Abstract
Group B Streptococcus (GBS) is a leading cause of bacterial sepsis and meningitis in newborns. GBS possesses a protein with homology to the pneumococcal virulence factor, NanA, which has neuraminidase (sialidase) activity and promotes blood-brain barrier penetration. However, phylogenetic sequence and enzymatic analyses indicate the GBS NanA ortholog has lost sialidase function – and for this distinction we designate the gene and encoded protein nonA/NonA. Here we analyze NonA function in GBS pathogenesis, and through heterologous expression of active pneumococcal NanA in GBS, potential costs of maintaining sialidase function. GBS wild-type and ΔnonA strains lack sialidase activity, but forced expression of pneumococcal NanA in GBS induced degradation of the terminal sialic acid on its exopolysaccharide capsule. Deletion of nonA did not change GBS-whole blood survival or brain microvascular cell invasion. However, forced expression of pneumococcal NanA in GBS removed terminal sialic acid residues from the bacterial capsule, restricting bacterial proliferation in human blood and in vivo upon mouse infection. GBS expressing pneumococcal NanA had increased invasion of human brain microvascular endothelial cells. Thus, we hypothesize that nonA lost enzyme activity allowing the preservation of an effective survival factor, the sialylated exopolysaccharide capsule.
Streptococcus agalactiae (Group B Streptococcus, GBS) is a Gram-positive bacterial pathogen that is a leading cause of sepsis, pneumonia, and meningitis during neonatal period and up to the first 90 days of life1,2. Each of the 10 different GBS capsular polysaccharide types1, though possessing different repeating subunits, share a terminal α-2-3-linked sialic acid (N-acetylneuraminic acid, Neu5Ac motif), which is identical to a sugar epitope capping many surface glycans on all mammalian cells3. Humans in particular express just the terminal α-2-3-linked Neu5Ac since they have lost the gene required to synthesize the alternative sialic acid, N-glycolylneuraminic acid (Neu5Gc) present in other mammals including primates3. The GBS sialylated capsule mimics a common presentation of Neu5Ac in the α-2-3-linkage, which contributes to evasion of the host immune system and promoting bacterial survival in vivo4. GBS capsular sialylation interferes with the host complement system to block C3b deposition and limit C5a deposition5,6, and inhibits neutrophil activation through interaction with inhibitory sialic acid-binding immunoglobulin-like lectin-9 (Siglec-9)7. The in vivo significance of these findings was corroborated in mice with and without Siglec-E, the closest homolog of human Siglec-9, which interacts with GBS in a sialic acid-dependent manner, triggering protein tyrosine phosphatase, SHP-1, recruitment to its intracellular domain and suppressing myeloid cell inflammatory responses8.
Streptococcus pneumoniae (pneumococcus) is a related Gram-positive pathogen and a major cause of pneumonia, sepsis, and meningitis9,10. Most severe S. pneumoniae diseases occur in children younger than 2 years and adults older than 65 years. The polysaccharide capsule of S. pneumoniae confers the antigenicity utilized to classify S. pneumoniae into at least 97 serotypes11. In contrast to GBS, no S. pneumoniae strains express sialic acid in its capsular polysaccharide. Instead, the bacterium expresses three sialic acid-cleaving enzymes or sialidases, NanA, NanB, and NanC11,12. The nanA and nanB genes are located in the same operon and detected in almost all clinical isolates, whereas the nanC gene is present in approximately half (51%) of isolates12. While the molecular functions of NanB and NanC in the pathogenesis are unclear, NanA has been identified as a multifunctional protein contributing to pneumococcal virulence13,14. NanA is a cell-wall-anchored protein and works as an invasin into human brain microvascular endothelial cells (hBMEC) through its LamG superfamily domain14,15,16. An isogenic S. pneumoniae ΔnanA mutant strain showed >90% reduction in adhesion and invasion efficiency compared to its parent strain; complementation of NanA expression on a plasmid vector restored the adherence/invasion phenotype. Furthermore, heterologous expression of NanA in Lactococcus lactis conferred an adhesion and invasion frequency ~10-fold greater than empty-vector-transformed control14. The NanA LamG domain induces inflammatory cytokine production from the brain endothelial cells, and the resulting cell activation promotes pneumococcal internalization16. In addition, desialylation of leukocyte cell surfaces by NanA resulted in MAP kinase phosphorylation and NF-κB activation through unmasking of Siglec-517.
Here, we identify through homology searching a putative ortholog of pneumococcal NanA that is present in GBS strains. The biological consequences for GBS of possessing a potential sialidase enzyme, while simultaneously expressing a sialylated capsule as an essential virulence determinant, were initially unclear. Our bioinformatics analysis suggested that, unlike pneumococcal NanA, the GBS orthologue has lost the LamG domain and cell wall-anchoring motif, and that there was a nonsense mutation in this gene in some GBS strains. Codon-based selection analysis indicated that pneumococcal nanA was under stronger negative selection than nonA. We find that the GBS strains do not possess neuraminidase activity, and for this distinction we designate the gene and encoded protein nonA/NonA. In contrast to earlier published findings with pneumococcal NanA mutants13,14,16, targeted deletion of the nonA gene in GBS did not alter resistance to human whole blood killing, brain microvascular endothelial cell invasion, or animal virulence. However, forced expression of active pneumococcal NanA in the GBS ΔnonA mutant removed terminal sialic acid from the GBS polysaccharide capsule, reducing GBS survival in whole blood, while promoting GBS invasion of brain microvascular endothelial cells. Taken together, our results strongly suggest that the loss of function as a sialidase in GBS NonA in contemporary GBS strains allowed the organism to preserve the selective advantage of sialylated capsule.
