Abstract
Glioblastoma multiforme (GBM) is the most commonly occurring primary brain cancer in adults, in whom its highly infiltrative cells prevent total surgical resection, often leading to tumor recurrence and patient death. Our group has discovered a gene therapy approach for GBM that utilizes high-capacity “gutless” adenoviral vectors encoding regulatable therapeutic transgenes. The herpes simplex type 1-thymidine kinase (TK) actively kills dividing tumor cells in the brain when in the presence of the prodrug, ganciclovir (GCV), whereas the FMS-like tyrosine kinase 3 ligand (Flt3L) is an immune-stimulatory molecule under tight regulation by a tetracycline-inducible “Tet-On” activation system that induces anti-GBM immunity. As a prelude to a phase I clinical trial, we evaluated the safety and efficacy of Food and Drug Administration (FDA)–approved doses of the tetracycline doxycycline (DOX) allometrically scaled for rats. DOX initiates the expression of Flt3L, which has been shown to recruit dendritic cells to the brain tumor microenvironment—an integral first step in the development of antitumor immunity. The data revealed a highly safe profile surrounding these human-equivalent doses of DOX under an identical therapeutic window as proposed in the clinical trial. This was confirmed through a neuropathological analysis, liver and kidney histopathology, detection of neutralizing antibodies, and systemic toxicities in the blood. Interestingly, we observed a significant survival advantage in rats with GBM receiving the 300 mg/day equivalent dosage of DOX versus the 200 mg/day equivalent. Additionally, rats rejected “recurrent” brain tumor threats implanted 90 days after their primary brain tumors. We also show that DOX detection within the plasma can be an indicator of optimal dosing of DOX to attain therapeutic levels. This work has significant clinical relevance for an ongoing phase I clinical trial in humans with primary GBM and for other therapeutic approaches using Tet-On transactivation system in humans.
Introduction
Glioblastoma multiforme (GBM) is a primary malignant brain cancer in adults with a dismal prognosis of 15–21 months.1 Therapeutic standard of care includes surgical resection, chemotherapy, and radiotherapy. Nevertheless, in spite of aggressive treatment regimes, these diffuse, invasive, and heterogeneous tumors invariably recur, ultimately resulting in the death of patients. Thus, there is an urgent need to develop novel and more efficacious therapeutic options.
Gene therapy has become an attractive therapeutic modality for cancer; improvements in vector safety and specificity make this approach readily translatable to the clinic.2–4 Replication-deficient first-generation adenoviral vectors (Ads) lacking the E1 gene, while effective at eliciting transgene expression, maintain low levels of viral gene expression. The result is an adaptive immune response against the vector leading to transient transgene expression. In recent years, safer vectors have been developed by removing all viral coding sequences, thereby decreasing toxicities associated with inflammation as well as preventing immune detection by the immune system of the host.5–7 These vectors have been termed “high-capacity,” “helper-dependent,” or “gutless” adenoviral vectors (HC-Ads). Finally, transgene expression driven from viral vectors can now be regulated by transcriptional “activators,” which can be used to control therapeutic transgene expression. For example, the Tet-On transactivation system can be turned “on” in the presence doxycycline (DOX), and switched “off” in its absence.7–12
To address GBM recurrence, we developed a novel combined gene therapy strategy utilizing the HC-Ads encoding the herpes simplex type 1-thymidine kinase (TK) transgene, which induces apoptosis in dividing cells in the presence of the prodrug, ganciclovir (GCV), and the FMS-like tyrosine kinase 3 ligand (Flt3L) transgene under tight regulation by the Tet-On transactivation system.13–24 Flt3L has been shown to recruit dendritic cells (DCs) to the site of TK-mediated tumor killing where they bind to the high-mobility-group box 1 (HMGB1) protein, an alarmin released by dying GBM cells within the tumor microenvironment, acting as an endogenous ligand for Toll-like receptor 2 (TLR2) on DCs.21,22 Once bound to HMGB1, these DCs migrate to the draining lymph nodes to prime CD4+ T-cells that in turn activate CD8 T-cells. We have shown that this adaptive immune response elicits long-term immunity in rats that not only eliminates large primary brain tumors, but also overcomes new tumor challenges in the opposite brain hemisphere, simulating tumor recurrence.19,20,22,24
Before the implementation of HC-Ad-mediated gene therapy in GBM patients, we sought to identify a dose of DOX that would be both efficacious and safe for clinical use. Despite the safety and accepted use of DOX as an antibiotic to treat infections in patients, the application of DOX as an activator of gene transcription via the Tet-On regulatable promoter system is not yet approved for use in humans. Thus, this would be an “off-label” use for this drug. Therefore, we assessed the DOX dose needed to elicit transgene expression from the Tet-On promoter system encoded within the HC-Ads and also the efficacy of the DOX-dependent therapy in increasing survival in GBM tumor-bearing rodents.11
DOX is an antibiotic used to treat various types of infections because of its specificity for inhibiting bacterial protein synthesis.25 When testing the therapeutic efficacy of HC-Ad-TK + HC-Ad-TetOn-Flt3L in the preclinical studies, DOX was administered in rat chow at a concentration of 2,000 parts per million (ppm). However, converting these doses to the human equivalent yields levels of DOX that could be considered unsafe for human use. Therefore, we elected to lower the dosage further to analyze the three most commonly used FDA-approved dosages of DOX (100, 200, and 300 mg/day) using the average adult human weight of 70 kg.26 This study allowed us to determine the efficacy of the Tet-On transcriptional activation system in the presence of a significantly lower dose of DOX, and also the safety of such doses when used within an identical clinical therapeutic window of one month. Additionally, demonstrating that the aforementioned doses of DOX were effective at inducing expression of the Flt3L transgene in a species with a complex immune system with similarities to the human immune system was essential in establishing the translation application of this therapeutic approach in patients.8
In this study, we investigated the efficacy of the DOX-driven therapy at improving survival in tumor-bearing rats and analyzed expression of Flt3L within the tumor microenvironment using human-equivalent doses of DOX (100, 200, and 300 mg/day assuming a 70 kg weight). In surviving animals, we assessed the impact of these human-equivalent doses on neurological, hepatic, renal, and hematological safety. Finally, we assessed the ability of the viral therapy to generate immunological memory in animals bearing intracranial tumors. Our results show that allometrically scaled doses of DOX, that is, equivalent doses to the ones recommended for use in humans to treat infections, can safely turn “on” Flt3L expression from the HC-Ad-TetOn-Flt3L vector in rodents. Our data warrant the use of DOX at doses that have previously been approved by the FDA for the treatment of infections in humans in an “off-label” application of this drug for the treatment of GBM. It also affirms the importance of DOX in eliciting sufficient levels of Flt3L expression to elicit immunological memory in order to block tumor recurrence. This work has significant translational relevance for our own upcoming clinical trial as well as for other researchers interested in implementing the Tet-On transcriptional activation-dependent expression of therapeutic transgenes within the brain.
Materials and Methods
Animals
Adult male Lewis rats (200–300 g; Harlan) were kept in controlled conditions of light (12 hr light–dark cycles) and temperature (20–25°C). Rats received water and standard rodent chow ad libitum. All animal procedures were carried out in accordance with NIH guidelines for the care and use of laboratory animals and approved by the University of Michigan Unit for Laboratory Animal Medicine.
