation of cFLIP. Oncogene. 2012;31:4677–4688. doi: 10.1038/onc.2011.614. [DOI] [PubMed] [Google Scholar]
- 53.Hingtgen S, Ren X, Terwilliger E, Classon M, Weissleder R, Shah K. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and temozolomide. Molecular Cancer Therapeutics. 2008;7:3575–3585. doi: 10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moosavi MA, Moasses Ghafary S, Asvadi-Kermani I, Hamzeiy H, Rahmati M, Ahmadi AH, Nikanfar A, Sanaat Z, Asadi-Khiavi M. Carbenoxolone induces apoptosis and inhibits survivin and survivin-DeltaEx3 genes expression in human leukemia K562 cells. Daru. 2011;19:455–461. [PMC free article] [PubMed] [Google Scholar]
- 55.Pivato LS, Constantin RP, Ishii-Iwamoto EL, Kelmer-Bracht AM, Yamamoto NS, Constantin J, Bracht A. Metabolic effects of carbenoxolone in rat liver. Journal of Biochemical and Molecular Toxicology. 2006;20:230–240. doi: 10.1002/jbt.20139. [DOI] [PubMed] [Google Scholar]
- 56.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anti-Cancer Agents in Medicinal Chemistry. 2011;11:191–201. doi: 10.2174/187152011795255911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akita M, Suzuki-Karasaki M, Fujiwara K, Nakagawa C, Soma M, Yoshida Y, Ochiai T, Tokuhashi Y, Suzuki-Karasaki Y. Mitochondrial division inhibitor-1 induces mitochondrial hyperfusion and sensitizes human cancer cells to TRAIL-induced apoptosis. International Journal of Oncology. 2014;45:1901–1912. doi: 10.3892/ijo.2014.2608. [DOI] [PubMed] [Google Scholar]
- 58.Ali DK, Oriowo M, Tovmasyan A, Batinic-Haberle I, Benov L. Late administration of Mn porphyrin-based SOD mimic enhances diabetic complications. Redox Biology. 2013;1:457–466. doi: 10.1016/j.redox.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells and Development. 2010;19:1885–1893. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 60.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. Journal of Dermatological Science. 2008;49:133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Lin HD, Fong CY, Biswas A, Choolani M, Bongso A. Human Wharton’s jelly stem cells, its conditioned medium and cell-free lysate inhibit the growth of human lymphoma cells. Stem Cell Reviews. 2014;10:573–586. doi: 10.1007/s12015-014-9514-3. [DOI] [PubMed] [Google Scholar]
- 62.Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Narita Y, Shibui S, Kayama T, Kitanaka C. Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Research. 2014;12:119–131. doi: 10.1016/j.scr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 63.Koh LW, Koh GR, Ng FS, Toh TB, Sandanaraj E, Chong YK, Phong M, Tucker-Kellogg G, Kon OL, Ng WH, Ng IH, Clement MV, Pervaiz S, Ang BT, Tang CS. A distinct reactive oxygen species profile confers chemoresistance in glioma-propagating cells and associates with patient survival outcome. Antioxidants & Redox Signaling. 2013;19:2261–2279. doi: 10.1089/ars.2012.4999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CBX modulates proteins involved in the apoptotic pathway (A) ΔGli36 cells were incubated with CBX (C); GZA (G); MSC-TRAIL-CM + CBX (TC) and MSC-TRAIL-CM + GZA (TG). 24 h post incubation, cells were harvested and their lysates were subjected to immunoblotting against (A) caspase-3; (B) Bid and tBid, Bax and Bcl-2. Densitometry quantification was done for all proteins and normalized to the actin internal loading control. Ratios emphasizing pro-apoptotic pathways were calculated, values listed in (A) and (B) and plotted in (C).