Results
Evolutionary analysis of a GBS nanA ortholog
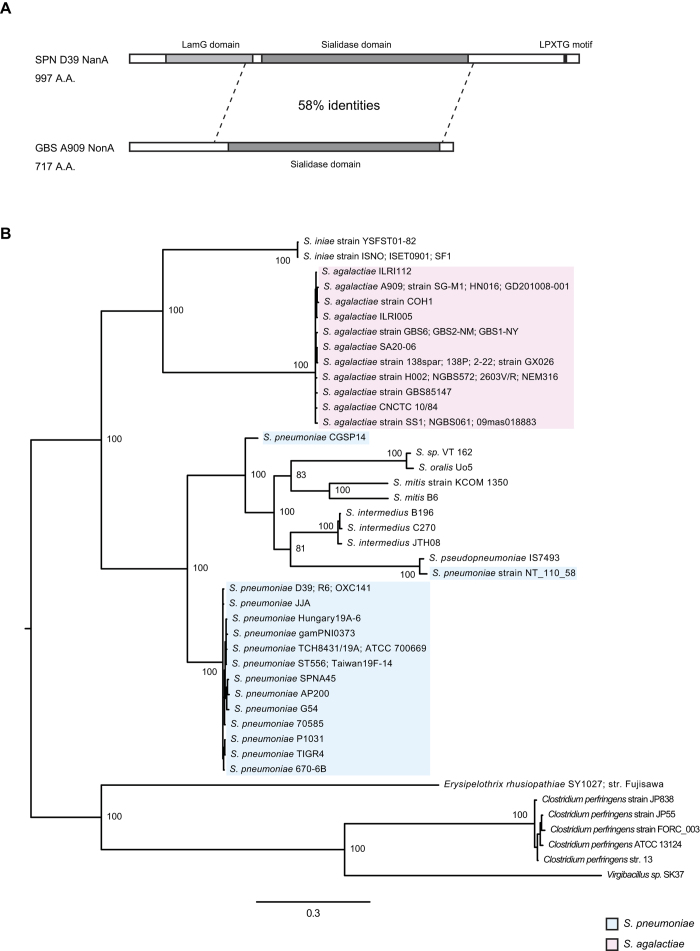
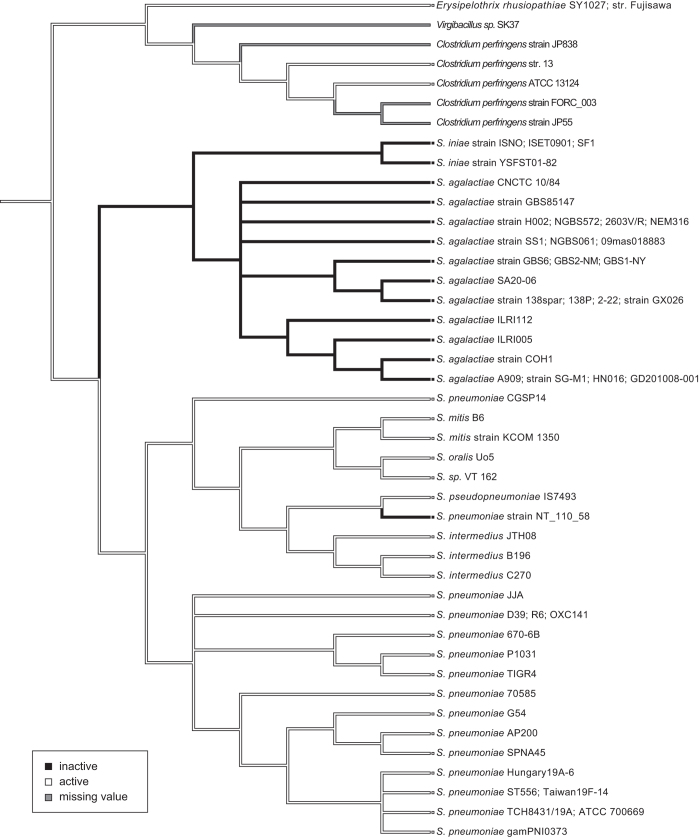
We performed a bioinformatics analysis on the nonA gene, an ortholog of pneumococcal nanA (SAK_RS09520 or SAK_1891), annotated in the published genome of GBS strain A909. Amino acid sequence alignment analysis showed that SAK_RS09520 contains a sialidase domain but lacks the conserved lectin like-domain LamG and cell wall anchoring motifs present in pneumococcal NanA (Fig. 1A). GBS NonA shared 58% amino acid sequence identity with pneumococcal NanA across the sialidase domain, with lesser degrees of sequence identity with pneumococcal NanB and NanC (27–28% amino acid sequence identities) (Supplementary Table 1). Next, tBLASTn analysis revealed that a subset of species in the genus Streptococcus contains nanA orthologs and a phylogenetic analysis was performed using orthologous bacterial nanA sequences. Both Bayesian- and maximum likelihood phylogenetic analyses of these orthologs revealed similar patterns of genetic classification with high posterior probabilities or bootstrap values (Fig. 1B, Supplementary Fig. 1 and Supplementary Table 2). The sialidase genes of Gram-positive and rod-shaped bacteria, Erysipelothrix rhusiopathiae, Clostridium perfringens, and Virgibacillus sp., were used to root, since with the exception of homologous genes from other streptococci, these genes exhibited the highest similarity with pneumococcal nanA. E. rhusiopathiae and C. perfringens are known to produce active sialidases18,19,20. Both trees indicate that the nanA ortholog genes of S. mitis and S. pseudopneumoniae diverged from each other, having shared a common ancestor. Of note, nanA genes of S. pneumoniae strains CGSP14 and NT_110_58 were distinct from those of other pneumococcal strains. The phylogenetic analysis revealed that the GBS nonA represents a single lineage in a cluster otherwise composed of S. iniae, which is a pathogen of fish and occasional nosocomial infections in humans21. NonA of both S. iniae and GBS lack an LPXTG motif, which is conserved in the NanA proteins of other streptococcus species. All streptococcal sialidases except GBS NonA possess the LamG domain (Fig. 1A and Supplementary Fig. 2). Further analysis of the genome database indicates that five GBS strains (GX026, SA20-06, 2-22, 138spar, and 138P) carry a nonA gene containing a nonsense mutation (Supplementary Table 3). In addition, we measured bacterial sialidase activities using streptococcal type strains and clinical isolates (Supplementary Fig. 3). Type strains of S. oralis, S. intermedius, and S. pseudopneumoniae showed positive sialidase activities. In contrast, the sialidase activity of S. mitis, GBS, and S. iniae strains was always below the detection limit. Previously, Killian et al. reported that 100% of 17 S. pneumoniae and 3 S. pseudopneumoniae strains and 69% of 54 S. mitis strains showed positive sialidase activity22. Some S. mitis strains appear to have reduced the genome sizes and may have lost virulence-associated factors including NanA in a reductive evolutionary process23,24. Thus, it is likely that S. mitis strains exhibit a diversity of sialidase activity. Furthermore, the result of an ancestral reconstruction technique suggests the possibility that sialidase activity was lost in the nonA lineage rather than gained in the nanA lineage (Fig. 2). Together these results suggest that streptococcal nanA orthologs diverged into two major groups, one consisting of S. mitis, S. intermedius and S. pneumoniae, and the other consisting of GBS and the S. iniae group. In the S. iniae/GBS group NonA appears to have lost its functional role.
Figure 1. Phylogenetic analysis of nanA orthologs.