Stereotactic intracranial injections
Three thousand CNS-1 tumor cells were injected into the striatum using a 5 μl Hamilton syringe fitted with a 26-gauge needle. The stereotactic coordinates were 1.0 mm anterior and 3.0 mm lateral to the bregma; the injection volume of 1.0 μl was delivered −4.5 mm from the dura. Re-challenged rats, along with 3 age-matched control rats received intracranial injections of 3,000 CNS-1 tumor cells 90 days after gene therapy using the injection procedure and stereotactic coordinates described above into the contralateral hemisphere. Rats were intracranially injected with 1 × 109 viral particles (vp) of HC-Ad-TetOn-Flt3L into the striatum. The vector was injected in a final volume of 1.5 μl of saline using a 5 μl Hamilton syringe fitted with 26-gauge needle. The stereotactic coordinates were 1.0 mm anterior and 3.0 mm lateral to the bregma; the injection volume of 1.5 μl was delivered in 3 locations (0.5 μl each) at −5.0, −4.5, and −4.0 mm from the dura. The appropriate dose of DOX (water for the control animals) was administered to each animal every 12 hr for 1 month as described below.
High-capacity adenoviral vector
Details of the molecular characterization, rescue, and amplification of the HC-Ad vector were published previously.11,27–33
Allometric dosing of DOX
Allometric doses of DOX for rats were calculated by dividing the human DOX dose by the average human weight of 70 kg, and then multiplying by the allometric conversion factor of seven.34 This number was calculated by taking into account differences in metabolic rate, pharmacokinetics, and body surface area between humans and rats. Therefore, the FDA-approved 300 mg dose of DOX in humans is equivalent to 30 mg/kg in the rat (see calculations in Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hgtb). Similarly, 200 and 100 mg doses of DOX in humans are equivalent to 20 and 10 mg/kg, respectively, in the rat. In the safety study, in order to express the Flt3L transgene, we prepared suspensions of the DOX solution (doxycycline hyclate; Sigma-Aldrich; Cat. # D9891) in H2O and administered the appropriate dose via oral gavage twice daily, 2 days before HC-Ad vector delivery (day −2), and lasting until 28 days after HC-Ad vector delivery. To create the 10 mg/ml oral gavage solution, 500 mg DOX was added to 50 ml Milli-Q H2O. The solution was stored at 4°C in the dark and prepared fresh every 5 days.
Ganciclovir administration
Rats were administered 25 mg/kg of the prodrug, GCV (Roche Laboratories), via intraperitoneal (IP) injection from the day of tumor implantation twice daily for 10 days. To prepare the 7 mg/ml stock solution, 20 ml of Milli-Q H2O was added to 250 mg of GCV, and pH was adjusted to 12 using 1 M NaOH such that the solution became clear. Next, the pH was lowered to 11 using 1 M HCl and Milli-Q H2O was added to obtain a final volume of 35.71 ml. The solution was stored at −20°C.
Perfusion and tissue harvesting
Rats were anesthetized with ketamine (75 mg/kg, IP) and dexmedetomidine (0.25 mg/kg, IP) before perfusion with oxygenated Tyrode's solution (132 nM NaCl, 1.8 mM CaCl2, 0.32 mM NaH2PO4, 5.56 mM glucose, 11.6 mM NaHCO3, 2.68 mM KCl, 0.1 U/ml heparin; pH 7.4) followed by fixation with 4% paraformaldehyde (4% paraformaldehyde, 137 mM NaCl, 2.7 mM KCl, 10 mM phosphate buffer; pH 7.4). Brains were post-fixed in 4% paraformaldehyde for 3 days before processing.32,35
Neuropathological analysis
Fifty-micrometer serial coronal sections were generated and analyzed from the immediate vicinity of the injection site using a Leica VT100S vibratome, and free-floating immunohistochemistry was performed as previously described24,35–37 with markers for oligodendrocytes and myelin sheaths (mouse monoclonal anti-MBP, 1:1,000; Chemicon; Cat. # MAB1580); dopaminergic nerve terminals (rabbit polyclonal anti-TH, 1:5,000; Calbiochem; Cat. # 657012); CD8+ T-cells (mouse anti-CD8, 1:1,000; Serotec; Cat. # MCA48G); macrophages and microglia (CD68/ED1, mouse anti-ED1, 1:1,000; Cat. # MCA341R; IBA1, polyclonal rabbit anti-IBA1, 1:1,000; Wako Pure Chemical Industries; Cat. # 019-19741); activated macrophages, microglia, and immune cells (mouse anti-MHC II, 1:1,000; Serotec; Cat. # MCA46G); and Flt3L (rabbit anti-Flt3L, 1:1,000; custom made).38 Nissl staining was performed to assess gross histopathological features of each brain as described previously.20 The stained sections were photographed with a Zeiss Optical Axioplan microscope using Axiovision Rel 4.6 and MOSAIX software (Carl Zeiss). All histology data and samples were reviewed by Dr. Henry Appelman, M.R. Abell Professor of Surgical Pathology at the University of Michigan.
Serum biochemistry
Collected blood was transferred to serum separation tubes (SC Micro Tube Ser-Gel PP; Biotang; Cat. # 41.1378.005); samples were left for 30 min at room temperature to allow for blood coagulation before centrifugation at 2,000 relative centrifugal force for 10 min and immediate analysis on a VetTest 8008 serum chemistry analyzer (IDEXX Laboratories).
Circulating neutralizing antiadenovirus antibodies
Plasma samples were heat-inactivated at 56°C for 30 min and serially diluted 2-fold in minimal essential medium (Invitrogen) containing 10% heat-inactivated fetal bovine serum (HI-FBS). Fifty-microliter plasma dilutions were incubated with 3 × 106 infectious units (iu) of first-generation Ads expressing β-galactosidase (Ad-β-Gal, 200 iu per cell) for 90 min at 37°C. A 50 μl sample of the plasma/Ad-β-Gal mix was added to wells of a 96-well plate containing 1.5 × 104 pre-seeded HEK 293 cells and incubated for 60 min at 37°C. Finally, an additional 50 μl containing 10% FBS was added to each well, and the cells were incubated at 37°C for 20 hr before fixation with 1% glutaraldehyde in PBS (pH 7.4) and staining with 5-bromo-4-chloro-indolyl-β-galactoside (X-gal; Sigma).8,26 ImageJ was used to quantify the percentage of β-Gal-positive cells in three representative images for every well. The percentages were averaged for these wells, and the average was subsequently compared with a control. The neutralizing antibody titer for each animal is given as the reciprocal of the highest dilution of serum at which 50% of maximal Ad-β-Gal-mediated transduction was inhibited. Serum from a pre-immunized animal was used as a positive control, and serum from a naïve animal was used as a negative control.
DOX concentration (ng/ml) in rat plasma
Blood was collected upon euthanasia and analyzed for DOX content by the Pharmacokinetics Core at the University of Michigan.
