Abstract
Progressive multifocal leukoencephalopathy (PML) has recently been described in psoriasis or multiple sclerosis patients treated with fumaric acid esters (fumarates), who had developed severe and long-standing lymphocytopenia (<500/mm3). We report a psoriasis patient who presented with progressive neurologic dysfunction and seizures after 2.5 years of fumarate therapy. Despite absolute lymphocyte counts remaining between 500–1000/mm3, his CD4+ and CD8+ T-cell counts were markedly low. MRI showed right hemispheric and brainstem lesions and JC virus DNA was undetectable in his cerebrospinal fluid. Brain biopsy revealed typical features of PML as well as JC virus-infected neurons. Clinicians should consider PML in the differential diagnosis of fumarate-treated patients presenting with brain lesions or seizures even in the absence of severe lymphocytopenia.
A 68-year-old white male noticed numb and tingling sensations on the left side of his face that quickly evolved into a painful “electric” sensation. Within a few weeks, these sensory symptoms spread to the left arm and the entire left half of his body. This prompted him to seek medical attention. His general physician ordered a cranial magnetic resonance tomogram (MRI) for a suspected vascular cause. The MRI showed a right-sided thalamic lesion, which was considered to be microangiopathic in etiology (Fig 1). He was sent to the emergency department for neurovascular assessment. On examination, he was alert without cognitive dysfunction. Memory, attention, verbal fluency, and concentration as well as mood were judged unaffected. No abnormalities of language and speech were noticed. Visual acuity and pupillary reaction were normal. Ocular pursuit was smooth and saccades were normometric. Hemicranial and especially facial sensibility was uniformly decreased on the left side, with prominent painful allodynia and dysesthesia. Facial muscles were unaffected, as was hearing. Bulbar cranial nerves were judged normal on examination. Muscle strength, tone, and bulk were normal, and deep tendon reflexes were symmetric. Both plantar responses were flexor. Left-sided hemihypesthesia for touch, pain, and temperature was observed together with dysesthesias of the affected regions. Gait was unaffected, and stance was steady. Carotid as well as transcranial Doppler and duplex ultrasonography did not show stenosis and showed only mild increase in intima–media thickness. A routine electrocardiogram did not show cardiac conduction abnormalities. The thalamic lesion was considered to be compatible with a microangiopathic lacunar stroke, and daily aspirin as well as further cardiac outpatient assessment were suggested.
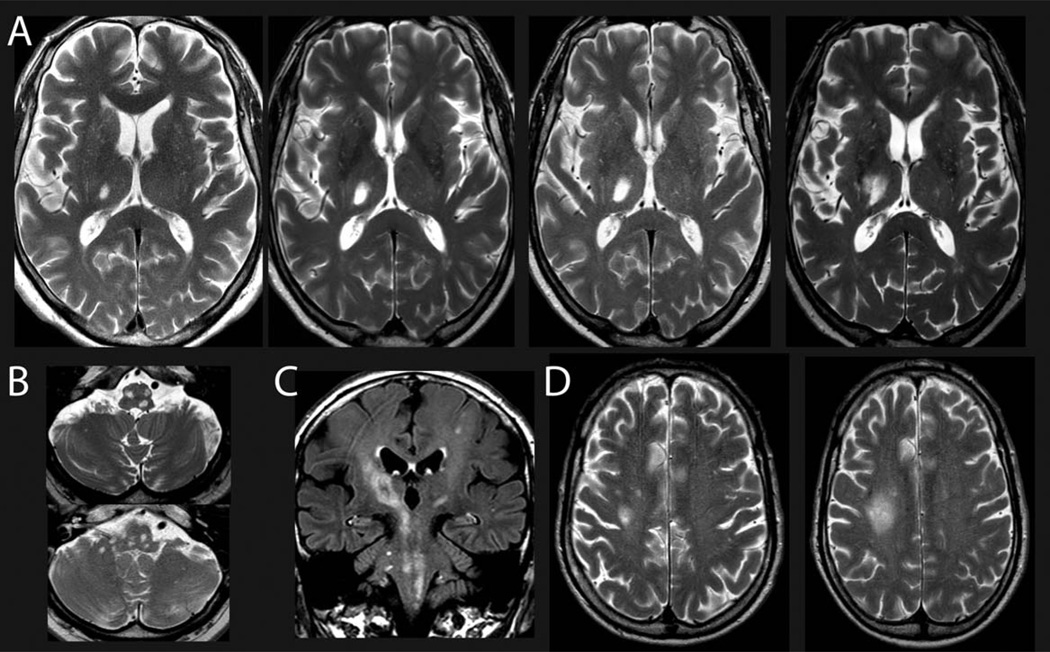
FIGURE 1.
(A, B) Representative magnetic resonance imaging showing the progressive enlargement of the lesion in the right thalamus (A; March 2013, July 2013, December 2013, March 2014) and multiple lesions in the mesencephalon, pons, cerebellar pedunculi, and medulla oblongata (B; March 2014) within the first 1.5 years after onset of neurological symptoms by T2-weighted imaging. (C) Fluid-attenuated inversion recovery imaging showing widespread and diffuse affection of the midbrain structures including the pyramidal tract and pontine and cerebellar structures (March 2014). (D) T2-weighted imaging (September 2014, December 2014) shows a progressive subcortical lesion that was chosen as biopsy target (see Fig 3). For time points of magnetic resonance imaging, please see also Figure 2.
The patient’s sensory complaints, however, progressively worsened over the next few months, and he additionally developed slowly progressive left-sided weakness and clumsiness. A follow-up MRI showed an enlargement of the thalamic lesion, and new small hyperintensities were detected in the brainstem and mesencephalon on T2 and fluid-attenuated inversion recovery imaging. He was admitted to the neurology service for diagnostic work-up 10 months after the emergence of symptoms.