(A) Schematic illustration of domains in S. pneumoniae NanA and GBS NonA. NonA lacks LamG domain and LPXTG motif conserved in NanA. (B) Bayesian phylogenetic tree of the nanA and nonA genes. The information on bacterial strains is listed in Supplementary Table 2. Strains with identical sequences are listed on the same branch. Percentage of posterior probabilities is shown near the nodes. The scale bar indicates nucleotide substitutions per site. S. pneumoniae nanA and GBS nonA genes are shaded in blue and red, respectively.
Figure 2. Ancestral state reconstructions based on the Bayesian phylogenetic tree.
Parsimony reconstruction using Mesquite for active or inactive sialidase phenotypes is shown as white or black lines, respectively. Gray lines indicate missing values.
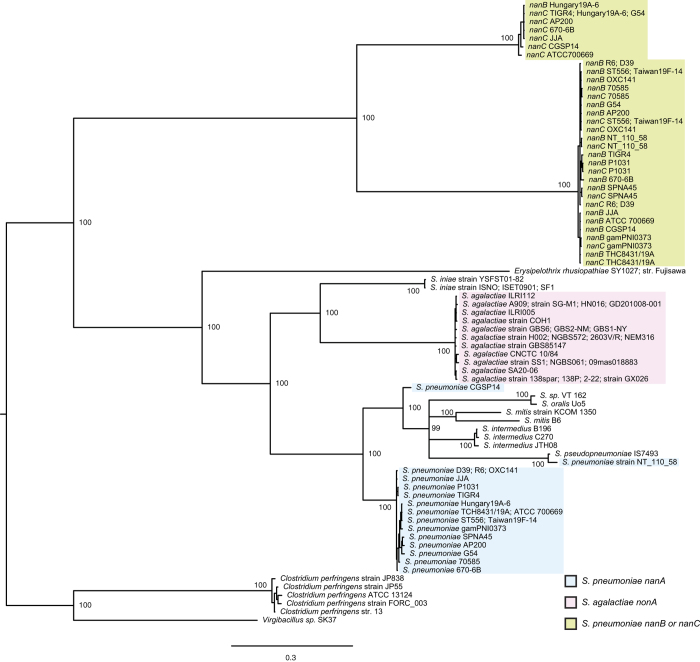
To examine the relationship of pneumococcal nanA, nanB, nanC, and GBS nonA, a phylogenetic analysis was performed using the genes. Bayesian- and maximum likelihood phylogenetic analyses of the genes revealed similar patterns of genetic classification with high posterior probabilities or bootstrap values (Fig. 3 and Supplementary Fig. 4). The pneumococcal nanB/nanC were well separated from pneumococcal nanA or GBS nonA. We performed an additional evolutionary analysis on nanA, nonA, nanB, nanC, bgaA, and strH genes. BgaA and StrH, another pneumococcal exoglycosidases, remove galactose that is β1-4 linked to N-acetylglucosamine, and N-acetylglucosamine that is β1 linked to mannose, respectively13. Selection analysis through non-synonymous/synonymous ratio calculations by Fixed Effects Likelihood (FEL) and Fast, Unconstrained Bayesian AppRoximation (FUBAR) analyses suggested similar results. There were more codons evolving under negative selection in the nanA genes of S. pneumoniae strains (Table 1, Supplementary Table 4 and Supplementary Figs 5–10). In contrast, fewer codons evolving under negative selection were detected in the nonA genes of GBS as well as the nanB and nanC genes. Similar results were obtained with the bgaA and strH genes, indicating that nanA is under strong selective pressure. On the other hand, there were no or very few codons that appear to be evolving under positive selection in these genes. We conducted a likelihood ratio test to investigate whether pneumococcal nanA and GBS nonA genes have the same distribution of substitution rates across sites (Table 2). The distributions of substitution rates indicate no significant differences in between pneumococcal nanA and GBS nonA genes. However, there was a significant difference in selective regimes (dN/dS and proportions), especially in the proportions of codons under selection. These results suggest that a functional change of NanA would be deleterious in S. pneumoniae. In fact, pneumococcal NanA is a multifunctional protein, that promotes bloodstream survival17 and penetration of host endothelial cell barriers system14. In contrast to pneumococcal NanA, the GBS NonA does not appear to be under strong selective pressure, which supports our hypothesis that NonA no longer functions in GBS.
Figure 3. Bayesian phylogenetic tree of nanA, nanB, nanC, and GBS nonA genes.
Percentage of posterior probabilities is shown near the nodes. Strains with identical sequences are listed on the same branch. The scale bar indicates nucleotide substitutions per site. Blue shows pneumococcal nanA and green is nanB or nanC. GBS nonA is shown as red.
Table 1. Evolutionary analyses of nanA, nonA, nanB, nanC, bgaA, and strH genes.
| Gene | Species | Number of Strains | dN/dS | Codons evolving under positive selection | Codons evolving under negative selection |
|---|---|---|---|---|---|
| nanA | S. pneumoniae | 16 | 0.231 | 0.268% (2/745) | 14.362% (107/745) |
| nonA | S. agalactiae | 16 | 0.315 | 0% (0/452) | 1.991% (9/452) |
| nanB | S. pneumoniae | 16 | 0.309 | 0% (0/454) | 0.881% (4/454) |
| nanC | S. pneumoniae | 6 | 0.192 | 0% (0/740) | 2.703% (20/740) |
| bgaA | S. pneumoniae | 14 | 0.194 | 0.313% (7/2233) | 5.867% (131/2233) |
| strH | S. pneumoniae | 17 | 0.595 | 0.152% (2/1319) | 0.455% (6/1319) |
Evolutionary analysis was performed using Baysian inference of aligned nanA, nonA, nanB, nanC, bgaA, or strH sequences from complete genomes of S. pneumoniae or S. agalactiae, with two rate FEL in the HyPhy software package. The dN/dS means ratio of non-synonymous changes to synonymous changes in overall analyzed genes. Individual codons with a statistically significant signature were also calculated and are expressed as a percentage of the total number of codons used in the analysis.
Table 2. Comparing codon selection between nanA and nonA genes.
| Tests | LR | DF | P-value |
|---|---|---|---|
| The distributions | 14.855 | 10 | 0.137 |
| Selective regimes (dN/dS and proportions) | 9.602 | 2 | 0.008 |
| Selection strength (dN/dS) | −0.020 | 1 | 1.000 |
| The proportions of codons under selection | 8.120 | 1 | 0.004 |
Comparing codon selection was performed using Baysian inference of aligned nanA or nonA sequences, and distribution comparison tests in the HyPhy software package. LR; Likelihood ratio. DF; degrees of freedom.