H&E staining
Liver and kidney tissue sections (5 μm thick) from DOX and saline-treated rats were stained with hematoxylin and eosin (H&E).8,39
Good laboratory practice guidelines
This study was not performed under good laboratory practice guidelines.
Results
Regulatable therapeutic transgene expression in response to allometrically scaled doses of DOX
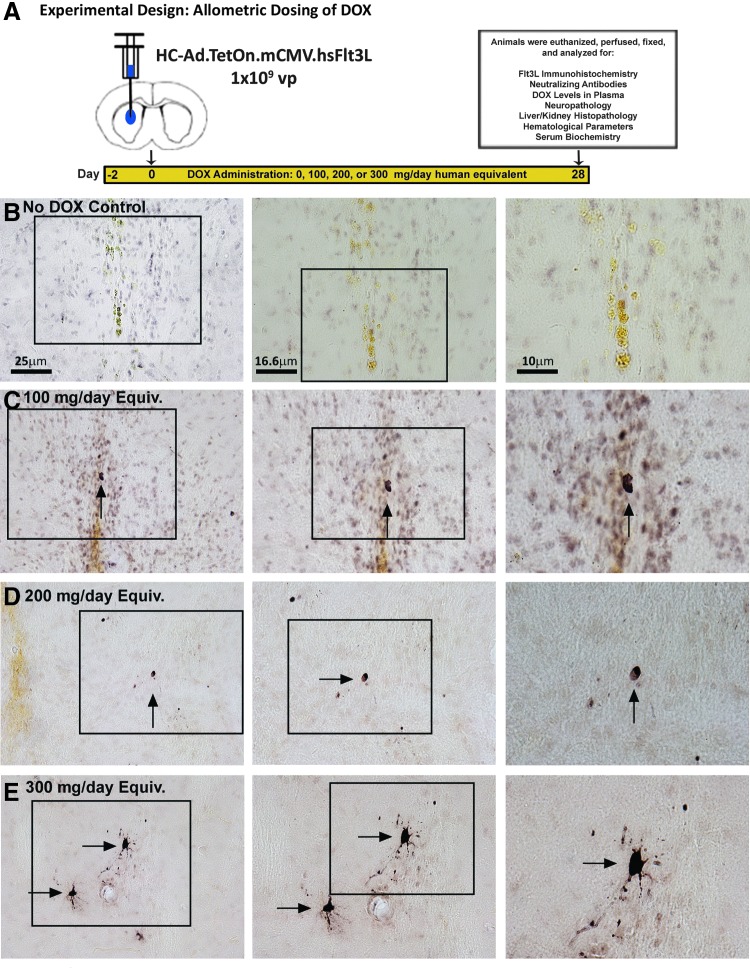
We first assessed if allometrically scaled doses of DOX, which would be compatible to doses already approved for use in humans, would be sufficient to turn on therapeutic Flt3L expression form the HC-Ad.TetOn.mCMV.hsFlt3L. To do this, DOX was delivered to naïve rats every 12 hr via oral gavage beginning 2 days before delivery of HC-Ad.TetOn.mCMV.hsFlt3L and lasting for 4 weeks (Fig. 1A)—identical to the course proposed in the phase 1 clinical trial. At the end of the 4 weeks, rats were perfused and the brain and other tissues were fixed for a complete neuropathological and histological analysis to assess any potential adverse side effects. When compared with the no DOX control, which received equivalent volumes of water delivered by oral gavage twice daily (Fig. 1B), the 100 mg/day equivalent (Fig. 1C), the 200 mg/day equivalent (Fig. 1D), and the 300 mg/day equivalent doses of DOX (Fig. 1E) revealed expression of Flt3L detected by 3, 3′-diaminobenzidine (DAB) immunohistochemical analysis. This demonstrates that each of the three most commonly used FDA-approved doses of DOX was sufficient to turn “on” the therapeutic transgene under the proposed DOX regimen.
Figure 1.
Inducible Tet-On transactivation of Flt3L expression with allometrically scaled human doses of doxycycline (DOX). (A) Experimental design showing a therapeutic window for DOX identical to that proposed in a pending clinical trial application to activate Flt3L transgene expression. Human-equivalent doses of DOX approved by the FDA for use in humans (100, 200, and 300 mg/day) were allometrically scaled for use in rats and administered beginning 2 days before treatment with the gene therapy HC-Ad vectors for 1 month. Brains from animals gavaged with water (B), 100 mg/day DOX equivalent (C), 200 mg/day DOX equivalent (D), and 300 mg/day DOX equivalent (E) were analyzed using immunohistochemical analysis to determine Flt3L expression. Color images available online at www.liebertpub.com/hgtb
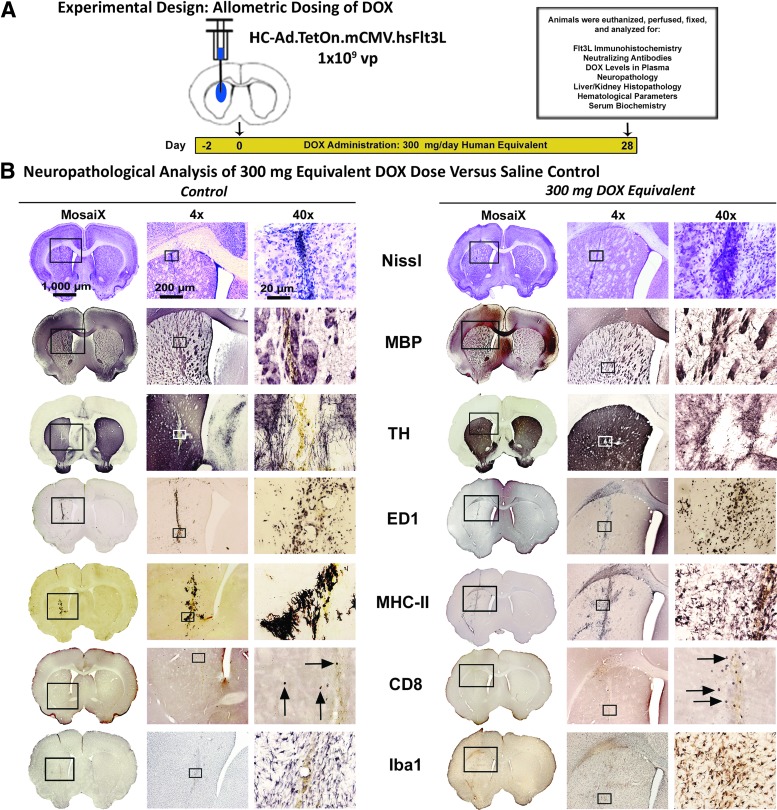
Neuropathological analysis and systemic effects of allometric doses of DOX after intracranial delivery of HC-Ad.TetOn.mCMV.hsFlt3L
Considering that the proposed clinical trial involves the delivery of the therapeutic gene-delivered therapy into the tumor cavity after the resection of the tumor mass, it is important to assess any potential neuropathological effects on normal brain parenchyma as a result of viral transduction and activation of therapeutic transgene expression via DOX administration. Under the same experimental design (Fig. 2A) mentioned in the previous paragraph, a complete neuropathological analysis of animals receiving the highest dose of DOX—300 mg/day for 1 month—was evaluated for signs of toxicity within the brain compared with a saline-treated control (Fig. 2B). Complete neuropathological analyses of the 100 mg/day equivalent (Supplementary Fig. S2) and the 200 mg/day equivalent (Supplementary Fig. S3) are also shown. Brains were processed for architectural integrity through an evaluation of the Nissl bodies (Nissl), white matter tracts and oligodendrocytes (MBP), and dopaminergic nerve fibers (TH). No differences were observed when comparing the saline-treated animals with the groups treated with 100, 200, or 300 mg/kg/day of DOX, with minor mechanical disturbances along the needle tract resulting in a localized increase in Nissl body prevalence. An increase in the white matter bundles formed along the needle tract in both groups was associated with a slight decrease in the dopaminergic nerve fiber presence. These effects can also attributed to the mechanical damage caused by the needle tract.
