Graphical abstract
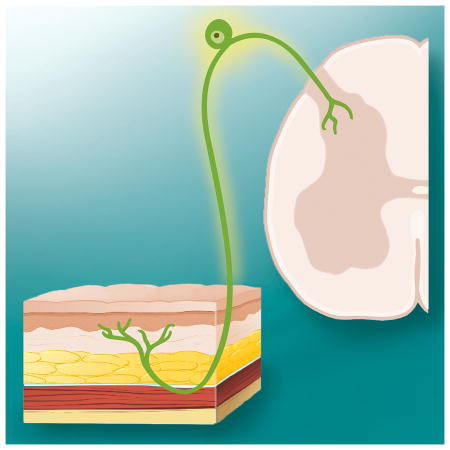
Sensory dysfunction and pain are among the most common treatment-limiting factors and most distressing physical symptoms of patients receiving any of the most commonly used cancer chemotherapy treatments. Symptoms range from mild tingling to a painful burning paresthesia that is refractory to effective remedy. The incidence and severity of chemotherapy induced pain is correlated with duration and dose such that nearly all patients experience discomfort by a third treatment cycle with vincristine, paclitaxel, bortezomib, cisplatin or oxaliplatin; and forces discontinuation of optimal therapy in up to half of patients. Treatment-related pain is therefore not only distressing but impacts survival. Symptoms also often persist long after treatment and so impacts rehabilitation and the return to productivity. By far the majority of studies into the underlying mechanisms of chemotherapy induced pain and dysesthesia have focused on those occurring in primary afferent nerves. Effects on DRG neurons and distal nerve endings in particular have been the focus of many studies given that these are the neural tissues seemingly most directly affected by chemotherapy drugs given their location outside the blood brain barrier and the poor penetrance of the majority of chemotherapy drugs to the central nervous system (CNS)1. Indeed, this bias is reflected in the commonly accepted name for this condition, chemotherapy-induced peripheral neuropathy (CIPN). Yet, the work by Huang et al in this issue reveals that CIPN may in fact involve important effects mediated by chemotherapeutics directly in the CNS on spinal cord neurons.
Huang et al show that a single systemic (i.p.) dose of 4.0mg/kg of oxaliplatin induces signs of acute pain within one hour after dosing in rats; and that this was accompanied by increased spinal cord responses evoked by sciatic nerve stimulation. They then used a very sensitive detection method, inductively coupled mass spectrometry, to reveal that albeit small, nevertheless significant amounts of oxaliplatin penetrate into spinal cerebrospinal fluid following the systemic dose used in the behavioral studies. Direct application of this small dose of oxaliplatin onto the spinal cord by intrathecal injection re-produced both the behavioral signs of hyperalgesia and the enhanced evoked responses of the spinal cord to sciatic nerve stimulation. Neither effect was re-produced by injection of oxaliplatin directly into the skin of the hindpaw at a concentration corresponding to the levels of oxaliplatin detected in this tissue using mass spec after systemic dosing. Finally, the investigators provide data suggesting that oxaliplatin acts within the CNS to produce its effects by the induction and release of the chemokine CX3CL1.
The findings by Huang et al suggesting CNS effects are involved in producing pain evoked by chemotherapeutics are not without precedent as a recent paper by Li et al reported complementary findings on the chemotherapeutic paclitaxel2. Paclitaxel, like oxaliplatin, though previously thought to not enter the CNS, does indeed penetrate to the CNS, albeit at very low concentrations, following systemic dosing that produces hyperalgesia in rats. Li et al tested the direct effects of paclitaxel at this very low concentration on the physiological responses of spinal cord neurons in vitro. They found that paclitaxel acts on Toll-like receptor 4 (TLR4) on spinal neurons to increase the signaling of the transient receptor potential vallinoid 1 channel (TRPV1); and intrathecal TPRV1 antagonists reduce paclitaxel-induced hyperalgesia in rats. Combined these studies show that CNS effects of very low concentrations of chemotherapeutics can no longer be excluded as having potentially important roles in generating CIPN.
Though the work by Huang et al may underscore previously little studied mechanisms in CIPN, it should remain mindful that as noted above there are numerous studies showing effects of chemotherapy drugs on dorsal root ganglion (DRG) neurons and distal nerve endings; and the potential efficacy of altering signaling in these tissues in preventing or reversing CIPN is well documented. Indeed, based on the data shown in this issue it cannot be excluded that the intrathecal dosing scheme used by Huang et al did in fact affect DRG neurons or their central terminals. It should also be noted that whereas an acute pain syndrome characterized by arthralgia and myalgia is reported for paclitaxel, acute mechanically-evoked pain is not a commonly reported symptom following oxaliplatin treatment in humans. Rather, acute sensitivity to skin cooling is the most common sensory side effect for oxaliplatin. Thus, the work by Huang et al may reveal potentially important new mechanisms of CIPN, yet the full context of this work will require further investigation.
Acknowledgments
This work was supported by NIH grant NS046606 and the H.E.B. Professorship in Cancer Research.
Footnotes
Commentary on, Cerebrospinal fluid oxaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin, Zhen-Zhen Huang, Ph.D., Dai Li, Ph.D., Han-Dong Ou-Yang, Ph.D., Cui-Cui Liu, Ph.D., Xian-Guo Liu, Ph.D., Chao Ma, Ph.D., Jia-You Wei, Ph.D., Yong Liu, Ph.D., Wen-Jun Xin, Ph.D.
The author is not supported by, nor maintain any financial interest in, any commercial activity that may be associated with the topic of this article.
Reference List
- 1.Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015;5:285–96. doi: 10.2217/pmt.15.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35:13487–500. doi: 10.1523/JNEUROSCI.1956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