Forced expression of NanA in GBS degrades terminal sialic acids of its capsule
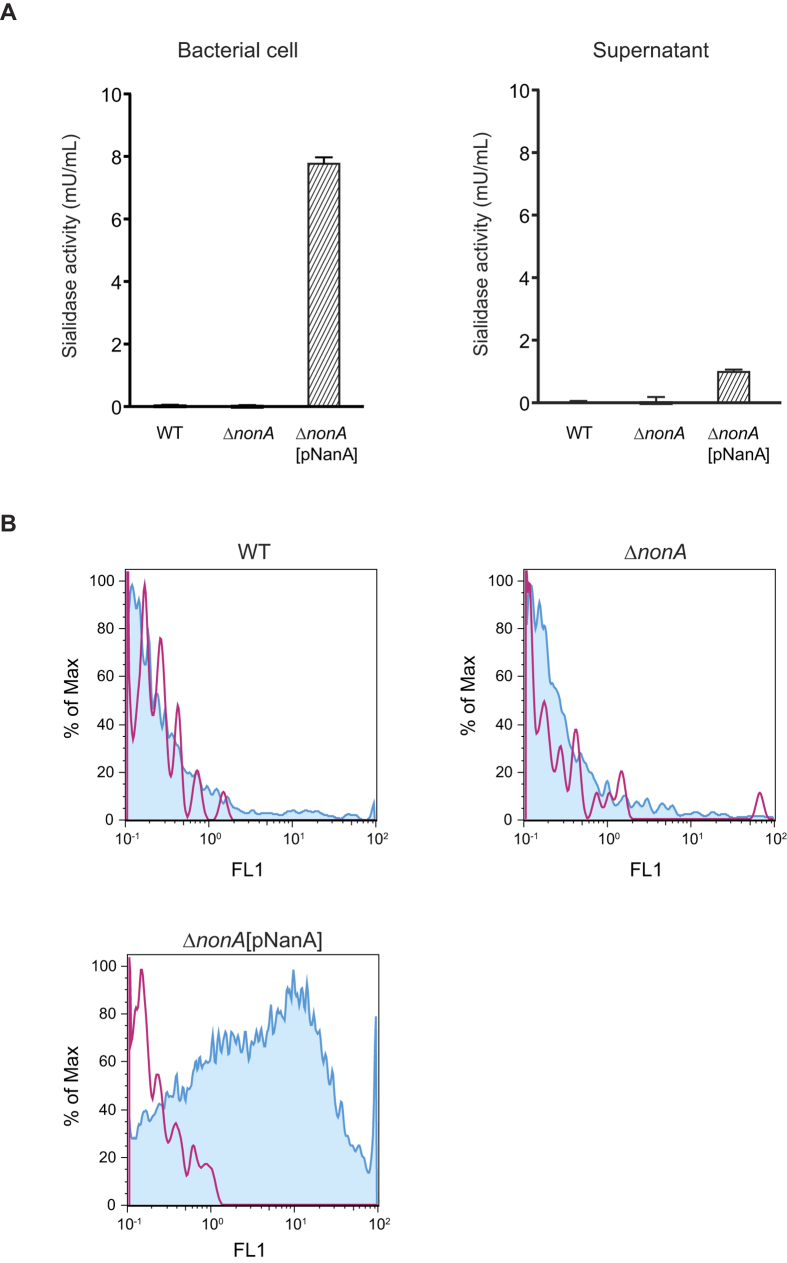
To investigate the role of NonA in bacterial pathogenesis, we constructed an isogenic GBS ΔnonA mutant strain and then complemented the ΔnonA strain with the functional pneumococcal NanA as described in the Methods section. The expression of the nonA gene in a GBS wild-type (WT) strain was higher than that of the well-characterized cylE gene encoding the GBS β-hemolysin/cytolysin (Supplementary Fig. 11). Sialidase activities of GBS WT, ΔnonA, and ΔnonA[pNanA] strains were determined using a fluorometric sialidase assay (Fig. 4A). Neither the WT nor ΔnonA GBS strains showed sialidase activity associated the bacterial cells or culture supernatants, but sialidase activity could be detected with heterologous expression of the pneumococcal enzyme.
Figure 4. NanA degrades terminal sialic acid displayed on GBS polysaccharide capsule.
(A) Sialidase activities of GBS cells and culture supernatant. After 2 h incubation at 37 °C, fluorescence of sialidase-degraded substrate was measured with excitation and emission wavelengths of 350 and 460 nm, respectively. Data are presented as the mean of sextuplets samples. S.E. values are represented by vertical lines. The sensitivity is 0.3 mU/mL. (B) FITC-labeled ECA binding to live GBS. Red line and blue histogram represents data for bacterial strains incubated without or with ECA, respectively.
We next investigated whether heterologous expression of a functional sialidase (NanA) would degrade the terminal sialic acid moiety on the GBS capsular polysaccharide repeating unit by flow cytometry with FITC-labeled Erythrina cristagalli agglutinin (ECA; Fig. 4B). ECA binds to terminal (unsialylated) galactose and the ECA binding level inversely reflects the level of sialylation on the GBS capsule. GBS WT and ΔnonA mutant strains showed similar histogram patterns and did not interact with the FITC-labeled ECA. On the other hand, the complemented GBS ΔnonA[pNanA] strain showed substantially higher fluorescence intensity when incubated with FITC-labeled ECA as compared to the strains incubated without FITC-labeled ECA. These results indicated that the GBS WT strain possessed no sialidase activity and the forced expression of the active sialidase in GBS could have the effect of degrading its own terminal sialic acid, a known immune evasion virulence factor of the pathogen with anti-complement, anti-phagocytic, and immunosuppressive properties4,5,6,7,8.
NonA does not contribute to GBS invasion into hBMECs
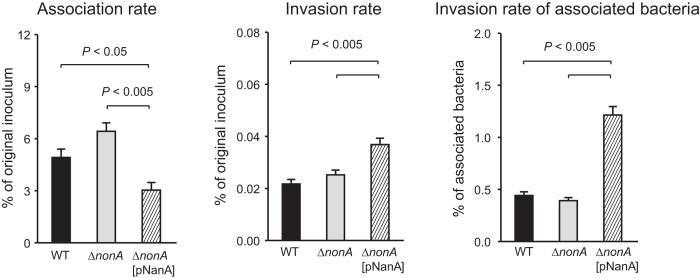
To examine the role of GBS NonA compared to the previously established role of pneumococcal NanA in the invasion of blood-brain barrier endothelium, we performed adherence/invasion assay using human brain microvascular endothelial cells (hBMECs) (Fig. 5). To quantify bacterial invasion, hBMECs were incubated with GBS strains for 1 hour (h) and further incubated for 1 h in medium containing antibiotics. WT GBS and the ΔnonA mutant did not differ in their adherence or invasion phenotypes to hBMEC (Fig. 5). The association of the ΔnonA[pNanA] strain was decreased as compared to that of other strains; however, invasion of the ΔnonA[pNanA] strains into human brain microvascular endothelial cells were significantly higher than that of GBS WT and ΔnonA strains. These results indicated that pneumococcal NanA, but not the endogenous GBS NanA homologue, can contributes to bacterial invasion of brain endothelial cells.