Figure 2.
Neuropathological assessment of 300 mg/day DOX dose. (A) Experimental design showing the chronology of the safety study evaluating the “off-label” use of DOX in rats to activate the Tet-On inducible system for Flt3L. After 1 month of the 300 mg equivalent of DOX, a neuropathological analysis (B) was performed to determine the safety of this combination in the rat brain versus the 0 DOX control. Architectural scaffolding in the brains of rats, shown by staining Nissl bodies, reveals no gross abnormalities versus the control. Further investigation of various structural components (axons and myelin sheaths [MBP]; dopaminergic nerve fibers [TH]) showed no differences in DOX-treated animals and controls. There appears to be an increase, however, in the immunological components of the brain, as CD68+ macrophages (ED1), major-histocompatibility complex-II antigen presenting cells (MHC-II), CD8+ T-cells (CD8), and activated macrophages and microglia (Iba1) revealed an increase in staining around the injection sites of DOX-treated animals when compared with controls.
Immune cell infiltration of rats receiving the 300 mg/day equivalent of DOX displayed a noticeable increase in infiltrating ED1+ macrophages (ED1), major histocompatibility complex II (MHC-II) antigen presenting cells, CD8+ T-cells, and activated microglia and macrophages (Iba1) when compared with the saline-treated control animals.
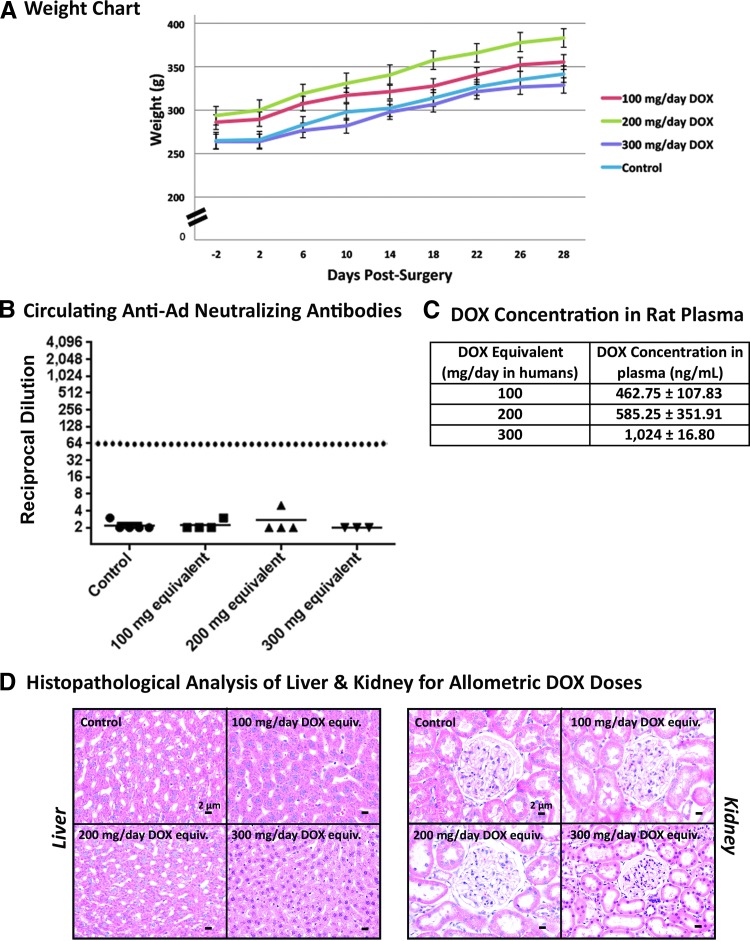
Animals receiving twice-daily oral gavages of allometrically scaled doses of DOX and water were monitored for changes in their body weight to detect possible systemic adverse off-target effects (Fig. 3A). The mean weights of animals showed a steady increase with a similar slope when compared with the controls for each experimental group, indicative of the safety of the doses of DOX used in this study.
Figure 3.
Absence of circulating anti-Ad antibodies post-surgery in rats receiving DOX. The body weight of Lewis rats receiving DOX was monitored during the course of the safety study and compared with a control rat receiving water (A). Blood from rats treated for 1 month with allometrically scaled doses of DOX was collected via cardiac puncture before perfusion and fixation and processed for (B) neutralizing antiadenovirus antibodies, which were quantified in the sera of rats treated with allometric equivalents of DOX in the safety analysis. (C) DOX concentration 5 hr after latest dosing for each dose. (D) A histopathological analysis of organs associated with metabolism and detoxification of DOX and its metabolites—the liver and kidneys—showed no gross changes in animals treated with the control or the 100, 200, or 300 mg/day DOX equivalent. Scale bars for liver and kidney histopathology: 2 μm.
One of the major limiting factors in gene therapeutic strategies is the presence of circulating neutralizing antiadenoviral antibodies in humans who have been pre-exposed to adenoviral infections. These circulating-neutralizing anti-Ad antibodies (Nabs) can hamper HC-Ad vector infection of target cells and thus hamper therapeutic efficacy. In order to assess the concentration of Nabs, we determined the titer of neutralizing antiadenoviral antibodies in the plasma of rats receiving either the water control or the allometrically scaled doses of DOX (Fig. 3B) and injected intracranially with HC-Ads. Our data show no noticeable induction of NAbs in rats receiving intrastriatal delivery of HC-Ads.
The use of DOX as the transcriptional activator of the Tet-On-inducible system ensures an added layer of safety in our gene therapeutic approach by allowing physicians to turn “on” and “off” gene expression upon addition or withdrawal of DOX, respectively. Quantifying the concentration of DOX in the plasma could allude to its efficacy to activate the expression of the therapeutic transgene, that is, Flt3L. To this end, we collected blood from rats 5 hr after receiving their morning administration of DOX, isolated the plasma, and quantified the circulating concentration of DOX. Rats receiving allometrically scaled doses of 100, 200, and 300 mg/day showed DOX concentrations of (mean ± standard error) 462.75 ± 107.83, 585.25 ± 351.91, and 1,024 ± 16.80 ng/ml, respectively (Fig. 3C).