His past medical history revealed a moderately differentiated adenocarcinoma of the rectum (pT2 pN1 cM0 L0 V0) diagnosed 8 years earlier. It was removed in toto and treated with adjuvant radiochemotherapy with 5-fluorouracil (30 cycles of 450mg/m2 corresponding to 830mg; cumulative dose = 24.900mg). He had been in routine follow-up, and clinical and radiological findings suggested stable remission. He had been treated with levothyroxine for hypothyroidism and citalopram for mild depression. He had a prior history of psoriasis, which had been diagnosed at the age of 12 years. His father had psoriasis as well. Otherwise the family history was noncontributory. The psoriasis had been previously treated with topical corticosteroids and ultraviolet light therapy (psoralen plus UV-A). Oral fumaric acid esters (Fumaderm) had been started 2.5 years before onset of neurological symptoms with a maximum daily dose of Fumaderm of 5 tablets (consisting of 120mg dimethyl fumarate [DMF] + 95mg of monoethyl fumarate). Following the treatment guidelines for fumaric acid esters in psoriasis, the uptitration of Fumaderm was performed using the recommended dosage regimen and treatment was closely monitored by regular laboratory studies including differential blood counts. He developed a moderate leukopenia (3,000/mm3) and his lymphocyte counts ranged between 500 and 1,000/mm3 (grade 2 lymphocytopenia; Fig 2).1
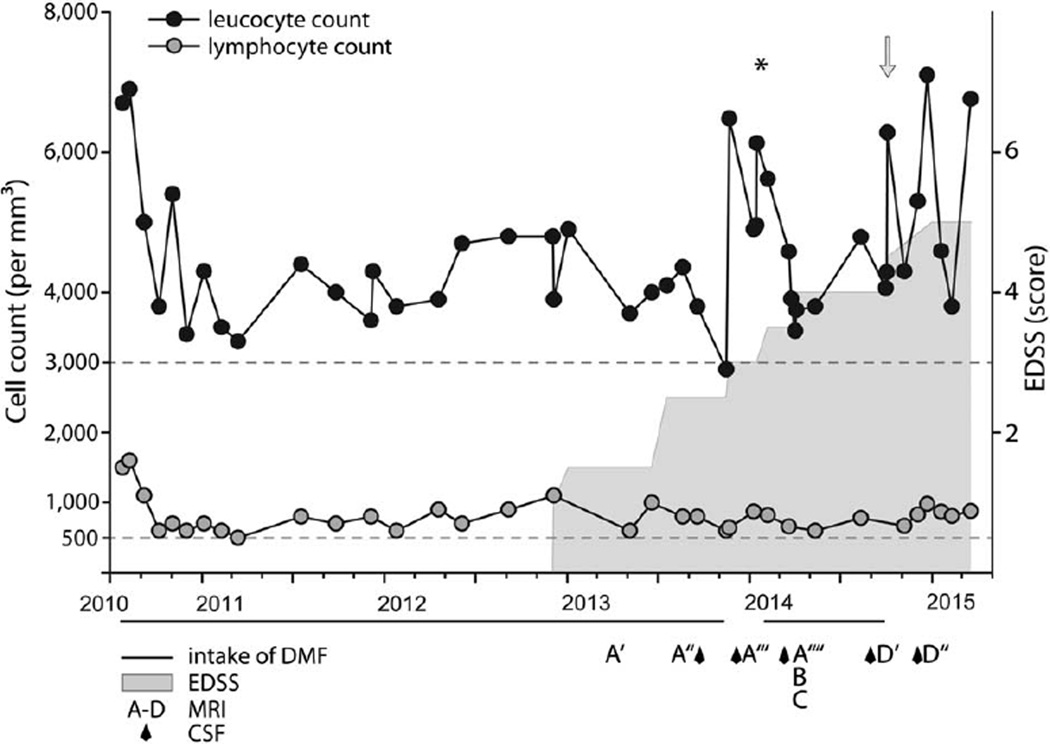
FIGURE 2.
Lymphocyte and leukocyte counts in relation with fumarate treatment and beginning of neurologic symptoms. Black circles = leukocyte count (per mm3); gray circles = lymphocyte count (per mm3); gray horizontal line = intake of Fumaderm tablets. Each tablet contains 120mg dimethyl fumarate (DMF) + 95mg monoethyl hydrogen fumarate. At the beginning of treatment in July 2010 and February 2014, fumaric acid was uptitrated using the established scheme to 5 × 120mg DMF + 95mg monoethyl fumarate daily. Gray area indicates onset and development of neurological disability over time using the Expanded Disability Status Scale (EDSS) score (for scaling, see y-axis). Asterisk indicates time point of intravenous treatment with cortisone (1,000mg/day for 5 days). Arrow indicates time point of brain biopsy. A–D = time point of magnetic resonance imaging (MRI) as shown in Figure 1 (another 4 MRIs are not shown). Diamonds indicate time points of cerebrospinal fluid (CSF) exams.
On neurological examination, cognitive testing did not show aphasia, apraxia, or impairment of higher cortical cognitive functions including memory. He was observed to have left-sided lower facial paresis, pronounced left facial hypesthesia and dysesthesia, and an attenuated left-sided corneal reflex. An ocular abduction deficit and diplopia upon horizontal gaze particular to the left with nystagmus were present. Motor examination showed a supranuclear tetraparesis predominantly on the left side (Medical Research Council grade = 3–4) with considerable left-sided spastic muscle tone with flexor positioning of the left arm. Deep tendon reflexes were generally brisk, more so on the left side, and plantar responses were flexor. Finger-to-nose test showed profound ataxia of the left arm and alternating movements were dyskinetic. Sensory examination revealed left-sided hemihypesthesia and dysesthesia. He was unsteady, with a spastic, wide-based, ataxic slow gait.
An extensive evaluation was performed that did not reveal any cause of acquired immunodeficiency or underlying infectious disease. Laboratory investigations are shown in Table 1. Cerebrospinal fluid (CSF) was noninflammatory, with a normal white blood cell (WBC) count and total protein, and without presence of oligoclonal bands. A comprehensive microbiologic screening for neuropathogenic bacteria, viruses, fungi, and parasites in serum and CSF remained negative. Polymerase-chain reaction (PCR) assays for the JC virus (JCV) in the CSF performed in 2 different laboratories (95% probability detection limits of 420 copies/ml and 117 copies/ml, respectively) were negative. An immunoglobulin heavy chain gene PCR analysis of CSF did not show signs for an underlying B cell lymphoma. Rectal and deep jejunal/duodenal biopsy did not provide evidence for amyloidosis or an infection with Tropheryma whipplei.
TABLE 1.
Laboratory Results
| Test | Value | Normal Range |
|---|---|---|
| At time of brain biopsy | ||
| Red blood cells | 5.13 × 106/µl | 4–5.65 |
| Platelets | 241 × 103/µl | 160–370 |
| Neutrophils | 2,730/mm3 | 1,500–77,000 |
| Monocytes | 780/mm3 | 100–900 |
| Eosinophils | 90/mm3 | 20–500 |
| Basophils | 20/mm3 | <210 |
| Leucocytes | 4,300/mm3 | 3.600–10.500 |
| Lymphocytes (abs) | 670/mm3 | 1,100–4,000 |
| CD3+ | 352/mm3 | 700–2,100 |
| CD4+ | 154/mm3 | 300–1,400 |
| CD8+ | 117/mm3 | 200–900 |
| 5 months after brain biopsy | ||
| Leucocytes | 6,700/mm3 | 3,600–10,500 |
| Lymphocytes (abs) | 880/mm3 | 1,100–4,000 |
| CD3+ | 519/mm3 | 700–2,100 |
| CD4+ | 278/mm3 | 300–1,400 |
| CD8+ | 140/mm3 | 200–900 |
| 8 months after brain biopsy | ||
| Leucocytes | 5,580/mm3 | 3,600–10,500 |
| Lymphocytes (abs) | 960/mm3 | 1,100–4,000 |
| CD3+ | 554/mm3 | 700–2,100 |
| CD4+ | 276/mm3 | 300–1,400 |
| CD8+ | 185/mm3 | 200–900 |
| Representative results during course of disease | ||
| Endocrinology | ||
| TSH | 4.48µlU/ml | 0.27–4.2 |
