Abstract
Hyperthermia from a central cause is associated with increased morbidity and mortality. Dysfunction of brainstem thermoregulatory pathways may explain the intractable rise in temperature. Antipyretics, dantrolene, bromocriptine, and surface and intravascular cooling devices have been attempted for temperature control. We report the case of a 54-year-old woman with history of hypertension who presented with pontine hemorrhage with extension into the midbrain and medulla. On days 8–9 of her hospital admission, she developed intractable fever and expired the same day despite aggressive treatment of hypothermia, including antipyretics, ice lavage, cold fluid boluses, surface cooling, dantrolene, and bromocriptine. Hyperthermia from brainstem hemorrhage can be difficult to manage with current treatment options. Early recognition of those patients who may develop hyperthermia could lead to early intervention and possibly better outcomes. More evidence from prospective randomized controlled trials will elucidate the risk–benefit profile of achieving normothermia with aggressive fever control in these patients.
Introduction
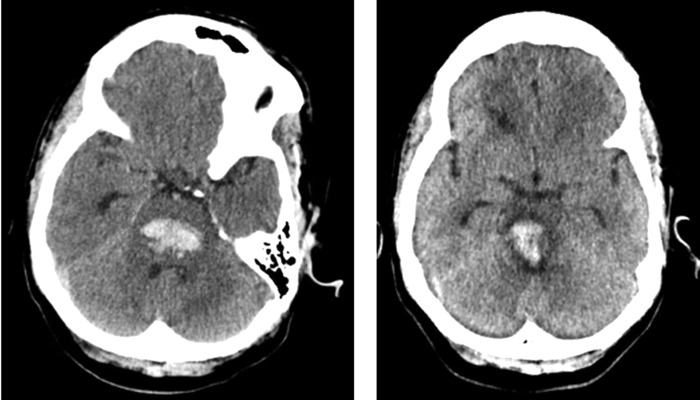
A 54-year-old African American woman with a medical history of untreated hypertension presented with generalized weakness and dizziness and a headache that worsened over the course of a few days. She called her son to come and assist her, but was found unresponsive ∼30 minutes later. Emergency medical services was called and she was transported to a local emergency department and intubated upon arrival. Her initial Glasgow Coma Scale score was 4 (E1 V1 M2) and her CT scan showed acute pontine hemorrhage (Fig. 1). She was subsequently transferred to the neurosciences ICU at our institution.
FIG. 1.
Patient head CT images showing hemorrhagic extension (initial CT to left, CT 5 days postpresentation to our institution to right).
Her initial blood pressure on arrival was 186/104 mmHg, and a nicardipine infusion was started with a goal systolic blood pressure of less than 160 mmHg. An MRI of the head showed a large pontine hemorrhage with mass effect on the fourth ventricle and cerebral edema tracking into the midbrain and medulla. In addition, the gradient echo sequence also showed several microhemorrhages in the subcortical white matter, with fluid-attenuated inversion recovery (FLAIR sequences) showing chronic microvascular ischemic changes (Fig. 2). Transthoracic echocardiogram showed concentric left ventricular hypertrophy with preserved systolic function and grade I diastolic dysfunction. Electroencephalogram showed diffuse, nonreactive theta activity without state changes or clear posterior dominant rhythm.
FIG. 2.
