Abstract
The urinary tract is constantly exposed to microorganisms that inhabit the gastrointestinal tract, but generally the urinary tract resists infection by gut microorganisms. This resistance to infection is mainly ascribed to the versatility of the innate immune defences in the urinary tract as the adaptive immune responses are limited, particularly when only the lower urinary tract is infected. In recent years, as the strengths and weaknesses of the immune system of the urinary tract have emerged and as the virulence attributes of uropathogens are recognized, several potentially effective and unconventional strategies to contain or prevent urinary tract infections have emerged.
The urinary tract comprises the kidneys, ureters, bladder and urethra and, with the exception of the urethra, most of this tract is perceived to be sterile. Protection from microbial colonization is mediated by various soluble factors that are secreted into urine and by anatomical barriers such as the glycoprotein plaque uroplakins1 and a layer of hydrated mucus2. In addition, the urinary tract is lined by epithelial cells and various resident immune cells that further protect against infection (FIG. 1). These barriers prevent pathogens from entering the urinary tract and from establishing persistent infection.
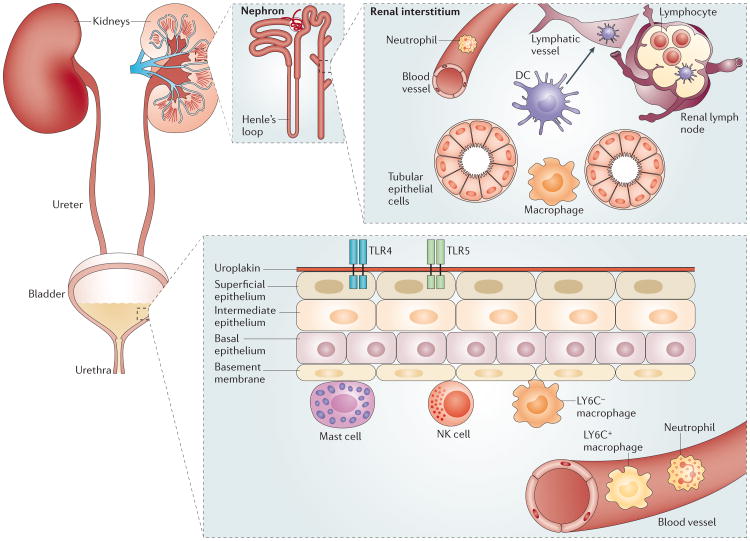
Figure 1. Organization of immune-competent cells along the urinary tract.
A variety of cell types are responsible for initiating immune responses along the urinary tract. The upper urinary tract is comprised primarily of the kidneys, and the filtration function of the kidneys is performed by hundreds of thousands of nephrons, each of which is composed of a glomerulus and a double hairpin-shaped tubule. Many of the immune-competent cells, including dendritic cells (DCs) and macrophages, are aggregated in the interstitium in close proximity to both the tubular epithelium and blood vessels. In addition, there is a large network of lymphatic vessels in these organs, which connects to the renal lymph nodes. The ureters, urethra and bladder constitute the lower urinary tract, and several layers of stratified epithelial cells that line the bladder function as the first line of defence. The major resident immune cells in the bladder include mast cells and LY6C− macrophages. These cells are located underneath the basal epithelium and function as sentinels to sense infection and recruit neutrophils and LY6C+ macrophages from the bladder. NK, natural killer; TLR, Toll-like receptor.
The majority of urinary tract infections (UTIs) in healthy individuals are caused by uropathogenic Escherichia coli (UPEC) that originate from the gut3. Uropathogens express common virulence factors that enable them to successfully establish infection in the urinary tract (BOX 1). However, most of the time the urinary tract resists infection, and if infection is established, it remains contained within this tract unless predisposing conditions exist4.
Box 1. Virulence attributes of uropathogens.