| fT3 | 3.0pg/ml | 2.0–4.4 |
| fT4 | 1.49ng/dl | 0.9–1.7 |
| Parathormone | 55.6pg/ml | 16–65 |
| Calcitonin | <2.00pg/ml | <18.2 |
| Immunology | ||
| Total IgE | 946kIU/l | 0–100 |
| Antinuclear abs | 1:160, homogenous and nucleolar |
<1:80 |
| pANCA | Negative | |
| cANCA | Negative | |
| Double-stranded DNA abs | 6.70U/ml | 0–20 |
| Antithyroglobulin | <10.0IU/ml | <115 |
| Antithyroperoxidase | 15.5ng/ml | <34 |
| TSH receptor abs | <0.3IU/l | |
| Histone abs | 6.00U/ml | 0–40 |
| CSF analysis (representative) | ||
| Cell count | 2/µl | <5 |
| Cytopathology | Few lymphocytes without atypical cells, no PAS-positive intra-/extracellular material |
|
| Total protein | 381mg/l | <500 |
| Glucose | 67mg/dl | 40–65 |
| Lactate | 1.6mmol/l | <2.1 |
| Test | CSF | Serum | Quotient, CSF/Serum × 103 |
|---|---|---|---|
| Albumin | 252mg/l | 43g/l | 5.9 |
| IgG | 17.7mg/l | 11.4g/l | 2.4 |
| IgA | 4.55mg /l | 3.26g/l | 1.4 |
| IgM | <0.14mg/l | 0.575g/l | <0.2 |
| Test | Findings |
|---|---|
| Oligoclonal bands | Negative |
| Molecular genetic analysis of CSF | No evidence of monoclonal B cells |
| Test | Method | Findings | Normal Value |
|---|---|---|---|
| HSV DNA | PCR | Negative | |
| JCV DNAa | PCR | Negative | |
| Aspergillus antigen | EIA | 0.01 | <0.5 |
| Borrelia IgG | EIA | <0.05U/ml | |
| Candida antigen | EIA | 0.01pg/ml | <62.5 |
| Cryptococcus antigen | EIA | Negative | |
| Aerobic bacteria | Culture | Negative | |
| Anaerobic bacteria | Culture | Negative | |
| Actinomyces | Culture | Negative | |
| Nocardia | Culture | Negative | |
| Mycobacteria | Microscopy, PCR, culture | Negative | |
| Echinococcus granulosus | IHA | Negative | <1:20 |
| E. multilocularis | IHA | Negative | <1:20 |
| Echinococcosis | IB | Negative | |
| Paragonimus uterobilateralis | IHA | Negative | <1:40 |
| Toxocara spp. | IB | Negative | |
| Cysticercosis | IB | Negative | |
| Tropheryma whipplei | PCR | Negative |
| Test | Findings |
|---|---|
| Microbiology (blood) | Negative for HIV-1/2 antibody, p24 antigen, adenovirus, CMV, EBV, enterovirus, rubeola, rubella, VZV, borrelia, Echinococcus antibody, toxoplasma, lues, Candida, Cryptococcus, Ascaris lumbricoides, echinococci, Fasciola hepatica, Paragonimus uterobilateralis, Strongyloides spp., Toxocara spp., cysticercosis, helminths (cestoda/Taenia solium) |
| Stool | Negative for eggs, enteropathogenic protozoa |
| Rectal/deep jejunal biopsy | No evidence for amyloidosis or Whipple disease |
JCV PCR 95% detection probability, laboratory 1: 420 copies/ml CSF Altona Diagnostics (Hamburg, Germany) Real Star JCV PCR Kit 1.0; laboratory 2: 117 copies/ml, custom made assay.57
abs = antibodies; cANCA = cytoplasmic antineutrophil cytoplasmic antibodies; CMV = cytomegalovirus; CSF = cerebrospinal fluid; EBV = Epstein–Barr virus; EIA = enzyme immunoassay; HIV = human immunodeficiency virus; HSV = herpes simplex virus; IB = immunoblotting; Ig = immunoglobulin; IHA = immunohistochemical assay; JCV = JC virus; pANCA = perinuclear antineutrophil cytoplasmic antibodies; PAS = periodic acid Schiff; PCR = polymerase chain reaction; TSH = thyroid-stimulating hormone; VZV = varicella-zoster virus.
Treatment with fumarates was initially discontinued but eventually reinstituted due to worsening psoriasis. In the following months, neurological symptoms slowly progressed, with worsening of the brainstem signs including emergence of dysphagia and dysarthria and worsening of the hemiparesis and hemiataxia. The patient described infrequent involuntary jerky movements of the left arm that were clinically assessed as focal motor seizures; however, repeated routine electroencephalography did not show generalized or focal slowing of baseline activity or epileptiform activity. Nevertheless, antiepileptic therapy with levetiracetam (3,000mg/day) and, after persistence of involuntary motor activity, carbamazepine (600mg/day) was started. Seizure activity decreased in the following weeks to 1 seizure event every 7 to 10 days.
Repeated follow-up MRI (see Fig 1) showed progressive multiple ringlike and contrast-enhancing lesions in predominantly an infratentorial location affecting the mesencephalon, pons, cerebellar pedunculi, and medulla oblongata, but also showing spotlike lesions in the left frontal operculum as well as a lesion in the right parietal lobe, subcortically. Magnetic resonance spectroscopy did not show altered metabolic spectra (creatine, N-acetylaspartate, choline) suggestive of necrosis or neoplasia. An MRI of the spinal cord was normal. Diagnostic subtraction angiography of cranial vessels was unremarkable. Repeated lumbar punctures continued to show acellular CSF without oligoclonal bands.
Subsequent treatment trials with corticosteroids (1,000mg intravenous prednisolone over 5 days) as well as trimethoprim/sulfamethoxazole for 1 year had no clinical or radiological effects. Because of continued clinical progression to a severe left-sided sensorimotor deficit combined with left-sided ataxia, gait disturbance, double vision, and other brainstem signs and progressive findings by MRI (see Fig 1), a brain biopsy of the right frontal subcortical T2 hyperintense and diffusion-restricted lesion was performed 2 years after the emergence of neurological symptoms (Fig 3).
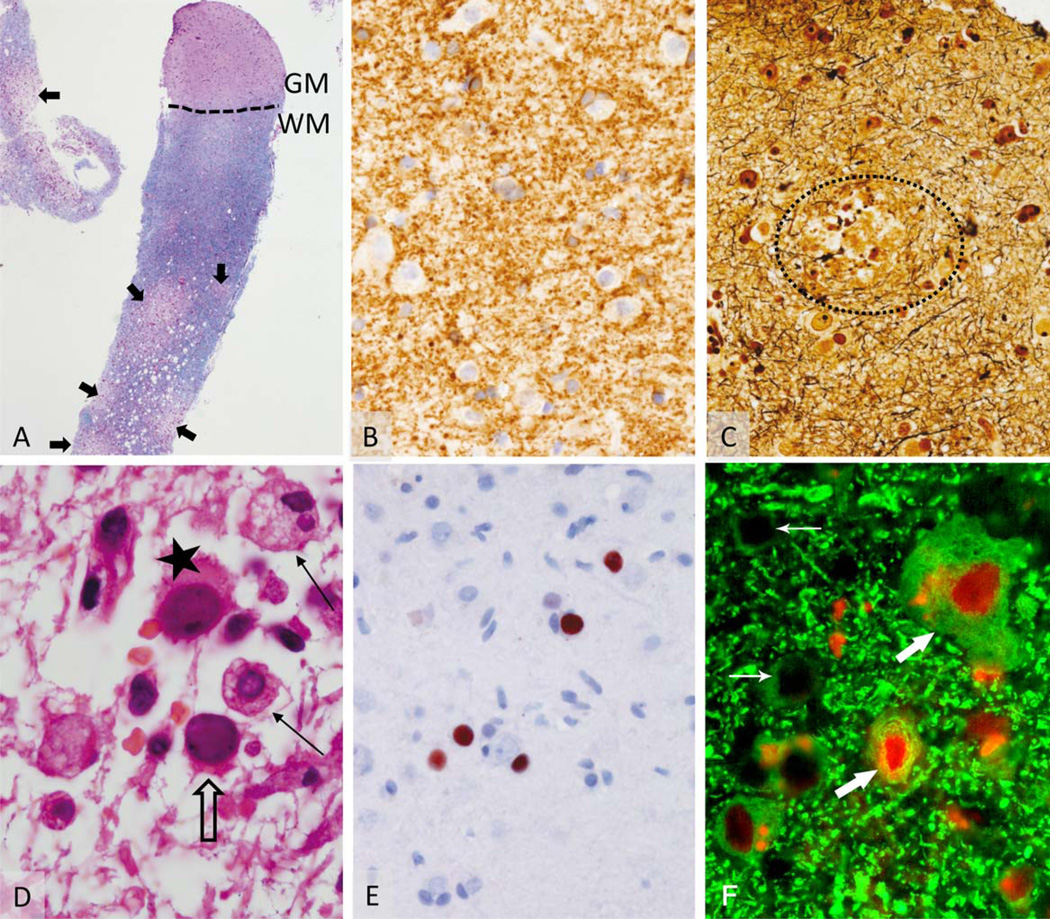
FIGURE 3.