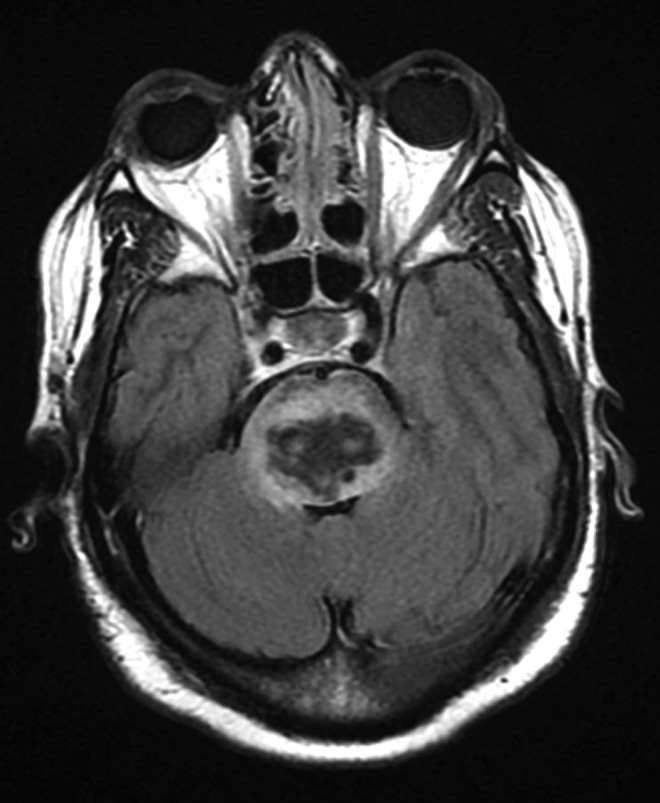
Patient MRI FLAIR sequence showing pontine hemorrhage with surrounding edema and sulcal effacement likely affecting brainstem thermoregulatory structures. FLAIR, fluid-attenuated inversion recovery.
Seven days after her initial symptoms, the patient's temperature rose to 38.5°C (101.3°F). Blood work showed a mild leukocytosis. Blood and urine cultures did not reveal growth of any microorganisms. Acetaminophen was given. Two days later, she developed intractable central hyperthermia. Her temperature increased rapidly from 37.1°C (98.9°F) to 40.9°C (105.6°F) over the course of a few hours. The core temperature (rectal) was 42.4°C (108.4°F). Her peripheral white blood cell count increased from 11.9 to 15.0 × 103/μL, and creatinine was also elevated to 1.00 mg/dL from baseline of around 0.8. Ice lavage and cold IV fluid bolus were given. A surface cooling device (Arctic Sun) was placed. Antipyretics, dantrolene and bromocriptine, were administered. Despite aggressive treatment, her temperature did not substantially decrease. Six hours later she underwent cardiac arrest and expired.
Discussion
Hyperthermia or hyperprexia in brainstem hemorrhage
Fever from a central cause, particularly intracerebral hemorrhage, is associated with increased morbidity and mortality (Commichau et al., 2003). As an independent variable, fever is consistently associated with worse outcomes in patients with neurologic injury (Greer et al., 2008). A retrospective study of 74 patients who developed hyperthermia (>39°C) in the neurosciences ICU poststroke was found to result in 70% mortality (Sung et al., 2009). Thus, it is an independent risk factor for poor clinical outcome. Brainstem, particularly pontine, hemorrhage was the direct cause of fever in 64% of patients, but all patients had brainstem involvement.
The intractability of fever caused by large-volume intracerebral hemorrhages is hypothesized to be due to direct compression of brainstem and hypothalamic thermoregulatory centers (Deogaonkar et al., 2005). Wijdicks and St Louis (1997), in a retrospective study of 38 patients, linked pontine hemorrhage to higher mortality if patients had a history of hypertension, presented in coma, or had absent oculocephalic reflexes and/or motor response. However, mortality was highest in those patients with CT evidence of extension into midbrain and thalamus (as our patient had), hyperthermia, tachycardia >100 beats/min, and acute hydrocephalus on admission. Only those patients who were alert on admission and with unilateral small pontine hemorrhage made good recovery in this series. In a retrospective study of 251 patients with spontaneous intracranial hemorrhage, duration of fever was independently associated with poor outcome in those who survived past 72 hours (Schwarz et al., 2000).
Fever in patients with brainstem hemorrhage is thus a sign of particularly poor prognosis. Previous reports have included fatal cases of extensive brainstem hemorrhage from midbrain to medulla, with characteristics similar to malignant hyperthermia, including rapid onset of hyperprexia, elevated creatinine kinase levels, and renal failure with precipitous clinical decline, despite no predisposition to malignant hyperthermia (Kitanaka et al., 1994). The central hyperthermia syndrome may also be marked by temperature fluctuation within a short period of time (Sung et al., 2009). Faster recognition of the etiology of fever in ICH/SAH patients may help to more effectively manage this complication.