Figure 5. Rate of GBS adhesion to and invasion of hBMECs.
GBS strain A909 and its isogenic mutant strains were examined for their adhesion and invasion activities. Adhesion rates were calculated by dividing CFU at 1 h after infection by CFU of original inoculum. Invasion rates were calculated by dividing CFU at 1 h after antibiotic addition by CFU of original inoculum. Data are presented as the mean of sextuplets samples. S.E. values are represented by vertical lines.
Expression of sialidase in GBS inhibits its survival
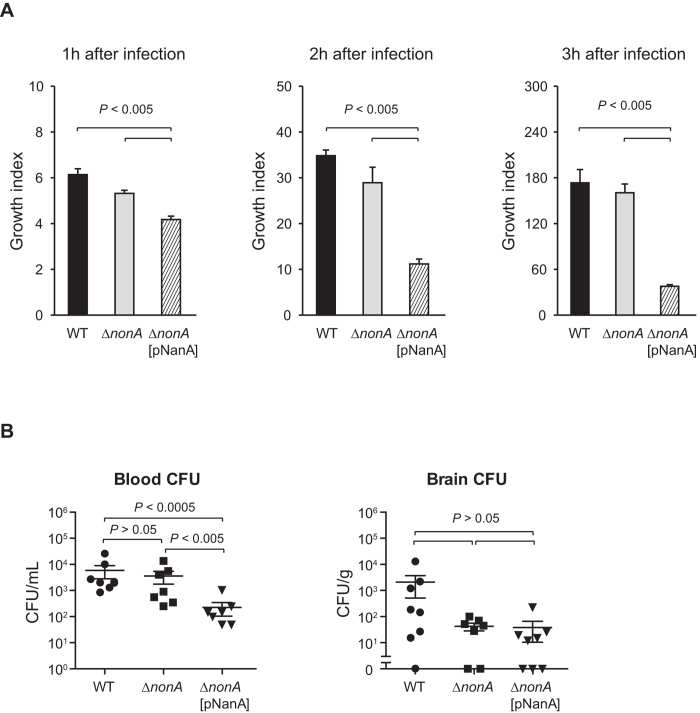
We compared bacterial survival rate ex vivo in human blood to compare the function of GBS NonA and active pneumococcal NanA in the GBS background (Fig. 6). The series of GBS strains were mixed with freshly collected human blood, and the mixture incubated for 3 h. Viable cell counts were determined hourly by plating diluted samples onto THY agar. No significant change in survival was noted comparing the isogenic ΔnonA mutant to the parent GBS WT strain. However, when NanA was introduced in this background, survival was approximately 72–79%, 39–44%, and 23% of WT levels after 1, 2, and 3 h, respectively. These results indicated that forced expression of NanA inhibits the survival of GBS in human blood, likely through the degradation of its terminal sialic acid on the capsule.
Figure 6. NanA expression diminishes GBS survival in human blood and mouse.
(A) Growth of GBS strains in fresh human blood was determined. Bacterial cells were incubated in blood for 1, 2, and 3 h at 37 °C in a 5% CO2 atmosphere. Next, the mixture was serially diluted and plated on THY agar. Following incubation, the number of CFU was determined. Growth index was calculated by dividing CFU after incubation by CFU of original inoculum. Data are presented as the mean of sextuplets samples. S.E. values are represented by vertical lines. (B) Mice were infected with ~3.5 × 107 CFU of GBS WT, ∆nonA, or ∆nonA[pNanA]. Blood and brain were collected at 20 h after infection. All mice were perfused with PBS prior to brain isolation. S.E. were represented by vertical lines. The difference between groups was analyzed using an Mann-Whitney U-test.
Finally, to investigate the potential role of NonA vs. an active sialidase in GBS pathogenesis, we infected GBS strains in mice intravenously, and compared bacterial CFU in blood and brains from mice 20 h after infection. Recovered CFUs of WT and ΔnonA strains in mouse blood were almost same. However, the ΔnonA[pNanA] expressing sialidase activity had on 14% the level of WT survival in the blood, consistent with the findings in the human blood survival assay.
Discussion
S. pneumoniae contains three sialidases, NanA, NanB, and NanC. NanB works as a virulence factor in pneumococcal infection and NanC catalyzes intermediate metabolic compounds which acts as sialidase inhibitor25,26. The nanC gene was reported to significantly associate with clinical isolates from invasive diseases12,27. Our evolutionary analysis indicated that ~15% of the codons in the pneumococcal nanA gene evolved under negative selection, while few codons of nanB and nanC evolved in positive or negative selection. The results suggest that selection pressures exist such that the key enzymatic functions of NanA may not change. In contrast to the conservations on nanA in S. pneumoniae, the GBS NanA homologue (NonA) appears to have lost its sialidase activity. Sialyltransferases are highly conserved in GBS strains of each serotype when compared to other glycosyltransferase genes in a same operon, and the difference in genetic diversity support a hypothesis that sialic acid is critical for GBS survival in the human host28. Our results showed that restoration of an active sialidase function inhibited GBS survival in human blood ex vivo and mouse blood in vivo. Therefore, sialidase activity would be deleterious to the fitness of GBS, and GBS nonA appears to be a non-functional gene.
We recently reported a similar relationship between bacterial capsule and glycosidase in another pathogenic streptococci, group A Streptococcus (GAS, Streptococcus pyogenes)29. Almost all serotypes of GAS express a hyaluronan exopolysaccharide capsule and contain an inactivated version of the hyaluronidase (HylA) with a single nucleotide mutation resulting in Asp to Val substitution at amino acid position 19930. However, serotype M4 strains express an active HylA, while lacking hyaluronan capsule biosynthesis operon. The operon was predicted to represent a more recent evolutionary acquisition in most serotypes. Although hyaluronan capsule is a major GAS virulence factor, heterologous expression studies to generate partial encapsulation of M4 wild-type strain and full encapsulation of an isogenic mutant ΔhylA strain did not increase virulence. In this human bacterial pathogen, the conflicts between polysaccharide capsule and glycosidase would exert conflicting selective pressures, and resulted in mutual exclusivity. In the present work, we find a similar mutual exclusivity between sialidase activity and the GBS polysaccharide capsule.