Histopathology of brain biopsy of the subcortical lesion (see Fig 1D). (A) Biopsy specimen (Luxol fast blue–periodic acid Schiff) consists predominantly of white matter (WM), with only 2 small areas of cortical gray matter (GM). Areas of patchy demyelination are indicated by arrows. (B) Within the GM no signs of demyelination were identified (cyclic nucleotide phosphodiesterase). (C) A small area of acute axonal damage in the gray matter could be demonstrated in Bielschowsky silver impregnation (encircled area). (D) Within the white matter lesion, a moderate T-cell infiltrate is accompanied by bizarre-looking astrocytes (star) and foamy macrophages (solid arrows). Oligodendrocytes (open arrow) exhibit nuclear alterations. (E) In situ hybridization confirms the infection with JC virus (JCV). (F) Interestingly, triple immunofluorescence staining for JCV proteins (T Ag, red; VP1, blue) and neurons (microtubule-associated protein 2, green) performed as previously described41 showed that some neurons in the cortex and gray–white matter junction expressed JCV T Ag (large arrows) interspersed with uninfected neurons (small arrows; original magnification, ×100). Neurons did not express JCV VP1, which was detected frequently in non-neuronal cells (not shown).
Dr Koralnik’s Discussion
I am aware of the diagnosis in this case. In summary, this 68-year-old man with psoriasis treated with fumarates for 2.5 years developed left hemisensory dysfunction, left hemiparesis, ataxia, brainstem signs, and focal motor seizures evolving progressively over a period of 2 years. Serial MRI showed initially a single nonenhancing right thalamic lesion, which slowly expanded into the right internal capsule, brainstem, and frontoparietal lobes. Other lesions appeared in the brainstem, cerebellar peduncles, and left frontal lobe, some of them enhancing after intravenous contrast administration. A thorough laboratory evaluation of his blood failed to reveal any inflammatory, infectious, metabolic, or endocrinologic etiologies, and showed only moderate lymphocytopenia. Serial CSF examinations showed normal cell count, protein, and glucose, no oligoclonal bands or malignant cells, and no evidence of viral, bacterial, fungal, or parasitic infections. Specifically, CSF JCV PCR was negative in 2 different clinical laboratories. The patient continued to worsen neurologically despite empirical treatment with corticosteroids and trimethoprim/sulfamethoxazole. Finally, a brain biopsy performed 2 years after onset of neurologic symptoms established the diagnosis of progressive multifocal leukoencephalopathy (PML). The histological examination showed subcortical demyelination with bizarre astrocytes and oligodendrocytes harboring nuclear alterations that were infected by JCV. Lipid-laden macrophages clearing the myelin debris completed the typical findings in PML. There was no demyelination in the cortex, but some neurons in the gray matter and gray–white matter junction were also infected by JCV.
This case is very informative at many levels and teaches us much about JCV biology and pathogenesis, the predisposing factors leading to viral reactivation and the development of PML, and the difficulty of establishing a correct diagnosis in atypical presentations. I will review those topics, starting with the clinical and radiological findings.
This patient presented initially with painful left dysesthesia and hypoesthesia, consistent with Dejerine–Roussy or thalamic pain syndrome, and the MRI confirmed the presence of an isolated right thalamic lesion that was thought to be microangiopathic in etiology despite absence of risk factors and an unrevealing stroke evaluation, leading to treatment with aspirin. This single lesion located in the basal ganglia—a gray matter structure— did not raise suspicion for PML, which highlights the misleading nature of the misnomer “PML.”2 PML is often initially unifocal and the demyelinating lesion may be entirely circumscribed within the gray matter. This apparent oxymoron—a white matter disease restricted to gray matter—can be easily explained by the fact that both cortical hemispheric and basal ganglia gray matter contain neurons whose axons are myelinated by oligodendrocytes, which are the main target for JCV. In addition, astrocytes, present both in white and gray matter, can be infected and destroyed by this virus.3
Thereafter, the lesion expanded laterally and vertically in surrounding white matter and new white matter lesions developed outside of defined vascular territories, raising the suspicion for PML. However, CSF JCV DNA was undetectable in 2 different clinical laboratories with sensitive PCR detection methods. The total WBC count had remained normal, and the absolute lymphocyte count (ALC) had been oscillating between 1,000 and 500/µl over >3 years on fumarates. A T-cell subset analysis was not performed until approximately 18 months later at the time of the brain biopsy, where the absolute CD4+ T-cell count was only 154/mm3 and CD8+ T-cell count was 117/mm3, whereas the ALC was 670/mm3.
The definite diagnosis of PML is based on clinical, radiological, and virological criteria. Although this patient clearly had a clinical presentation and, in time, MRI findings consistent with PML, JCV PCR remained negative in his CSF. Does this rule out the diagnosis? Whereas the sensitivity of JCV CSF PCR for the diagnosis of PML in patients with acquired immunodeficiency syndrome (AIDS) was 72 to 92% in the early part of the human immunodeficiency virus (HIV) epidemic,4,5 it dropped to 58% after antiretroviral medications became widely available.6,7 This suggests that improvement of the immune response may decrease JCV load in CSF below the level of detection of the PCR assay. Low or undetectable JCV CSF load has also been reported in multiple sclerosis patients with natalizumab-associated PML,8–10 whose degree of immunosuppression is arguably not as profound as in patients with AIDS. Finally, the location of the lesion away from CSF spaces may have precluded PCR detection in this case. Nevertheless, the clinical and radiological findings alone were sufficient to establish the diagnosis of “possible PML,” based on a consensus statement of the American Academy of Neurology Neuroinfectious Disease Section.11 However, a key to determining that clinical findings are consistent with PML is to realize that the patient is immunosuppressed.
Our immune system is globally divided into the innate portion, providing immediate response to pathogens in a generic way, comprised mainly of the complement cascade, mast cells, neutrophils, monocytes, macrophages, basophils, eosinophils, and natural killer cells; and the adaptive immune system, the source of immunological memory, which is divided into the humoral and the cellular immune response. Unlike its innate counterpart, the adaptive immune system is highly specific for a given pathogen. B lymphocytes are the effectors of the humoral immune response and produce antibodies. In the case of JCV, antibodies do not appear to be instrumental in the prevention or containment of PML. Most cases of PML had preexisting antibodies against JCV. PML patients have high JCV-antibody titers in blood and evidence of intrathecal antibody production, and this has not been associated with either a protective effect or better outcome. Rather, antibody titers are now used as part of risk stratification strategies for natalizumab-treated multiple sclerosis patients.12–14
Nevertheless, B cells function as antigen-presenting cells for T lymphocytes, and their depletion, as seen in treatment with rituximab, may be indirectly associated with the development of PML in rare instances.15
The cellular immune response is comprised mainly of CD4+ and CD8+ T lymphocytes. CD4+ lymphocytes have various helper functions that serve to activate the humoral immune response, the phagocytic capabilities of monocytes/macrophages, and the cytotoxic function of CD8+ T lymphocytes. Those CD8+ T lymphocytes are the ultimate effector cells of the cellular immune response, and they can recognize and destroy virus-infected cells through production of toxic chemokines, thereby preventing further spread of the disease. Historically, individuals at higher risk of developing PML are those presenting with decreased T-cell immunity such as patients with AIDS, hematologic malignancies, and idiopathic lymphocytopenia, transplant recipients, and individuals treated with corticosteroids or immunomodulatory medications for a variety of inflammatory or autoimmune disorders.3,16
A very crude way to measure the cellular immune response is by monitoring the absolute lymphocyte count in peripheral blood, calculated as the total number of WBCs × percentage of lymphocytes/100. In adults, lymphocytopenia is divided into grade 1 (ALC = 800–1,000/mm3), grade 2 (ALC = 500–799/mm3), grade 3 (ALC = 200–499/mm3), and grade 4 (ALC < 200/mm3), with higher grades being associated with higher risk of infections. Contrary to the infection precautions that are required for patients whose neutrophil count drops below 500/mm3, no specific protective measures are mandated for lymphopenic patients, and in the absence of an underlying condition typically associated with immunosuppression such as AIDS, the lymphocytopenia is often ignored, or goes unrecognized. Failure of recognition—or agnosia—is a well-defined neurologic syndrome involving body parts, objects, shapes, smells, or sounds. I propose that failure to recognize that patients with lymphocytopenia are at risk for infections be from now on called “lymphocytopeniagnosia,” as a gentle reminder for their physicians.