Brainstem temperature regulation
The central neural thermoregulatory system is not completely understood. Integration of temperature information from spinothalamocortical pathways as well as from the lateral parabrachial nucleus, located at the junction of pons and midbrain, occurs in the hypothalamic preoptic area (POA) (Morrison and Nakamura, 2011). POA neurons are uniquely sensitive to the influence of pyrogenic mediators, such as prostaglandin E2, and this area controls body temperature set point (Nakamura et al., 1999).
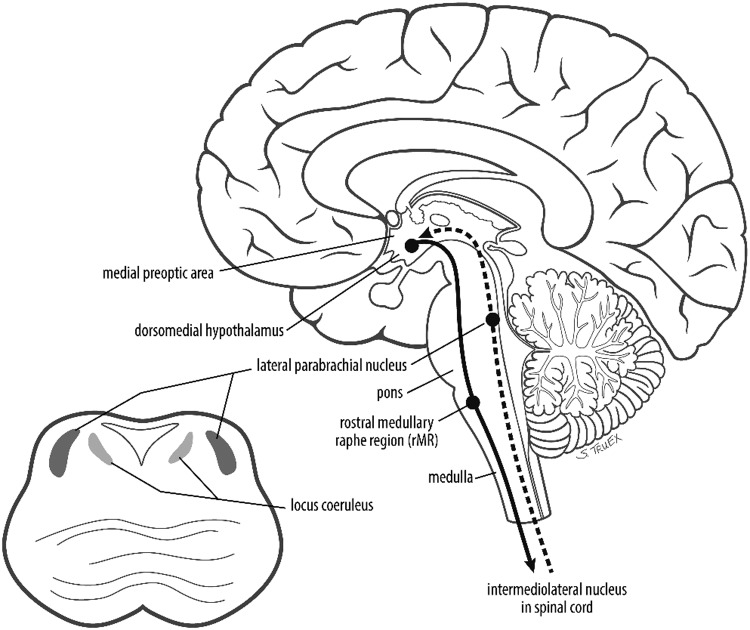
Thermogenic signals result in activation of warm-sensitive neurons in the median preoptic nucleus within the POA. Brown adipose tissue thermogenesis is affected through afferents to key brainstem regions such as the rostral ventromedial medulla. By contrast, neuronal activation in the ventrolateral medulla or the nucleus of the solitary tract can decrease brown adipose tissue thermogenesis (Morrison et al., 2008). In addition, the midbrain rostral ventromedial periaqueductal gray area has been implicated in tonic inhibition of thermogenesis (Rathner and Morrison, 2006). Hence, multiple brainstem regions are important in central temperature regulation (Fig. 3). Injury to the brainstem has directly been shown to affect normal mammalian thermostasis, as evidenced by altered temperature regulation in cats with pontine tegmental lesions and hyperthermia in rats that have undergone prepontine knife cut (Benzi et al., 1988).
FIG. 3.
Brainstem areas responsible for temperature regulation; see text for further explication.
Avenues for treatment and future research
Extensive brainstem hemorrhage is a poor prognostic factor. Central hyperthermia is costly to manage because of extended hospital and ICU lengths of stay; more targeted and effective therapies might help to address this issue (Reaven et al., 2009). Conventional fever control, including antipyretics, can be tried initially. If these fail to produce normothermia, aggressive temperature management can be achieved by external cooling systems or by intravascular cooling techniques. Empirically, bromocriptine and baclofen have been reported to help abolish central fever; dantrolene may provide neuroprotection in patients at risk for intractable central hyperthermia (Huang et al., 2009; Muehlschlegel and Sims, 2009; Yu et al., 2013).