It is widely thought that pathogenic microbes may explain some human polymorphisms31,32. Sialylated pathogens can dampen the immune response through interaction with Siglecs, and this molecular mimicry is considered to be one of the primary forces in the rapid evolution of human Siglecs4,33,34,35. For example, Siglec-13 and -17 may have been genetically eliminated during hominid evolution, because of interactions with pathogenic bacteria, including GBS, that cause invasive infections33. In addition, Siglec-14 and -5 expressed on neutrophils and monocytes appear to have evolved to provide a balanced response to pathogens and infants with Siglec-14 deficiency were the most prone to GBS immune subversion36. Thus, there exists a multifaceted interaction between pathogen and human evolution at the molecular level. The synergy of evolutionary bioinformatics and functional analysis may help to investigate the interplay between pathogen and host within an evolutionary framework and to identify new genetically stable therapeutic targets within pathogens and/or their human hosts.
Methods
Phylogenetic and evolutionary analysis
Phylogenetic and evolutionary analyses were performed as previously described with minor modifications37. Homologues of nanA were searched for using tBLASTn of NCBI BLAST38. Sequences from complete genomes with e-values <2 × 10−85 and >40% query coverage were selected for phylogenetic tree analysis. The sequences were aligned using MAFFT ver. 7.221 with FFT-NS-i strategy39 and edited using by Jalview40. Regions coding sialidase domain were used for further phylogenetic analysis. Edited sequences were aligned again using MAFFT with L-INS-i strategy. The best-fitting codon evolutionary models for maximum likelihood and Bayesian phylogenetic trees were determined by Kakusan441. Maximum likelihood phylogenetic trees with bootstrap values were generated by RAxML ver. 8.1.2042. To validate phylogenetic inferences, Bayesian Markov chain Monte Carlo (MCMC) analyses were performed in MrBayes ver. 3.2.543, sampling 106 generations with a confirmation that the standard deviation of split frequencies was <0.01. Phylogenetic trees were drawn by FigTree ver. 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/) based on calculated data.
Tests for evolutionary analysis were performed on aligned common codon sequences of nanA, nonA, nanB, nanC, bgaA, or strH genes. Complete identical sequences were excluded. Whole gene non-synonymous/synonymous (dN/dS) ratio calculations, as well as statistical tests for negative or positive selection for individual codons, were performed using two-rate Fixed Effects Likelihood (FEL) and Fast Unconstrained Bayesian AppRoximation (FUBAR) in the HyPhy software package44,45. Comparing codon selection between nanA and nonA genes was performed using LR tests in the HyPhy44.
Ancestral states for bacterial sialidases were reconstructed in Mesquite version 3.0446 using a parsimony model with characters treated as unordered. The reconstruction was performed on the phylogenetic tree generated by MrBayes. States of active or inactive sialidase were assigned “0” or “1” for each taxon. Unavailable data were coded as missing.
Bacterial strains and cell lines
Streptococcal strains listed in Supplementary Table 5 were cultured in Todd-Hewitt broth (BD Biosciences) supplemented with or without 0.2% yeast extract (BD Biosciences) (THY or TH medium) at 37 °C. Streptococcus pseudopneumoniae ATCC BAA-960 (also called as SK1069 or CCUG 49455)47 was kindly provided by Dr. T. Hoshino, Nagasaki University, Japan. The Escherichia coli strain TOP10 (Invitrogen) was used as a host for derivatives of plasmids pSET4s, pDCerm, and pDESTerm. All E. coli strains were cultured in Luria-Bertani (LB) broth at 37 °C with agitation. For selection and maintenance of mutants, antibiotics were added to the media at the following concentrations: ampicillin (Wako), 100 μg/ml for E. coli; kanamycin (Sigma-Aldrich), 50 μg/ml for E. coli; chloramphenicol (Sigma-Aldrich), 10 μg/ml for E. coli; spectinomycin (Wako), 100 μg/ml for E. coli and 150 μg/ml for GBS; and erythromycin (Sigma-Aldrich), 400 μg/ml for E. coli and 5 μg/ml for GBS. Human brain endothelial cell line (hBMEC) was maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS, 10% NuSerum (BD), and 1% MEM nonessential amino acids, and were incubated at 37 °C in 5% CO2.
Construction of mutant strain
The construction of in-frame deletion mutants was conducted using a temperature-sensitive shuttle vector, pSET4s, as reported previously48,49. During the course of construction, a merodiploid strain was created after the first allelic replacement and then resolved to possess either mutant or wild type alleles after the second allelic replacement. To minimize the effect of secondary mutations and epigenetic changes that may have arisen during mutagenesis, a clone possessing the wild type allele was used as a wild-type strain. Both the wild-type and an in-frame deletion mutant strain arose from the same merodiploid ancestor. The correct in-frame deletion of genes was confirmed by site-specific PCR using purified chromosomal DNA. To create NonA and NanA-swapped GBS strain, ΔnonA[pNanA], pNanA plasmid was introduced respectively into GBSΔnonA strain by electroporation14. pNanA was constructed by ligating nanA gene from S. pneumoniae strain D39 into pDESTerm plasmid50.
Sialidase activity assay
Sialidase activities of bacterial cells and supernatants were determined by Neuraminidase assay kit (abcam). Streptococcal strains were grown to the mid-log phase (OD600 = 0.4–0.5) and centrifuged. To prepare bacterial cell fraction, the bacterial pellet was washed by PBS and resuspended in PBS. The supernatant was used as a supernatant fraction. The samples were incubated for 2 hours at 37 °C and fluorescence intensity was measured at Ex/Em = 350 nm/460 nm.
Real-time reverse transcription-PCR (RT-PCR) assay
Total RNA of GBS strains grown to the exponential phase (OD600 = 0.5) was isolated with RNeasy mini kit and RNase-Free DNase Set (Qiagen). Then, cDNA was synthesized with Transcriptor First Strand cDNA Synthesis Kit (Roche). Real-time RT-PCR analysis was conducted using StepOnePlus Real-Time PCR system (Thermo Fisher Scientific) and KAPA SYBR Fast qPCR Kit (KAPA Biosystems). Data for gyrA were used as internal control. Primers are listed in Supplementary Table 6.