More than 80% of urinary tract infections (UTIs) in healthy individuals are caused by uropathogenic Escherichia coli (UPEC), with the remainder being caused by other enteric Gram-negative bacteria and some Gram-positive bacteria3. Most of these bacteria originate from the gut and reach the urinary tract by progressive colonization via the perineal region3. Uropathogens express certain key virulence factors that enable them to successfully establish infection in the urinary tract; these include adhesive fimbriae, which enable bacteria to adhere avidly to specific receptors on the urothelium, and flagella that enable bacteria to swim along the urinary tract including ‘upstream’ from the bladder to the kidneys67. Uropathogens also secrete toxins, such as haemolysin and cytotoxic necrotizing factor, which disrupt the epithelial barrier and enable access to the underling tissue68, and siderophores, which enable bacteria to chelate iron that is important for growth69. Bacterial survival in the urinary tract is also promoted by their expression of cell surface capsules, which enable them to resist the bactericidal actions of complement and phagocytic cells70. Arguably, the capacity to penetrate the highly impregnable bladder epithelial barrier and seek refuge in bladder epithelial cells (BECs) is a critical initiating step of infection in the urinary tract. Uropathogens seem to achieve this feat by hijacking the innate capacity of BECs to regulate bladder volume. Once UPEC become intracellular, they are protected from elimination by urine or the immune system, enabling them to initiate an acute infection. If untreated, UTIs tend to be self-limiting and naturally resolve within several days71. However, studies in mice reveal that even after the infection is resolved, an appreciable population of UPEC persists within BECs in a quiescent phase for extended periods of time70. It is assumed that recurrent infections occur when some of these intracellular bacteria escape into the bladder where they rapidly grow in the urine to initiate another bout of infection.
An important function of the urinary tract is to store urine for substantial periods of time, and as urine contains toxic and noxious compounds, it has to be contained within a tight epithelial barrier. Thus, the immune responses of the urinary tract need to balance the response to microbial challenge with the need to rapidly curtail inflammatory responses to maintain the structural integrity of the epithelial barrier. This ‘balancing act’ can result in premature termination of inflammatory responses, leading to the persistence of residual bacteria, and can potentially give rise to chronic or recurrent infections, which are remarkably common in the urinary tract5.
UTIs are more prevalent in women than men, and the incidences have increased substantially over the past 30 years6, mainly owing to an increase in the population of elderly and immune-compromised individuals, as well as the rise in the use of indwelling urinary catheters7. The annual cost of managing and treating UTIs in the United States is now estimated at approximately US$3 billion4, and the treatment of UTIs has become a serious clinical challenge due to the growing incidences of multi-drug resistant uropathogens and the high frequency of recurrent UTIs6. Thus, there is keen interest in improving our understanding the nature of the immune responses in the urinary tract and determining whether some of these activities could be targeted to combat UTIs. Here, we review the components of the immune system in the urinary tract and discuss potential novel approaches for targeting the immune system to prevent and limit UTIs. As the immune responses of the kidneys are fairly well characterized and reviewed elsewhere8,9, we focus our discussion on the bladder. The immune responses of the bladder have a central role in determining the outcome of UTIs, as the bladder stores urine — which is a potentially permissive medium for bacterial growth — for extended periods of time.
Innate immune responses
The innate immune system in the urinary tract comprises various resident and recruited cells that express a wide range of pattern recognition receptors (PRRs) — such as Toll-like receptor 2 (TLR2), TLR4, TLR5 and TLR11 — which enable early recognition of the pathogen and transduce this signal to induce a rapid and robust pro-inflammatory immune response. These signalling events have been reviewed in detail10,11 and are therefore not described here. The importance of these signalling events is illustrated by the fact that individuals with genetic defects in components of these pathways have increased susceptibility to UTIs12. Although these immune responses are important, they have to be tightly controlled to ensure the retention or rapid recovery of the epithelial barrier. In this section, we review the main innate immune cells in the urinary tract. We focus on their unique antimicrobial activities and discuss the regulatory mechanisms that control over-reactive innate immune responses.
Epithelial cells
The epithelial cells lining the urinary tract are the first line of defence against pathogens. These cells secrete a plethora of soluble compounds ranging from pro-inflammatory cytokines to antibacterial agents. Interleukin-1 (IL-1), IL-6 (REF. 13) and IL-8 (REF. 14) are often the first cytokines to be detected in urine following infection, and they are important for the recruitment of phagocytes into the infected bladder or kidney tissue15. Uromodulin (also known as Tamm–Horsfall urinary glycoprotein) is specifically produced in the urinary tract by epithelial cells lining the ascending limb of Henle's loop in kidney nephrons16. Upon binding to UPEC, uromodulin prevents bacteria from interacting with the epithelial cell surface while simultaneously inducing them to aggregate, which facilitates early removal of UPEC in the urine17. Uromodulin has also been suggested to directly trigger TLR4 and induce the maturation of certain cells such as myeloid dendritic cells (DCs)18. Thus, uromodulin may also have an immune-modulatory function.