Both surface and intravascular cooling devices have been shown by prospective randomized controlled trial to be superior to conventional therapies in controlling temperature (Mayer et al., 2004; Broessner et al., 2009). However, although cooling devices can be extremely effective in producing normothermia, the benefit is offset by the metabolic consequences of shivering, as well as the increased risk of infection with normothermia (Scaravilli et al., 2011). Although hyperthermia associated with hemorrhage is known to be deleterious to neurologic injury patients, it is unclear whether an aggressive push for normothermia results in better clinical outcomes for these patients. Prospective studies of patients with hemorrhage-related central hyperthermia with specific related hypotheses are necessary to understand how to proceed with temperature control, hyperthermia prevention, and resuscitative measures, and to better counsel families regarding prognosis.
Conclusion
Intractable central hyperthermia related to brainstem hemorrhage is associated with high morbidity and mortality. Distinguishing central hyperthermia from other causes of hyperthermia in the neurosciences ICU is important to optimize treatment. An improved understanding of the mechanisms that lead to intractability, with evidence from large prospective randomized controlled trials, could aid earlier recognition of patients who are more likely to progress to this state and implementation of earlier management. Good prospective evidence would also elucidate the risk–benefit profile of maintaining normothermia.
Author Disclosure Statement
No competing financial interests exist.
References
- Benzi RH, et al. . Prepontine knife cut-induced hyperthermia in the rat. Effect of chemical sympathectomy and surgical denervation of brown adipose tissue. Pflugers Arch 1988;411:593–599 [DOI] [PubMed] [Google Scholar]
- Broessner G, et al. . Prophylactic, endovascularly based, long-term normothermia in ICU patients with severe cerebrovascular disease: Bicenter prospective, randomized trial. Stroke 2009;40:e657–e665 [DOI] [PubMed] [Google Scholar]
- Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the neurologic intensive care unit. Neurology 2003;60:837–841 [DOI] [PubMed] [Google Scholar]
- Deogaonkar A, et al. . Fever is associated with third ventricular shift after intracerebral hemorrhage: Pathophysiologic implications. Neurol India 2005;53:202–206; discussion 206–207. [DOI] [PubMed] [Google Scholar]
- Greer DM, et al. . Impact of fever on outcome in patients with stroke and neurologic injury: A comprehensive meta-analysis. Stroke 2008;39:3029–3035 [DOI] [PubMed] [Google Scholar]
- Huang YS, et al. . Baclofen successfully abolished prolonged central hyperthermia in a patient with basilar artery occlusion. Acta Neurol Taiwan 2009;18:118–122 [PubMed] [Google Scholar]
- Kitanaka C, et al. . Malignant brain stem hyperthermia caused by brain stem hemorrhage. Stroke 1994;25:518–520 [DOI] [PubMed] [Google Scholar]
- Mayer SA, et al. . Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32:2508–2515 [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 2008;93:773–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlschlegel S, Sims JR. Dantrolene: Mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care 2009;10:103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, et al. . Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett 1999;260:117–120 [DOI] [PubMed] [Google Scholar]
- Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: A source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res 2006;1077:99–107 [DOI] [PubMed] [Google Scholar]
- Reaven NL, Lovett JE, Funk SE. Brain injury and fever: Hospital length of stay and cost outcomes. J Intensive Care Med 2009;24:131–139 [DOI] [PubMed] [Google Scholar]
- Scaravilli V, Tinchero G, Citerio G. Fever management in SAH. Neurocrit Care 2011;15:287–294 [DOI] [PubMed] [Google Scholar]
- Schwarz S, et al. . Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000;54:354–361 [DOI] [PubMed] [Google Scholar]
- Sung CY, Lee TH, Chu NS, Central hyperthermia in acute stroke. Eur Neurol 2009;62:86–92 [DOI] [PubMed] [Google Scholar]
- Wijdicks EF, St Louis E. Clinical profiles predictive of outcome in pontine hemorrhage. Neurology 1997;49:342–1346 [DOI] [PubMed] [Google Scholar]
- Yu KW, et al. . Effectively managing intractable central hyperthermia in a stroke patient by bromocriptine: A case report. Neuropsychiatr Dis Treat 2013;9:605–608 [DOI] [PMC free article] [PubMed] [Google Scholar]