ECA-binding assay
ECA-binding assay was performed as previously described51. GBS strains were grown to the mid-log phase and resuspended in PBS to adjust OD600 to 0.1. The bacteria were incubated on ice with FITC-conjugated Erythrina cristagalli agglutinin (ECA; Vector Laboratories, CA) at 10 μg/mL for 30 min. And then, bacterial cells were washed and resuspended in PBS. The ECA-binding activities on the surface of live bacterial cells were analyzed with a CyFlow SL flow cytometer.
hBMEC association and invasion assay
The bacterial association to and invasion of hBMEC were quantified with minor modifications as described previously52,53,54. GBS strains were grown to mid-log phase (OD600 = 0.5) and resuspended in PBS (OD600 = 0.1). hBMECs were seeded at 2 × 105 cells per well in RPMI1640 supplemented with 10% FBS in 24-well plates 1 d prior to bacterial infection. In each well, ~2.0 × 106 CFU of bacteria was added to infect with ~2.0 × 105 hBMECs at an multiplicity of infection (MOI) of 10 in a final volume of 500 μl, and the plate was centrifuged at 1600 rpm for 5 min to initiate their contact. To determine bacterial adhesion, the infected cells were incubated for 1 h, washed three times with PBS, and harvested with a trypsin and 0.025% Triton X-100 solution. The number of bacterial association was quantified by serial dilution plating. To examine bacterial invasion, hBMECs were washed following 1 h-incubation, and 500 μL of media containing 100 μg/mL of gentamicin was added and cells were incubated for an additional 1 h. The cells were washed and lysed, and the number of bacterial invasion was quantified. The bacterial association or invasion rate was calculated by dividing the number of bacterial association/invasion by the number of original inoculums. The invasion rate of bacterial association was also calculated by dividing the number of bacterial invasion by the number of bacterial association.
Blood bactericidal assay
A blood bactericidal assay was performed as previously described52,55,56. Blood was obtained via venopuncture from healthy donors. It was performed under written informed consent according to a protocol approved by the institutional review boards of Osaka University Graduate School of Dentistry. The GBS cells grown to the mid-log phase were washed and resuspended in PBS, and OD600 was adjusted to 0.1. Bacterial cells (10 μl) were combined with fresh human blood (190 μl), and then the mixture was incubated at 37 °C in 5% CO2 for 1, 2, and 3 hours. Viable cell counts were determined by plating diluted samples onto THY agar. Growth index was calculated as the number of CFU at the specified time point/number of CFU in the initial inoculum.
Mice infection assay
All mouse experiments were conducted in accordance with animal protocols approved by the Animal Care and Use Committees at Osaka University Graduate School of Dentistry (24-025-2). CD-1 (ICR: IGS) mice (6 weeks, female; Oriental) were infected with 3.5 × 107 CFU of GBS via the tail vein. After 20 h post-infection, blood aliquots were collected from mice just after general euthanasia. The samples of brain/meninges were collected following perfusion with PBS. Bacterial counts in blood and brain homogenates were determined by plating serial dilutions. Bacterial counts in brain were corrected for differences in each brain weight.
Statistical analysis
Statistical analysis of in vitro and in vivo experiments was performed using a nonparametric analysis, Mann-Whitney U test. The tests were carried out with Graph Pad prism version 6.0 e (GraphPad Software, Inc.). In evolutionary analysis, P < 0.1 was regarded as a significant difference as well as HyPhy default setting.
Additional Information
How to cite this article: Yamaguchi, M. et al. Evolutionary inactivation of a sialidase in group B Streptococcus. Sci. Rep. 6, 28852; doi: 10.1038/srep28852 (2016).
Supplementary Material
Acknowledgments
This study was supported in part by a research grant from NOVARTIS Foundation (Japan) for the Promotion of Science (to M. Y.), as well as a grant from Takeda Science Foundation (to M. Y.), Naito foundation (to S. K.), Grants-in-Aid for Scientific Research (B) (15H05012 to S. K.), and Young scientists (B) (26861546 to M. Y.) from the Japan Society for the Promotion of Science (JSPS), the U.S. National Institutes of Health-Funded UCSD Program for Excellence in Glycosciences (HL107150 to V. N.) and NIH Grant HL125352 (V. N.). M. Y. was the JSPS research fellows and a recipient of an award from the Iwadare scholarship foundation. Y. H. was a recipient of Iwadare scholarship from the Iwadare scholarship foundation.
Footnotes
Author Contributions M.Y., S.K. and V.N. designed the study. M.Y. and Y.Y. performed bioinformatics analysis. M.Y. and S.U. performed enzyme assay. M.N. and S.U. performed real-time RT-PCR assay. M.Y. performed adhesion and invasion assay, and bactericidal assay. M.Y., Y.H. and K.G. performed mouse infection assay. M.N., M.Y., S.U. and A.L.L. constructed mutant strains. M.Y., M.N., T.S., A.L.L., S.K. and V.N. wrote the manuscript.
References
- Edwards M. S., Nizet V. & Baker C. J. In Remington and Klein’s infectious diseases of the fetus and newborn infant (eds Christopher B. Wilson et al.) Ch. 12, 411–456 (Elsevier/Saunders, 2016). [Google Scholar]
- Le Doare K. & Heath P. T. An overview of global GBS epidemiology. Vaccine 31 Suppl 4, D7–12, 10.1016/j.vaccine.2013.01.009 (2013). [DOI] [PubMed] [Google Scholar]
- Varki A. Multiple changes in sialic acid biology during human evolution. Glycoconj J 26, 231–245, 10.1007/s10719-008-9183-z (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C. & Nizet V. The interplay between Siglecs and sialylated pathogens. Glycobiology 24, 818–825, 10.1093/glycob/cwu067 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M. B., Kasper D. L., Pangburn M. K. & Wessels M. R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun 60, 3986–3993 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Aoyagi Y., Adderson E. E., Okuwaki Y. & Bohnsack J. F. Capsular sialic acid limits C5a production on type III group B streptococci. Infect Immun 67, 1866–1870 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin A. F. et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113, 3333–3336, 10.1182/blood-2008-11-187302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C. et al. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog 10, e1003846, 10.1371/journal.ppat.1003846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K. L. et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902, 10.1016/S0140-6736(09)61204-6 (2009). [DOI] [PubMed] [Google Scholar]
- McIntyre P. B., O’Brien K. L., Greenwood B. & van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet 380, 1703–1711, 10.1016/S0140-6736(12)61187-8 (2012). [DOI] [PubMed] [Google Scholar]
- Geno K. A. et al. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev 28, 871–899, 10.1128/CMR.00024-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew M. M., Fennie K. P., York M. P., Daniels J. & Ghaffar F. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect Immun 74, 3360–3365, 10.1128/IAI.01442-05 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia A. B., Standish A. J. & Weiser J. N. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect Immun 78, 2108–2116, 10.1128/IAI.01125-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S. et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med 206, 1845–1852, 10.1084/jem.20090386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofling J., Vimberg V., Battig P. & Henriques-Normark B. Cellular interactions by LPxTG-anchored pneumococcal adhesins and their streptococcal homologues. Cell Microbiol 13, 186–197, 10.1111/j.1462-5822.2010.01560.x (2011). [DOI] [PubMed] [Google Scholar]