In healthy individuals, the ALC is made of approximately 70% CD3+ T cells, 20% CD19+ and CD20+ B cells, and 10% CD56+ natural killer cells. Because CD3+ T cells usually include ~70% CD4+ and 30% CD8+ T cells, a rough rule of thumb in healthy individuals is that the absolute number of CD4+ T cells can be estimated as ~50% of ALC. Why is this important in the setting of PML? In HIV-infected patients, opportunistic infections including PML occur mainly when the CD4+ T-cell count drops below 200/mm3, warranting the diagnosis of AIDS by CD4+ criteria. Although immunosuppression associated with HIV-infection is more profound than that of HIV-seronegative individuals with a comparable lymphocytopenia, physicians should remember that any individual with CD4+ T-cell count < 200/mm3 is at high risk of PML. Because this patient had an ALC oscillating between 1,000 and 500/mm3, one might have thought that his CD4+ T-cell count remained between 500 and 250/mm3, still outside of the “danger zone.” This was, however, not the case, because the absolute CD4+ T-cell count at the time of the brain biopsy was 154/mm3 despite an ALC of 670/mm3 (see Table 1). Why was the CD4+ T-cell count in this patient only 23% of the ALC when it can be expected to be ~50% in healthy individuals? This can be explained, in part, by the effect of fumarates on T lymphocytes. In a small prospective study of 10 psoriasis patients with normal blood count at baseline treated with DMF over a 12-month period, all developed lymphocytopenia, with a stronger suppression of CD8+ than CD4+ T lymphocytes. After 1 year of therapy, there was an average 53.2% reduction in CD4+ T cells (range = 130–250/mm3), an 87.4% reduction of CD8+ T cells (range = 30–100/mm3), and concomitant 175.6% increase in the CD4+/CD8+ ratio.17 That DMF affects CD8+ more than CD4+ T cells in this small group of patients is of importance, because we and others have shown time and again that CD8+, more than CD4+ T cells, are crucial in the containment of JCV and clinical outcome of PML.18–20 In the present case, because we do not have baseline T-cell subsets prefumarates treatment, it is difficult to determine whether CD8+ T cells were more severely affected at the time of the biopsy (117/mm3), with a CD4+/CD8+ ratio at 1.3. However, 5 months after biopsy and discontinuation of fumarates, the ALC had climbed to 880/mm3 and CD4+ T-cell counts to 278/mm3 (80% increase), compared to only a 20% increase of CD8+ T-cell counts to 140/mm3, with a 51% increase of CD4+/CD8+ ratio to 1.98. The exact mechanism of the selective reduction of CD8+ T cells and the delay in recovery after interruption of treatment is unknown. An in vitro study of the effect of 48-hour incubation of T cells from blood donors with DMF showed a dose- and time-dependent upregulation of an apoptosis marker and DNA fragmentation by DMF, especially in activated T cells, suggesting that DMF could induce apoptosis in human T cells. Nevertheless, CD4+ and CD8+ T cells were not tested separately.21 Other mechanisms of action of DMF and its metabolites include a shift from Th1 to Th2 immune response,22 inhibition of nuclear factor kappa B signaling,23 and reduced binding of peripheral blood mononuclear cells (PBMCs) to vascular adhesion molecule-1, thereby limiting extravasation of leukocytes.24,25 Those effects are beneficial in psoriasis and multiple sclerosis (MS), but could possibly preclude effective immunosurveillance in the central nervous system and trigger the reactivation of JCV.
Whether fumarates contained in Fumaderm or those produced by compound pharmacies affect lymphocytes of psoriasis patients26,27 in different ways than delayed-release DMF (Tecfidera; Biogen Idec, Cambridge, MA) affects those of MS patients has only been recently investigated. Of note, grade 3 lymphocytopenia (ALC < 500/mm3) occurred in only 1.9% (47 of 2,470) of MS patients treated for 6 months or longer, but T-cell subsets were not reported.28 In controlled MS clinical trials, delayed-release DMF reduced the mean ALC by 30% during the first year. ALC increase was observed 4 weeks after discontinuation of the drug, albeit without return to baseline.29,30 Two observational studies in 25 and 256 delayed-release DMF-treated patients with relapsing–remitting MS reported the evolution of T-cell subsets before and up to 12 months after initiation of treatment, showing a predominant depletion of CD8+ T cells.25,31 Although grade 3 lymphocytopenia was observed in only 6.6% of patients and CD4+ T-cell count dropped below 200/mm3 in only 9%, CD8+ T-cell counts dropped below 200/mm3 in 54% of them after 1 year on the drug.25 Similarly, there was a greater decrease in CD8+ compared to CD4+ T-cell counts over 1 year in the other study (54.6% vs 39.2%).31 Concomitantly, the CD4/CD8 ratio increased by 35.5 to 38.5% from a baseline of 2.5–2.6 to 3.4–3.6, a difference that was highly significant in both studies.25,31
As noted in Table 2, in all cases of DMF-associated PML cases in psoriasis and MS patients where T-cell subsets were measured, CD4+ and CD8+ T-cell deficiencies were noted. In the absence of a baseline value pre-DMF and evolution after DMF discontinuation, however, the nature and kinetics of T-cell reduction associated with DMF leading to PML remains to be fully determined. Borrowing from the popular wisdom, I propose that the saying “you can’t tell where you’re going if you don’t know where you’ve been” applies to DMF-treated patients as well.
TABLE 2.