Some secreted factors from epithelial cells inhibit bacterial growth by eliminating important bacterial growth factors in the urine. The neutrophil gelatinase-associated lipocalin (NGAL) protein binds bacterial siderophores such as enterochelin19,20. NGAL, which is produced by α-intercalated cells in the kidney, was recently shown to restrict the growth of UPEC in the urinary tracts of both humans and mice21. During UPEC infection, mice deficient in NGAL or α-intercalated cells showed increased susceptibility to infection compared with wild-type mice21. A variety of antimicrobial peptides (AMPs) — such as cathelici-din-related AMPs (for example, LL-37 (also known as CAMP))22, β-defensin 1 (REF. 23) and ribonuclease 7 (REF. 24) — are also secreted into the urine to restrict bacterial growth. In response to bacterial infection, bladder epithelial cells (BECs) and α-intercalated cells function as the initial source of AMPs in the urinary tract; at a later stage, AMPs are secreted by recruited neutrophils (FIG. 2). The contribution of AMPs to protection against UTIs is indicated by the observation that cathelicidin-deficient mice are highly susceptible to both cystitis and pyelonephritis22. AMPs can also have immune-modulatory effects, such as increasing cytokine production and promoting neutrophil infiltration25. Pentraxins are an evolutionarily conserved protein family that can function as soluble PRRs. The level of pentraxin-related protein 3 (PTX3) in urine during UTIs is strongly associated with the severity of symptoms26, and genetic polymorphisms in the human PTX3 locus correlate with increased susceptibility to acute pyelonephritis26. PTX3 is thought to bind bacterial surfaces, which leads to complement-mediated killing and uptake by phagocytes26.
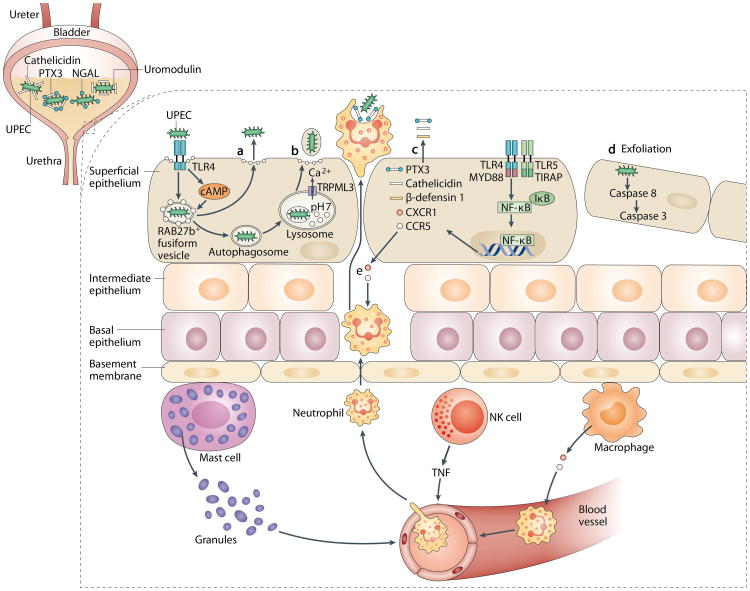
Figure 2. Scope of innate immune responses in the bladder.