The liver and kidneys are the most commonly implicated sites associated with the metabolism and elimination of DOX and its potential metabolites,40 and are also the most commonly implicated organs in viral sequestration.41,42 We examined the livers and kidneys of control and DOX-treated animals after processing and staining the tissue with H&E for signs of systemic toxicities (Fig. 3D). Livers from all animals revealed healthy hepatocytes and kidneys revealed normal nephrons in all the groups with no indication of inflammation or toxicities.
Systemic toxicities were further analyzed through a complete hematological and serum biochemical analysis (Supplementary Table S1) in rats from all the groups before and after the 4-week treatment with allometrically scaled doses of DOX. All parameters were compared with control water-treated animals. No change in the cellular components of the blood was noted through complete blood counts for animals receiving DOX when compared with the water-treated controls, nor were there any significant changes in important enzymes involved in liver (ALT, ALKP, AST) and kidney (BUN, CRE) physiology.
Efficacy of HC-Ad.TK + HC-Ad.TetOn.mCMV.hsFlt3L in response to allometrically scaled doses of DOX in a transplantable rat glioma model
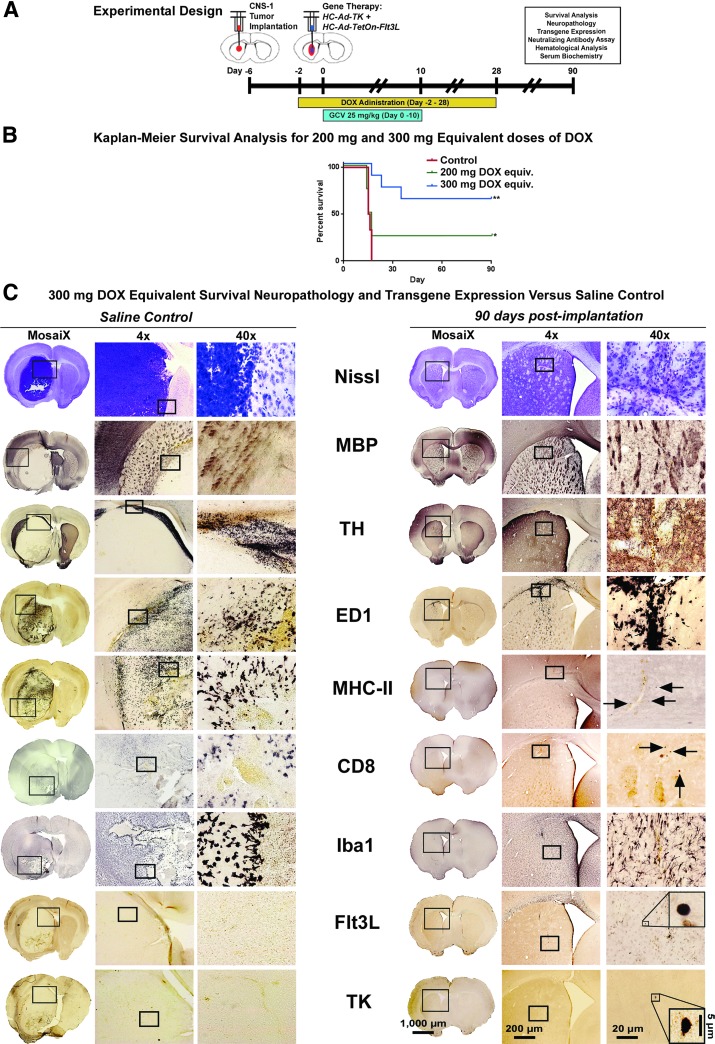
To examine the efficacy associated with the different allometrically scaled doses of DOX, the 200 and 300 mg/day were used as the variable in a set of survival analyses in rats bearing syngeneic CNS-1 tumors, receiving the HC-Ad-mediated TK/Flt3L combined gene therapy in conjunction with 10 days of GCV administration and 4 weeks of twice-daily administration of DOX (Fig. 4A).
Figure 4.
In vivo efficacy of 300 mg/day DOX dose in tumor-bearing rats. (A) Experimental design showing the chronology of a survival analysis experiment, starting with the implantation of 3,000 CNS-1 tumor cells into the right striatum (ipsilateral hemisphere) of male Lewis rats 6 days before gene therapy. DOX was administered twice daily via oral gavage beginning 2 days before the gene therapy using the human equivalent doses of 200 or 300 mg/day in rats and lasting for 1 month. Ganciclovir (GCV) was administered twice daily via intraperitoneal injection (7 mg/kg) beginning on the day of gene therapy and lasting for 10 days. (B) Kaplan–Meier survival analysis of Lewis rats receiving gene therapy and human equivalent doses of DOX. (C) A neuropathological and transgene expression analysis in rats receiving no gene therapy (saline control, left panel), and rats receiving the gene therapy along with the human equivalent of 300 mg/day of DOX for 1 month; 90 days after gene therapy. Architectural scaffolding in the brains of rats, shown by staining Nissl bodies, reveals the complete invasion by tumor cells of the striata of control rats, whereas minor evidence of intervention can be seen in the treated rats. Other structural components showed a similar pattern, with axons and myelin sheaths (MBP), and dopaminergic nerve fibers (TH) completely displaced by the tumor mass, with no change to the treated animals. Immunological components, like CD68+ macrophages (ED1), major-histocompatibility complex-II antigen presenting cells (MHC-II), CD8+ T-cells (CD8), and activated macrophages and microglia (Iba1) are shown throughout the tumor and surrounding parenchyma, whereas only minor remnants of these cell types can be found in the treated animals 90 days after receiving gene therapy. Additionally, evidence of the Flt3L and TK transgenes was detected using DAB immunohistochemical analysis in rats treated with the gene therapy. Scale bars: 1,000 μm for full brain sections, 200 μm for 4× images, 20 μm for 40× images, and 5 μm for 100× insets.
We observed a significant survival advantage when using the allometrically scaled 300 mg/day human equivalent dose of DOX when compared with the 200 mg/day equivalent (p = 0.0304) (Fig. 4B). Over 62% of rats receiving the 300 mg/day equivalent dose of DOX survived the initial tumor threat, compared with only 25% of rats receiving the 200 mg/day equivalent. Previous survival analyses were conducted by our group using DOX chow at a concentration of 2,000 parts per million (ppm) consumed ad libitum (685.2 mg/day) by tumor-bearing rats treated with the combined gene therapy,9,43 which showed a comparable overall survival of rats (∼70%). Our results indicate that there is no survival benefit in escalating the recommended doses of DOX past 300 mg/day, but shows that there is a significant survival advantage for the 300 mg/day dosage over the 200 mg/day dosage.