Published Cases of Progressive Multifocal Leukoencephalopathy in Patients Treated with Fumarates
| Study | Disease | Treatment and Duration |
Oncological History |
Prior Immune Treatment |
Lymphopenia | CD4/8 Counts | JCV PCR | Neurological Status Iris |
MRI Manifestation |
|---|---|---|---|---|---|---|---|---|---|
| Ermis 201326 | Psoriasis | Fumaderm, 3 yr | None | Methotrexate 3 yr before |
Grade 3 | CD3+, CD4+ T- cell deficiency, CD4/CD8 ratio > 2 |
90 copies/ml CSF |
Progressive sensory aphasia; IRIS |
Subcortical/ juxtacortical white matter |
| Van Oosten 201327 |
Psoriasis | Psorinovo, 5 yr | None | None | Grade 3 | CD3+, CD4+, CD8+ T-cell defi- ciency, CD4/CD8 ratio = 2 |
Positive | Progressive right- sided hemiparesis, IRIS |
Small, deep white matter lesions and 1 large progressive lesion |
| Stoppe 201451 | Psoriasis | Fumaderm, 3 yr | Superficial malignant melanoma 3 yr before |
Efalizumab 3 yr before |
Grade 1–2 | CD4+ T-cell defi- ciency (131/µl), CD4/CD8 ratio < 2 |
>2,000 copies/ ml CSF |
Left-sided hemiataxia, dysarthria, unstable walking, no IRIS |
Lesions in the left cerebellar peduncle, hemisphere and pons (Gd+) |
| Nieuwkamp 201553 |
Psoriasis | Psorinovo, 2 yr | None | None | Grade 1 | Not given | Initially negative, positive during IRIS |
Apraxia, progressive hemiparesis, somnolence, IRIS, fatal outcome |
Multiple subcortical white matter lesions (Gd+), herniation |
| Rosenkranz 201528 |
Multiple sclerosis |
Tecfidera, 4.5 yr | None | Glatiramer acetate |
Grade 3 | Not given | Positive | Severe gait and speech disorder, ataxia, fatal outcome |
Confluent and progressive lesions in cerebellar peduncles, pons, and left cerebellar hemisphere |
| Hoepner 201552 |
Psoriasis | Fumaderm, 4 yr | None | Toxic bone marrow damage, increased excretion of kappa light chains |
Grade 2 | Not given | 16 & 42 copies/ ml CSF |
Right hemiparesis and aphasia, IRIS |
Subcortical left hemispheric lesion |
| Current study |
Psoriasis | Fumaderm, 2.5 yr | Adenocarcinoma in rectum (pT2 pN1 cM0 L0 V0) 8 yr earlier |
Adjuvant radiochemotherapy with 5-fluorouracil 8 yr earlier |
Grade 2 | CD3+, CD4+, CD8+ T-cell defi- ciency, CD4/CD8 ratio < 2 |
Negative | Diplopia, nystagmus, dysphagia, dysarthria, tetraparesis (PG 3– 4), spasticity, ataxia, IRIS |
Lesions in mesencephalon, pons, cerebellar pedunculi, and medulla oblongata (Gd+), right thalamus and subcortical lesions |
Grade 3 lymphopenia, ALC < 500; grade 2, ALC = 500–799; grade 1, ALC = 800–1,000.1 Fumaderm = 120mg dimethyl fumarate + 95mg of monoethyl fumarate); Psorinovo = dimethyl fumarate; Tecfidera = delayed-release dimethyl fumarate.
ALC = absolute lymphocyte count; CSF = cerebrospinal fluid; Gd+ = gadolinium-enhancing lesions; IRIS = immune reconstitution inflammatory syndrome; JCV = JC virus; MRI = magnetic resonance imaging; PCR = polymerase chain reaction; PG = paresis grade.
Why is it important to obtain a measurement of baseline T-cell subsets prior to initiating therapy with fumarates, in addition to measuring the ALC? In contrast to the dogmatic view that PML only occurs in severely immunosuppressed individuals, we and others have observed patients who develop PML despite minimal or occult immunosuppression.32,33 Among those are individuals with idiopathic CD4+ lymphocytopenia (ICL). These people are entirely asymptomatic and may have low CD4+ T-cell counts for years until they present with catastrophic opportunistic infections, including PML. Because T-cell subsets are not checked routinely in healthy individuals and HIV-negative patients in general, the prevalence of this condition is unknown. ICL is likely a heterogeneous disorder that may also affect CD8+ T cells, CD19+ B cells, and/or CD56+ natural killer cells,34,35 or CD8+ T cells in isolation.36 Hence, it is possible that the 1.9% of patients who developed severe lymphocytopenia on delayed-release DMF had abnormal T-cell subsets at baseline despite normal WBC counts and ALC.
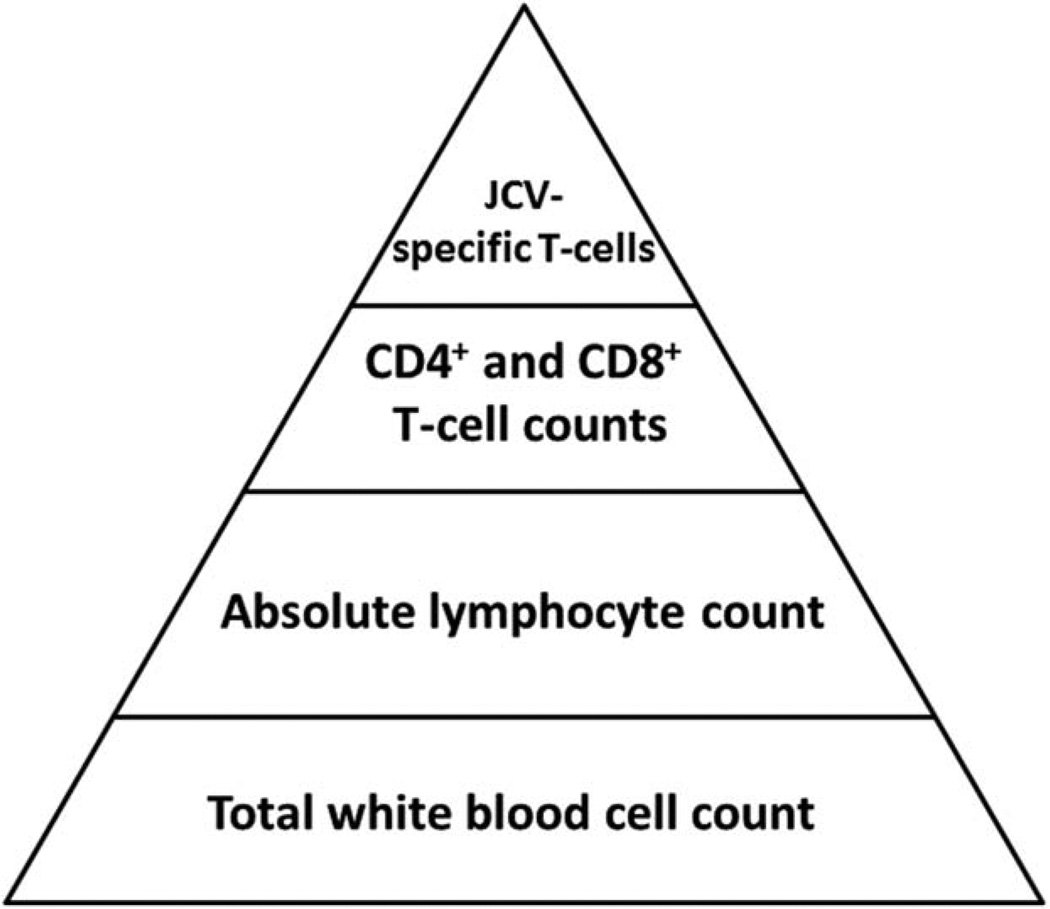
Is counting CD4+ and CD8+ T cells sufficient to determine whether a given individual is at particular risk of developing PML? Prior exposure to JCV or any other pathogen leads to the development of an immune memory, carried by the appropriately named memory T cells. In the setting of JCV reactivation, only those cells will be able to get activated and effectively recognize and destroy JCV-infected cells. Contrary to herpes viruses, which are very immunogenic, polyomaviruses elicit only a relatively weak immune response mediated by a limited numbers of cells. In healthy individuals, the number of JCV-specific CD8+ cytotoxic T lymphocytes (CTLs) ranged between 1 in 2,500 and 1 in 100,000 PBMCs.37 In PML patients, JCV epitope-specific CTLs could be detected by tetramer staining in only 1 of 4,545 to 1 in 100,000 CD8+ T cells isolated from peripheral blood.38 These studies show that the CTLs that can actively recognize and destroy JCV-infected cells are really few and far between in the peripheral blood. Therefore, the number of JCV-specific CD4+ and CD8+ T cells, rather than the total number of CD4+ and CD8+ T cells, should be determined when assessing the risk of developing PML in fumarate-treated patients. The multiple layers of WBC counts used as surrogate markers of the immune response and the determination of PML risk are shown in Figure 4.