- Banerjee A. et al. Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization. Cell Microbiol 12, 1576–1588, 10.1111/j.1462-5822.2010.01490.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Uchiyama S., Varki A. & Nizet V. Leukocyte inflammatory responses provoked by pneumococcal sialidase. MBio 3, 10.1128/mBio.00220-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji Y. Pathogenicity of Erysipelothrix rhusiopathiae: virulence factors and protective immunity. Microbes Infect 2, 965–972 (2000). [DOI] [PubMed] [Google Scholar]
- Boraston A. B., Ficko-Blean E. & Healey M. Carbohydrate recognition by a large sialidase toxin from Clostridium perfringens. Biochemistry 46, 11352–11360, 10.1021/bi701317g (2007). [DOI] [PubMed] [Google Scholar]
- Chiarezza M. et al. The NanI and NanJ sialidases of Clostridium perfringens are not essential for virulence. Infect Immun 77, 4421–4428, 10.1128/IAI.00548-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew W. & Barnes A. C. Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet Microbiol 122, 1–15, 10.1016/j.vetmic.2007.03.002 (2007). [DOI] [PubMed] [Google Scholar]
- Kilian M. et al. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 3, e2683, 10.1371/journal.pone.0002683 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Riley D. R., Jensen A., Bruggemann H. & Tettelin H. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. MBio 5, e01490–01414, 10.1128/mBio.01490-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. et al. Detection of large numbers of pneumococcal virulence genes in streptococci of the mitis group. J Clin Microbiol 48, 2762–2769, 10.1128/JCM.01746-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco S. et al. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect Immun 74, 4014–4020, 10.1128/IAI.01237-05 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. et al. Three Streptococcus pneumoniae sialidases: three different products. J Am Chem Soc 133, 1718–1721, 10.1021/ja110733q (2011). [DOI] [PubMed] [Google Scholar]
- Janapatla R. P. et al. Necrotizing pneumonia caused by nanC-carrying serotypes is associated with pneumococcal haemolytic uraemic syndrome in children. Clin Microbiol Infect 19, 480–486, 10.1111/j.1469-0691.2012.03894.x (2013). [DOI] [PubMed] [Google Scholar]
- Cieslewicz M. J. et al. Structural and genetic diversity of group B Streptococcus capsular polysaccharides. Infect Immun 73, 3096–3103, 10.1128/IAI.73.5.3096-3103.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningham A. et al. Mutual exclusivity of hyaluronan and hyaluronidase in invasive group A Streptococcus. J Biol Chem 289, 32303–32315, 10.1074/jbc.M114.602847 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes W., Johnson C. & Stokes M. A single nucleotide mutation results in loss of enzymatic activity in the hyaluronate lyase gene of Streptococcus pyogenes. Microb Pathog 47, 308–313, 10.1016/j.micpath.2009.09.008 (2009). [DOI] [PubMed] [Google Scholar]
- Fumagalli M. et al. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet 7, e1002355, 10.1371/journal.pgen.1002355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M., Cagliani R., Forni D. & Clerici M. Evolutionary insights into host-pathogen interactions from mammalian sequence data. Nat Rev Genet 16, 224–236, 10.1038/nrg3905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Specific inactivation of two immunomodulatory SIGLEC genes during human evolution. Proc Natl Acad Sci USA 109, 9935–9940, 10.1073/pnas.1119459109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V. et al. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J 28, 1280–1293, 10.1096/fj.13-241497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley M. S., Crocker P. R. & Paulson J. C. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 14, 653–666, 10.1038/nri3737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. R. et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med 211, 1231–1242, 10.1084/jem.20131853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. et al. Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature 518, 98–101, 10.1038/nature13965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz E. M., Yu Y. K., Agarwala R., Schaffer A. A. & Altschul S. F. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol 4, 41, 10.1186/1741-7007-4-41 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K. & Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30, 772–780, 10.1093/molbev/mst010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A. M., Procter J. B., Martin D. M., Clamp M. & Barton G. J. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191, 10.1093/bioinformatics/btp033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe A. S. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol Ecol Resour 11, 914–921, 10.1111/j.1755-0998.2011.03021.x (2011). [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313, 10.1093/bioinformatics/btu033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61, 539–542, 10.1093/sysbio/sys029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond S. L., Frost S. D. & Muse S. V. HyPhy: hypothesis testing using phylogenies. Bioinformatics 21, 676–679, 10.1093/bioinformatics/bti079 (2005). [DOI] [PubMed] [Google Scholar]
- Murrell B. et al. FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Mol Biol Evol 30, 1196–1205, 10.1093/molbev/mst030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison W. P. & Maddison D. R. Mesquite: a modular system for evolutionary analysis. Version 3.04 http://mesquiteproject.org. (2015).
- Hoshino T., Fujiwara T. & Kilian M. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J Clin Microbiol 43, 6073–6085, 10.1128/JCM.43.12.6073-6085.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu D., Osaki M. & Sekizaki T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46, 140–148, 10.1006/plas.2001.1532 (2001). [DOI] [PubMed] [Google Scholar]
- Nakata M. et al. Assembly mechanism of FCT region type 1 pili in serotype M6 Streptococcus pyogenes. J Biol Chem 286, 37566–37577, 10.1074/jbc.M111.239780 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel A. S. et al. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe 4, 170–178, 10.1016/j.chom.2008.07.002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiman S. et al. Genetic and biochemical modulation of sialic acid O-acetylation on group B Streptococcus: phenotypic and functional impact. Glycobiology 19, 1204–1213, 10.1093/glycob/cwp111 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Terao Y., Mori Y., Hamada S. & Kawabata S. PfbA, a novel plasmin- and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis. J Biol Chem 283, 36272–36279, 10.1074/jbc.M807087200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C. et al. Glycosaminoglycan binding facilitates entry of a bacterial pathogen into central nervous systems. PLoS Pathog 7, e1002082, 10.1371/journal.ppat.1002082 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M. et al. Streptococcus pneumoniae invades erythrocytes and utilizes them to evade human innate immunity. PLoS One 8, e77282, 10.1371/journal.pone.0077282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M. et al. Role of Streptococcus sanguinis sortase A in bacterial colonization. Microbes Infect 8, 2791–2796, 10.1016/j.micinf.2006.08.010 (2006). [DOI] [PubMed] [Google Scholar]
- Mori Y. et al. α-Enolase of Streptococcus pneumoniae induces formation of neutrophil extracellular traps. J Biol Chem 287, 10472–10481, 10.1074/jbc.M111.280321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.