To study tumor regression after delivery of HC-Ad.TK + HC-Ad.TetOn.mCMV.hsFlt3L, we performed a neuropathological analysis comparing the brains of moribund saline-treated control animals to the brains of rats 90 days after receiving the combined TK/Flt3L therapy (Fig. 4C). Saline-treated controls show ipsilateral hemispheres that are filled with Nissl bodies, along with infiltrating immune cells, such as macrophages (ED1), MHC-II-expressing cells (MHC-II), CD8+ T-cells (CD8), and activated microglia and macrophages (Iba1). The tumor mass completely displaced the white matter tracts (MBP) and dopaminergic nerve terminals (TH) without any evidence of the Flt3L or TK transgene expression (saline was delivered to these animals in place of the HC-Ad vectors). Tumor-bearing rats analyzed 90 days after gene therapy showed little to no evidence of any disturbance near the site of tumor implantation or viral therapy. Nissl stain showed no remnants of either the tumor or the injection needle tract. A slight increase in the density of white matter tracts (MBP) surrounding the injection site indicates continued repair of the earlier mechanical damage and regression of a large tumor mass. Dopaminergic nerve terminals (TH) are slightly displaced in the direct vicinity of the brain tumor implantation. There were still remnants of ED1+ macrophages where the injection needle penetrated the corpus callosum, whereas MHC-II and CD8 cells were scarcely present. Iba1 showed slight activation of microglia and macrophages along needle tract made from the tumor implantation and gene therapy. Finally, the TK and Flt3L transgenes delivered by the HC-Ad vector were detected 90 days after gene therapy in normal parenchyma in the ipsilateral hemisphere (Fig. 4C).
HC-Ad.TK + HC-Ad.TetOn.mCMV.hsFlt3L gene therapy prevents tumor recurrence in a rat glioma model in response to treatment with 300 mg/kg of DOX
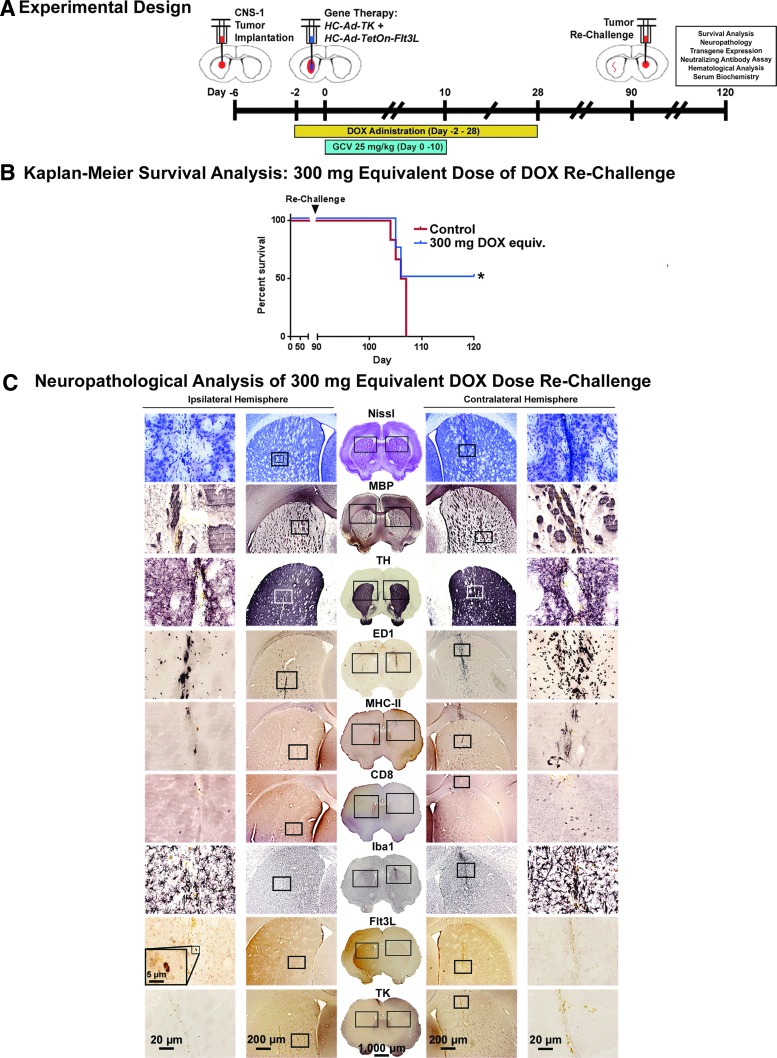
Long-term-surviving rats from the 300 mg/day DOX equivalent group discussed in the previous section were re-challenged 90 days after gene therapy with a second tumor threat implanted into the contralateral hemisphere (Fig. 5A). Fifty percent of recurrent brain tumors were eliminated, indicating the development of a robust immunological memory in response to the immune-stimulatory gene therapeutic approach mediated by DOX-induced Flt3L expression (Fig. 5B).
Figure 5.
Adaptive immunity eliminates recurrent brain tumors in rats with 300 mg/day equivalent of DOX. (A) Experimental design showing the chronology of a survival analysis experiment (see Fig. 4). Long-term survivors from this experiment were re-challenged 90 days after the initial surgery with 3,000 CNS-1 cells implanted into the contralateral striata. (B) Kaplan–Meier survival analysis of rechallenged Lewis rats receiving gene therapy and human equivalent doses of DOX. (C) A complete neuropathological immunohistochemical analysis was performed on survivors of both the initial brain tumor and survivors were then re-challenged, simulating a recurrent GBM. These animals responded to the initial gene therapeutic approach by developing an adaptive immunological response that cleared new tumor threats. Neuropathological analysis was performed on surviving rats to evaluate the integrity of critical brain components following primary (ipsilateral hemisphere) and secondary (contralateral hemisphere). Staining for Nissl bodies showed slight mechanical aberrations from the needle tracts, with more intense localized staining in the more recent re-challenge tumor than the primary tumor implantation and subsequent gene therapy. MBP shows an increased density of axonal bundles surrounding the injection sites in both hemispheres, whereas TH staining showed slight displacement of dopaminergic nerve fibers in the vicinity of the injection sites. Immunological components (CD68+ macrophages [ED1], major-histocompatibility complex-II antigen presenting cells [MHC-II], CD8+ T-cells [CD8], and activated macrophages and microglia [Iba1]) all revealed an increase in staining around the injection site for the secondary tumor with lesser staining surrounding the earlier tumor challenge. Finally, the Flt3L transgene was detected in the hemisphere receiving the gene therapy several months after treatment. Scale bars: 1,000 μm for full brain sections, 200 μm for midmagnification images, 20 μm for the highest magnification images, and 5 μm for 100× insets.
Figure 5C shows the neuropathological analysis of rats that cleared a primary brain tumor threat (Ipsilateral Hemisphere) through the gene therapeutic approach with the allometrically scaled 300 mg/day dose of DOX, and subsequently eliminated a “recurrent” brain tumor challenge (contralateral hemisphere) without any additional treatment. Nissl shows largely unaffected gross tissue morphology with areas of increased cell density near needle tract from delivery of the re-challenge tumor into the contralateral hemisphere 1 month after injection. The ipsilateral hemisphere shows no evidence of heightened cell density 4 months after gene therapy. Similarly, DAB immunohistochemical analysis on free-floating sections shows increased MBP staining of axonal bundles surrounding the site of tumor re-challenge, indicative of oligodendrocyte activation. Meanwhile, the ipsilateral hemisphere shows little indication of alterations in the white matter surrounding the injection sites. These sites are identified only through the presence of hemosiderin staining, a result of macrophage phagocytosis of the iron-containing heme cofactor of red blood cells caused by mechanical damage of the needle. TH staining shows highly localized displacement of dopaminergic nerve endings along the needle tract in both the contralateral and ipsilateral hemispheres.