FIGURE 4.
Pyramidal representation of white blood cell populations for determination of progressive multifocal leukoencephalopathy (PML) risk. The pyramid contains all white blood cell populations that can be counted in fumarate-treated patients to estimate their degree of immunosuppression. Low cell counts in higher layers are more indicative of PML risk, but they cannot be extrapolated accurately from the cell counts in the layers below. JCV = JC virus.
Another interesting aspect of this patient’s presentation is epilepsy. He presented with focal motor seizures involving the left upper extremity within 24 months from onset of neurological symptoms, which were incompletely controlled despite 2 antiepileptic medications. Seizures are considered to be a manifestation of cortical dysfunction, and their occurrence is not expected in the setting of PML. However, a retrospective review of PML patients seen at our institution from 1995 to 2005 indicated that seizures occurred in 18% of them. The median time for seizure development from onset of symptoms was 2.5 months (range = 1–108 months), and they were associated with PML lesions immediately adjacent to the cerebral cortex.39 A more recent evaluation revealed that up to 34.7% of PML patients developed seizures, which were associated with a hyperintense cortical signal on precontrast T1-weighted MRI images. This MRI finding corresponded histologically to leukocortical encephalitis, characterized by striking JCV-associated demyelination of cortical and subcortical U fibers, significant macrophage infiltration, and a pronounced reactive gliosis in the deep cortical layers.40 These findings suggest that the presence of JCV-induced demyelination and inflammation in the cerebral cortex is a key component of epileptogenesis in PML. Furthermore, we have also observed that in addition to glial cells, JCV could also infect cortical pyramidal neurons within demyelinating PML lesions of the gray matter in 50% of cases, and in gray matter outside demyelinated areas in 11% of patients.41 Interestingly, the right frontal lobe PML lesion of this patient that was biopsied was likely responsible for his focal motor seizures. This lesion involved the gray–white matter junction and contained a number of JCV-infected neurons (see Fig 3). The discovery of JCV-infected neurons in a seizure-inducing PML lesion is tantalizing, and suggests that neuronal infection should be included as a mechanism of JCV-induced epileptogenesis.
Another aspect worth discussing is that this patient developed contrast-enhancing lesions on MRI. Enhancement of brain parenchyma after injection of contrast material indicates breakdown of the blood–brain barrier, and is a surrogate marker of inflammation. Although typical PML lesions are usually nonenhancing, contrast enhancement has long been recognized as an occasional feature of PML,42 and is frequently observed during an immune reconstitution inflammatory syndrome (IRIS).43 IRIS occurs in the setting of immune recovery in PML patients with AIDS treated with antiretroviral medications, or in natalizumab-treated MS patients who developed PML, after discontinuation of the drug or after treatment with plasma exchange,44,45 with more than half of those developing seizures.46 This patient had oscillations of his ALC between 500 and 1,000/mm3 over the course of PML. We can only surmise the occurrence of a similar variation of his CD4+ and CD8+ T-cell subsets, including JCV-specific T cells, perhaps triggering multiple episodes of IRIS over a 2-year period. This would provide a unifying explanation for the protracted course of the disease, undetectable JCV in CSF, contrast enhancement on MRI, and occurrence of seizures.
In summary, this case teaches us that fumarate-treated psoriasis patients may be at risk of PML despite having an ALC that remains above 500/mm3, and that ALC is not a good indicator for CD4+ and CD8+ T-cell counts. PML should be included in the differential diagnosis of any brain lesion occurring in these patients, regardless of their number or location in gray or white matter. The diagnosis of “possible PML” should be retained in those with negative JCV CSF PCR who have MRI lesions and associated neurological dysfunction consistent with PML. This should trigger immediate discontinuation of fumarates and consideration for brain biopsy. Contrast enhancement and seizures occur frequently in PML and may be associated with IRIS.
Psoriasis patients have been treated with fumarates in Germany since 1994, but cases of PML have only been newly reported (see Table 2). It is therefore possible that PML had been previously overlooked in this patient population and that recent recognition may be caused by heightened awareness due to the occurrence of PML in 563 MS patients treated with natalizumab through June 3 2015 (https://medinfo.biogen.com/secure/pmlresource) and 1 with delayed DMF to date.28 Education of patients and physicians, diagnostic algorithms, and risk mitigation strategies are needed to better diagnose, manage, and ultimately prevent PML in psoriasis and MS patients treated with fumarates.
Follow-up
After the diagnosis of PML, treatment with mirtazapine (45mg/day) and mefloquine (loading dose of 250mg for 3 consecutive days and a maintenance dose of 250mg/wk) was initiated.47 Experimentally, it has been shown that the serotoninergic receptor 5HT2AR acts as one of the cellular receptors for JCV on human glial cells and facilitates virus entry into glial cells, and blocking the receptor by 5HT2AR antagonists inhibited infection with JCV in vitro.48 Thus, several case reports reported the use of the 5HT2AR blocker mirtazapine in PML,49,50 including in fumarate-associated PML, albeit with varying results.26,51–53 This treatment option has frequently been used in combination with mefloquine, which has been shown to inhibit viral replication after entry into glial cells.54 However, a recent randomized, parallel-group study comparing patients with PML did not show a significant effect of mefloquine over standard of care.55 The neurological condition of our patient further progressed after brain biopsy for approximately 3 months with worsening of paresis, gait disturbances, and ataxia. MRI showed progression of the right frontal lesion with edema. Three months after discontinuation of fumarate, an IRIS was suspected; however, the patient declined to receive steroid treatment. After 4 months, the clinical condition gradually stabilized and frequent MRI controls showed atrophy of the frontal lesion, no new lesions, and no contrast enhancement. Seizure activity was insufficiently controlled by a combination of levetiracetam and carbamazepine, with about 1 seizure every 7 to 10 days, which motivated replacement of carbamazepine with lamotrigine. Absolute lymphocyte values gradually increased but remained <1,000 mm3 up to 8 months after the diagnosis of PML. Similarly, the CD3+, CD4+, and CD8+ subset values increased but remained below the reference range (see Table 1).17 Interestingly, retrospective analysis of paired CSF and serum samples showed a significant increase in intrathecally produced JCV-specific antibodies measured by the JCV ASI.13 At the onset of neurological symptoms, JCV antibody specificity index (ASI) was 1.02 (<1.5) and increased to 4.04, 1.61, and 12.56, respectively, 3, 6, and 12 months later. This supports the notion that measuring intrathecal JCV-specific antibody synthesis in suspected cases with negative JCV PCR should be added to the diagnostic evaluation.56
Acknowledgments
Supported by the German Cluster of Excellence Inflammation-at-Interfaces (ExC 306, T.B., F.L.); Faculty of Medicine, University of Kiel, Germany (T.B., F.L.); NIH (NINDS R01 NS 047029, NS 074995, I.J.K.); and German Research Council (SFB 855, DE 438/14-1, 16-1, G.D.; SFB 654, FOR 2093, T.B.).
We thank Dr R. Gold for helpful discussions and expert advice.
Potential Conflicts of Interest
F.L.: speaking fees, Grifols, Teva, Biogen Idec. O.A.: personal fees, Biogen Idec. W.B.: grants, Teva Pharma, Novartis, Biogen Idec; personal fees, Teva Pharma, Novartis, Merck Serono, Bayer, Biogen Idec; nonfinancial support, Teva Pharma, Novartis, Bayer, Biogen Idec. G.D.: speaking fees, UCB, Medtronic, Desitin; consultancy, Medtronic, Sapiens, Boston Scientific, Britannica; royalties, Thieme; government employee, receives funding through institution from German Research Council, German Ministry of Education and Health, Medtronic. I.J.K.: consultancy, Biogen Idec, Johnson & Johnson, Genzyme, Eisai, Medimmune; royalties, UpToDate; grant, Biogen Idec.