The immunological components of both striata show similar patterns, with ED1, MHC-II, CD8, and Iba1 immunohistochemical analyses showing increased levels of infiltrating immune cells at the site of the most recent tumor implantation (contralateral hemisphere), and lower levels of these markers in the ipsilateral hemisphere. Particularly noteworthy is the relatively large population of CD8+ T-cells in the contralateral hemisphere of animals that recently eliminated “recurrent” brain tumors.
Finally, the Flt3L transgene was detected in the ipsilateral hemisphere of rats cured of primary and secondary brain tumors using the combined gene therapeutic approach, showing the enhanced stability of transgenes via the HC-Ad platform for long periods of time.9,44 The TK transgene was not detected in the section analyzed. This transgene does not persist in surviving animals, as a large number of cells transduced with TK are tumor cells eliminated in the process of GCV-induced cell death.
A complete hematological and serum biochemical analysis was run on animals surviving the secondary tumor and compared with blood drawn from a long-term surviving animal harvested 90 days post-injection (dpi) (Supplementary Table S2). These data show no significant change in any of the cellular components of the blood, or any of the proteins associated with normal liver and kidney health.
Discussion
Our group has pioneered the development of regulatable HC-Ads for GBM immune-mediated gene therapy.9,43,44 We previously constructed and characterized a novel HC-Ad vector platform using a modified tetracycline-dependent inducible system (TetOn) that enables efficient and tightly regulated transgene expression in vitro and also in the central nervous system in vivo.10,11,31,45 The use of DOX as the transcriptional activator of the Tet-On-inducible system ensures an added layer of safety in the gene therapeutic approach proposed by allowing therapeutic transgene expression, that is, Flt3L to be turned “on” or “off” in the presence or absence of DOX, respectively, and in line with medical need.
We also previously demonstrated the therapeutic efficacy and high safety profile of the combined, conditional cytotoxic/immune stimulatory gene therapy strategy using these regulatable HC-Ads (HC-Ad-TK + HC-Ad-TetOn-Flt3L) in syngeneic, orthotopic rat models of GBM.9 Additionally, as a requirement before initiating a phase 1 clinical trial for GBM, we performed a dose-escalation and biodistribution study of HC-Ad-TK + HC-Ad-TetOn-Flt3L to assess the maximum tolerated dose (MTD) of these viral vectors in the brain in vivo.44 We found that dose to be 1 × 109 viral particles (vp). Using real-time quantitative PCR we did not observe evidence of biodistribution of vector genomes to peripheral organs, behavioral testing revealed no abnormalities, and serum biochemistry was also normal.9,44
As a prelude to the phase I clinical trial using this vector platform, we needed to examine the amount of DOX required to induce transgene activation from the TetOn transactivation system. In previous preclinical studies testing the therapeutic efficacy of HC-Ad-TK + HC-Ad-TetOn-Flt3L, rat chow containing DOX at a concentration of 2,000 ppm was provided ad libitum to animals for a period of 1 month, which is equivalent to 685.2 mg/day.9,44 Although our results showed an increase in survival rate for animals treated with the combined gene therapy versus animals injected with saline, converting this DOX concentration to human equivalents yielded doses (685.2 mg/day) that had never been tested in humans and thus their safety could come into question. To address this, we elected to use the three most common doses of DOX approved by the FDA for use in humans, 100, 200, and 300 mg/day, assuming a body weight of 70 kg, and assessed their efficacy at inducing tumor regression and immunological memory in a syngeneic GBM model in rodents. The use of DOX to induce transgene expression via a TetOn transactivation system is not currently approved for use in humans; this study proposes an “off-label” application for this drug.
Our data suggest that FDA-approved doses of DOX can successfully induce expression of the Flt3L transgene without eliciting systemic toxicities (Fig. 3). Monitoring of post-surgery body weight shows a steady increase in weight over time for both DOX-receiving animals and controls. A histopathological analysis of the liver and kidneys of rats receiving HC-Ad-TK + HC-Ad-TetOn-Flt3L reveals no gross morphological changes; furthermore, staining of the tissues with H&E shows normal nephrons and hepatocytes in all three tested DOX doses when compared with control animals. Neutralizing antibodies have not been detected in the circulation of GBM-bearing animals treated with HC-Ad-TetOn-Flt3L + HC-Ad-TK delivered into the brain tumor mass. Although opsonizing factors could direct the HC-Ad vector to phagocytes,46 diminishing therapeutic transgene expression, previous work from our group has not encountered this possibility after delivery of the vectors into either the normal brain parenchyma, or into a tumor mass growing within the brain, even in the presence of a systemic immunization against the adenovirus vectors.5,11,30–32 In addition, hematological and biochemical parameters were all normal in both DOX-treated animals and controls (Supplementary Table S1).
Having determined that allometrically scaled doses of DOX approved by the FDA for use in human patients were able to turn on therapeutic transgene expression from HC-Ad-TetOn-Flt3L, we set out to examine their therapeutic efficacy in a tumor-bearing animal model of GBM in preparation for a phase I clinical trial. Using the 200 and 300 mg/day human equivalent doses of DOX, we analyzed the survival rates for tumor-bearing animals. The data indicate a significant survival advantage of the 300 mg/day DOX dose over the 200 mg/day dose (Fig. 4B). The survival rate of animals in the 300 mg/day cohort (∼62%) was comparable to the survival rate (∼70%) of animals in previous preclinical experiments that received doses of DOX higher than those approved by the FDA.9,44 Furthermore, we observed no neuropathological adverse side effects as a result of HC-Ad transduction or transgene activation. These results indicate that these allometrically equivalent doses of DOX are effective to evoke tumor regression and that substantially higher DOX doses do not confer a meaningful survival advantage over the FDA-approved doses.
Human GBM is characterized by a high rate of tumor recurrence, despite an aggressive current standard of care, including gross tumor resection whenever possible, ionizing radiation treatments, and adjuvant chemotherapy. For this reason, it is critical to develop a therapy that not only overcomes the initial tumor burden, but also provides a lasting efficacy against tumor recurrence. Thus, we next demonstrated that the allometrically scaled doses of DOX elicited expression of sufficient levels of Flt3L to enable mounting effective anti-GBM immunological memory. Long-term surviving animals were re-challenged with tumor cells in the contralateral hemisphere 90 days after initial administration of the combined gene therapy (Fig. 5A) and did not receive any further treatment. By the end of the 120-day trial, half of the re-challenged animals rejected the second tumor, indicating the stimulation of immunological memory (Fig. 5B). These data provide strong support for implementing phase I clinical trial for GBM using HC-Ad-TK + HC-Ad-TetOn-Flt3L as an effective and safe therapeutic gene delivery platform, which has the added safety feature of DOX-mediated regulated transgene expression. This work has significant translational relevance for our own ongoing clinical trial as well as for other researchers interested in implementing the Tet-On transcriptional activation-dependent expression of therapeutic transgenes within the brain.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants U01-NS052465, U01-NS052465-S1, R01-NS074387, and R01-NS057711 to M.G.C.; NIH/NINDS Grants R01-NS054193, R01-NS061107, and R01-NS082311 to P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH UL1-TR000433 and MICHR U040007; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863. Pharmacokinetic analysis was supported in part by an NIH grant made to the University of Michigan Comprehensive Cancer Center, P30 CA046952 NIH. We gratefully acknowledge Mr. Philip Jenkins and the Department of Neurosurgery at the University of Michigan Medical School for their support of our work. We are also grateful to Dr. Karin Muraszko for her academic leadership, and D. Tomford, S. Napolitan, and M. Dahlgren for superb administrative support.