References
- 1.Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler. 2015;21:796–797. doi: 10.1177/1352458514559299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: has the disease outgrown its name? Ann Neurol. 2006;60:162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 3.Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- 4.Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253–260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 5.Cinque P, Scarpellini P, Vago L, et al. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS. 1997;11:1–17. doi: 10.1097/00002030-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Antinori A, Cingolani A, Lorenzini P, et al. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA) J Neurovirol. 2003;9(suppl 1):47–53. doi: 10.1080/13550280390195388. [DOI] [PubMed] [Google Scholar]
- 7.Marzocchetti A, Di Giambenedetto S, Cingolani A, et al. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43:4175–4177. doi: 10.1128/JCM.43.8.4175-4177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhle J, Gosert R, Buhler R, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 2011;77:2010–2016. doi: 10.1212/WNL.0b013e31823b9b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazda ME, Brosch JR, Wiens AL, et al. A case of natalizumab-associated progressive multifocal leukoencephalopathy with repeated negative CSF JCV testing. Int J Neurosci. 2013;123:353–357. doi: 10.3109/00207454.2012.760561. [DOI] [PubMed] [Google Scholar]
- 10.Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology. 2011;76:1697–1704. doi: 10.1212/WNL.0b013e31821a446b. [DOI] [PubMed] [Google Scholar]
- 11.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–812. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warnke C, von Geldern G, Markwerth P, et al. Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:792–801. doi: 10.1002/ana.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscidi RP, Khanna N, Tan CS, et al. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin Infect Dis. 2011;53:711–715. doi: 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 2012;64:3043–3051. doi: 10.1002/art.34468. [DOI] [PubMed] [Google Scholar]
- 16.Wollebo HS, White MK, Gordon J, et al. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol. 2015;77:560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoxtermann S, Nuchel C, Altmeyer P. Fumaric acid esters suppress peripheral CD4− and CD8-positive lymphocytes in psoriasis. Dermatology. 1998;196:223–230. doi: 10.1159/000017903. [DOI] [PubMed] [Google Scholar]
- 18.Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85:7256–7263. doi: 10.1128/JVI.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–1558. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du Pasquier RA, Kuroda MJ, Zheng Y, et al. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127(pt 9):1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 21.Treumer F, Zhu K, Glaser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121:1383–1388. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 22.de Jong R, Bezemer AC, Zomerdijk TP, et al. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur J Immunol. 1996;26:2067–2074. doi: 10.1002/eji.1830260916. [DOI] [PubMed] [Google Scholar]
- 23.Vandermeeren M, Janssens S, Wouters H, et al. Dimethylfumarate is an inhibitor of cytokine-induced nuclear translocation of NF-kappa B1, but not RelA in normal human dermal fibroblast cells. J Invest Dermatol. 2001;116:124–130. doi: 10.1046/j.1523-1747.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 24.Gergely P. Drug-induced lymphopenia: focus on CD4+ and CD8+ cells. Drug Saf. 1999;21:91–100. doi: 10.2165/00002018-199921020-00003. [DOI] [PubMed] [Google Scholar]
- 25.Khatri B, Garland J, Berger J, et al. The effect of dimethyl fumarate (Tecfidera™) on lymphocyte counts: a potential contributor to progressive multifocal leukoencephalopathy risk. Mult Scler Relat Disord. 2015;4:377–379. doi: 10.1016/j.msard.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med. 2013;368:1657–1658. doi: 10.1056/NEJMc1211805. [DOI] [PubMed] [Google Scholar]
- 27.van Oosten BW, Killestein J, Barkhof F, et al. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368:1658–1659. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 28.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–1478. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 29.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 30.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 31.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2:e76. doi: 10.1212/NXI.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81:247–254. doi: 10.1136/jnnp.2009.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naess H, Glad S, Storstein A, et al. Progressive multifocal leucoencephalopathy in an immunocompetent patient with favourable outcome. A case report. BMC Neurol. 2010;10:32. doi: 10.1186/1471-2377-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med. 2013;3:37–47. doi: 10.4103/2231-0770.114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regent A, Autran B, Carcelain G, et al. Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow-up of 40 patients. Medicine (Baltimore) 2014;93:61–72. doi: 10.1097/MD.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuire JL, Fridman V, Wuthrich C, et al. Progressive multifocal leukoencephalopathy associated with isolated CD8+ T-lymphocyte deficiency mimicking tumefactive MS. J Neurovirol. 2011;17:500–503. doi: 10.1007/s13365-011-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Pasquier RA, Schmitz JE, Jean-Jacques J, et al. Detection of JC virus-specific cytotoxic T lymphocytes in healthy individuals. J Virol. 2004;78:10206–10210. doi: 10.1128/JVI.78.18.10206-10210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima MA, Marzocchetti A, Autissier P, et al. Frequency and phenotype of JC virus-specific CD8+ T lymphocytes in the peripheral blood of patients with progressive multifocal leukoencephalopathy. J Virol. 2007;81:3361–3368. doi: 10.1128/JVI.01809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 40.Khoury MN, Alsop DC, Agnihotri SP, et al. Hyperintense cortical signal on magnetic resonance imaging reflects focal leukocortical encephalitis and seizure risk in progressive multifocal leukoencephalopathy. Ann Neurol. 2014;75:659–669. doi: 10.1002/ana.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012;71:54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger JR, Levy RM, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol. 1998;44:341–349. doi: 10.1002/ana.410440309. [DOI] [PubMed] [Google Scholar]
- 43.Gheuens S, Ngo L, Wang X, et al. Metabolic profile of PML lesions in patients with and without IRIS: an observational study. Neurology. 2012;79:1041–1048. doi: 10.1212/WNL.0b013e318268465b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72:779–787. doi: 10.1002/ana.23676. [DOI] [PubMed] [Google Scholar]
- 45.Wattjes MP, Richert ND, Killestein J, et al. The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2013;19:1826–1840. doi: 10.1177/1352458513510224. [DOI] [PubMed] [Google Scholar]
- 46.Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013;84:1068–1074. doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroder A, Lee DH, Hellwig K, et al. Successful management of natalizumab-associated progressive multifocal leukoencephalopathy and immune reconstitution syndrome in a patient with multiple sclerosis. Arch Neurol. 2010;67:1391–1394. doi: 10.1001/archneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- 48.Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 49.Verma S, Cikurel K, Koralnik IJ, et al. Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis. 2007;196:709–711. doi: 10.1086/520514. [DOI] [PubMed] [Google Scholar]
- 50.Cettomai D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. 2009;66:255–258. doi: 10.1001/archneurol.2008.557. [DOI] [PubMed] [Google Scholar]
- 51.Stoppe M, Thoma E, Liebert UG, et al. Cerebellar manifestation of PML under fumarate and after efalizumab treatment of psoriasis. J Neurol. 2014;261:1021–1024. doi: 10.1007/s00415-014-7311-1. [DOI] [PubMed] [Google Scholar]
- 52.Hoepner R, Faissner S, Klasing A, et al. Progressive multifocal leukoencephalopathy during fumarate monotherapy of psoriasis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e85. doi: 10.1212/NXI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieuwkamp DJ, Murk JL, van Oosten BW, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med. 2015;372:1474–1476. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- 54.Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–1849. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. 2013;19:351–358. doi: 10.1007/s13365-013-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koralnik IJ. Finger pointing to JC virus: a tale of two indexes. Ann Neurol. 2014;76:789–791. doi: 10.1002/ana.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067–1074. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]