Author Disclosure
No competing financial interests exist.
References
- 1.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 2010;16:2443–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro MG, Candolfi M, Wilson TJ, et al. Adenoviral vector-mediated gene therapy for gliomas: Coming of age. Expert Opin Biol Ther 2014;14:1241–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonato M, Bennet J, Boulis NM, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol 2013;9:277–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroeger KM, Muhammad AK, Baker GJ, et al. Gene therapy and virotherapy: Novel therapeutic approaches for brain tumors. Discov Med 2010;10:293–304 [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenstein PR, Mandel RJ, Xiong WD, et al. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: The role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther 2007;7:347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowenstein PR, Kroeger KM, Castro MG. Immunology of neurological gene therapy: How T cells modulate viral vector-mediated therapeutic transgene expression through immunological synapses. Neurotherapeutics 2007;4:715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goverdhana S, Puntel M, Xiong W, et al. Regulatable gene expression systems for gene therapy applications: Progress and future challenges. Mol Ther 2005;12:189–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanderVeen N, Paran C, Appelhans A, et al. Marmosets as a preclinical model for testing “off-label” use of doxycycline to turn on Flt3L expression from high-capacity adenovirus vectors. Mol Ther Methods Clin Dev 2014. Abstract [DOI] [PMC free article] [PubMed]
- 9.Muhammad AK, Puntel M, Candolfi M, et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther 2010;88:204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtin JF, Candolfi M, Puntel M, et al. Regulated expression of adenoviral vectors-based gene therapies: Therapeutic expression of toxins and immune-modulators. Methods Mol Biol 2008;434:239–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong W, Goverdhana S, Sciascia SA, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol 2006;80:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JC, Stone D, Smith-Arica JR, et al. Regulated, adenovirus-mediated delivery of tyrosine hydroxylase suppresses growth of estrogen-induced pituitary prolactinomas. Mol Ther 2001;4:593–602 [DOI] [PubMed] [Google Scholar]
- 13.Ali S, King GD, Curtin JF. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res 2005;65:7194–7204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mineharu Y, Kamran N, Lowenstein PR, et al. Blockade of mTOR signaling via rapamycin combined with immunotherapy augments antiglioma cytotoxic and memory T-cell functions. Mol Cancer Ther 2014;13:3024–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candolfi M, Yagiz K, Wibowo M, et al. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res 2014;20:1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mineharu Y, Muhammad AK, Yagiz K, et al. Gene therapy-mediated reprogramming tumor infiltrating T cells using IL-2 and inhibiting NF-kappaB signaling improves the efficacy of immunotherapy in a brain cancer model. Neurotherapeutics 2012;9:827–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candolfi M, Curtin JF, Yagiz K, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia 2011;13:947–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mineharu Y, King GD, Muhammad AK, et al. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: Implications for clinical trial design. Clin Cancer Res 2011;17:4705–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King GD, Muhammad AK, Larocque D, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther 2011;19:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: Humoral and cellular immunity lead to tumor regression. Clin Cancer Res 2009;15:6113–6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Candolfi M, Yagiz K, Foulad D, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: Efficacy and neurotoxicity. Clin Cancer Res 2009;15:4401–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtin JF, Candolfi M, Xiong W, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 2009;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King GD, Kroeger KM, Bresee CJ, et al. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther 2008;16:682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King GD, Muhammad AK, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol 2008;10:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DRUGDEX®, Thompson Reuters
- 26.VanderVeen N, Paran C, Krasinkiewicz J, et al. Effectiveness and preclinical safety profile of doxycycline to be used “off-label” to induce therapeutic transgene expression in a phase I clinical trial for glioma. Hum Gene Ther Clin Dev 2013;24:116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Southgate T, Kroeger KM, Liu C, et al. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci 2008:4231–4.23.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer DJ, Ng P. Methods for the production of helper-dependent adenoviral vectors. Methods Mol Biol 2008;433:33–53 [DOI] [PubMed] [Google Scholar]
- 29.Palmer DJ, Ng P. Rescue, amplification, and large-scale production of helper-dependent adenoviral vectors. Cold Sping Harb Protoc 2011;2011:857–866 [DOI] [PubMed] [Google Scholar]
- 30.King GD, Muhammad AK, Xiong W, et al. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J Virol 2008;82:4680–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong W, Candolfi M, Kroeger KM, et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther 2008;16:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barcia C, Jimenez-Dalmaroni M, Kroeger KM, et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: Clinical implications. Mol Ther 2007;15:2154–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puntel M, Curtin JF, Zirger JM, et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum Gene Ther 2006;17:531–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voisin M, Ruthsatz M, Collins JM, et al. Extrapolation of animal toxicity to humans: Interspecies comparisons in drug development. Regul Toxicol Pharmacol 1990;12:107–116 [DOI] [PubMed] [Google Scholar]
- 35.Puntel M, Kroeger KM, Sanderson NS, et al. Gene transfer into rat brain using adenoviral vectors. Curr Protoc Neurosci 2010;Chapter 4: Unit 4 24 [DOI] [PMC free article] [PubMed]
- 36.King GD, Muhammad AK, Xiong W, et al. High-Capacity adenoviral vector-mediated anti-glioma gene therapy in the presence of systemic anti-adenovirus immunity. J Virol 2008;82:4680–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candolfi M, Curtin JF, Xiong W, et al. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cell. Mol Ther 2006;14:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtin JF, King GD, Barcia C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol 2006;176:3566–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: Neuropathological characterization and tumor progression. J Neurooncol 2007;85:133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bocker R. Analysis and quantitation of a metabolite of doxycycline in mice, rats, and humans by high-performance liquid chromatography. J Chromatogr 1983;274:255–262 [DOI] [PubMed] [Google Scholar]
- 41.Shayakhmetov DM, Gaggar A, Ni S, et al. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 2005;79:7478–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Short JJ, Rivera AA, Wu H, et al. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol Cancer Ther 2010;9:2536–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puntel M, Muhammad AK, Candolfi M, et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol 2010;84:6007–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhammad AK, Xiong W, Puntel M, et al. Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: Implications for a glioma phase 1 clinical trial. Hum Gene Ther Methods 2012;23:271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curtin JF, Candolfi M, Xiong W, et al. Turning the gene tap off; implications of regulating gene expression for cancer therapeutics. Mol Cancer Ther 2008;7:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevenson M, Boos E, Herbert C, et al. Chick embryo lethal orphan virus can be polymer-coated and retargeted to infect mammalian cells. Gene Ther 2006;13:356–368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.