A robust and complicated network of innate immune responses can be initiated in the bladder in response to infection. a | Following invasion by uropathogenic Escherichia coli (UPEC) and encapsulation within RAB27b+ vesicles in bladder epithelial cells (BECs), Toll-like receptor 4 (TLR4) recognizes intracellular UPEC and increases intracellular cyclic AMP (cAMP) levels. This leads to exocytosis of RAB27b+ vesicles harbouring UPEC and expulsion of the intracellular UPEC back into the lumen of the bladder. b | When intracellular UPEC escape the first wave of expulsion by breaking the RAB27b+ vacuole, these bacteria are targeted by autophagy and delivered into the lysosomes, which in turn are manipulated by UPEC to lose their degradative capacity. These malfunctioning lysosomes are sensed by a lysosomal transient receptor potential (TRP) mucolipin 3 channel (TRPML3) and trigger lysosome exocytosis, resulting in bacterial expulsion. c | Upon sensing the presence of pathogens by TLR4 and subsequent signalling a wide range of soluble factors are also secreted by BECs, including antimicrobial peptides (such as cathelicidin and β-defensin 1), antimicrobial proteins (such as pentraxin 3 (PTX3)) and chemokines (such as CXCL1 and CCL5). d| Bacterial infection initiates caspase 3-and caspase 8-dependent apoptosis of infected BECs, which shed into the bladder lumen; this represents another effective mechanism to reduce bacterial load. e | Coordinated cellular immune responses in the bladder are shown. The resident sentinel immune cells — such as mast cells, natural killer (NK) cells and macrophages — can sense the presence of the infections and secrete various cytokines to recruit other innate immune cells from the bloodstream, especially neutrophils to clear the infections. IκB, NF-κB inhibitor; MYD88, myeloid differentiation primary response protein 88;NGAL, neutrophil gelatinase-associated lipocalin; NF-κB, nuclear factor-κB; TIRAP, Toll/IL-1R domain-containing adaptor protein; TNF, tumour necrosis factor.
Following UPEC invasion of BECs, the bacteria are encapsulated in RAB27b+ fusiform vesicles, which intrinsically have exocytic properties, and as a consequence, BECs can then expel intracellular bacteria back into the extracellular medium without loss of viability27. In vitro studies of UPEC-infected BECs reveal that bacterial expulsion can be detected within minutes of infection; the rate seems to peak at 4 hours post-infection and by 24 hours, up to 70% of the infecting bacteria are expelled from BECs27. The underlying mechanism involves mobilization of multiple exocytic activities within BECs. One pathway involves TLR4 signalling that leads to increased intracellular levels of the second messenger molecule cyclic AMP, which in turn triggers spontaneous expulsion of RAB27b+ vesicles (some of which contain bacteria)28. A second exocytic pathway is activated when intracellular bacteria that escaped the first wave of expulsion, by breaking out of RAB27b+ vacuoles, are recognized and captured by autophagy29. Autophagy normally leads to the degradation of bacteria, but UPEC can block acidification and survive within lysosomes29. Studies in cultured human BECs have shown that the presence of malfunctioning lysosomes containing UPEC is promptly sensed by transient receptor potential mucolipin 3 (TRPML3), which is a cation channel that is expressed on the lysosome, and this sensing triggers the spontaneous exocytosis of these lysosomes29. This capacity of BECs to exocytose lysosomal contents seems to be an innate homeostatic mechanism to remove lysosomal contents that are not degraded. In contrast to bacteria exocytosed in the first wave of expulsion, bacteria expelled from lysosomes are encapsulated within host membranes, which prevents reattachment of UPEC to the bladder wall and ensures bacterial removal in urine29. Thus, infected BECs seem to use various components of their export machinery to reduce bacterial load, and in vivo studies in mice show that inhibition of autophagy or blockade of calcium flux can substantially increase the bacterial load in the bladder and, conversely, boosting the activity of other components, such as intracellular cAMP, can markedly reduce bacterial load27.
A more drastic mechanism used by the bladder to reduce bacterial load is shedding of the superficial epithelial cell layer. Observations in experimental mouse models and in patients with UTIs reveal that during the acute phases of UTIs, when the superficial BECs become heavily infected, BECs spontaneously shed in large numbers into the urine, which leads to significantly reduced bacterial numbers in the bladder30. Conceivably, massive shedding of BECs may also be a homeostatic action to temper their inflammatory responses, as excessive release of pro-inflammatory mediators can be harmful. Shedding of BECs leads to exposure of the underlying tissue to toxic components within urine, which could have severe consequences. To limit these harmful effects, the loss of the superficial epithelial cell layer is promptly followed by a shift of the urothelium from a quiescent phase to a highly proliferative state to restore the epithelial barrier31. When the stem-cell like progenitors at the basal layer of the urothelium sense injury in the overlying epithelium caused by exfoliation, they secrete sonic hedgehog (SHH), which activates the WNT signalling pathway. In turn, activation of WNT stimulates the proliferation of urothelial cells and stromal cells and the restoration of a tight epithelial barrier32 (FIG. 3). This rapid turnover of cells protects the deeper tissue from toxic substances in the urine, and it enables efficient bacterial clearance while limiting pro-inflammatory responses.
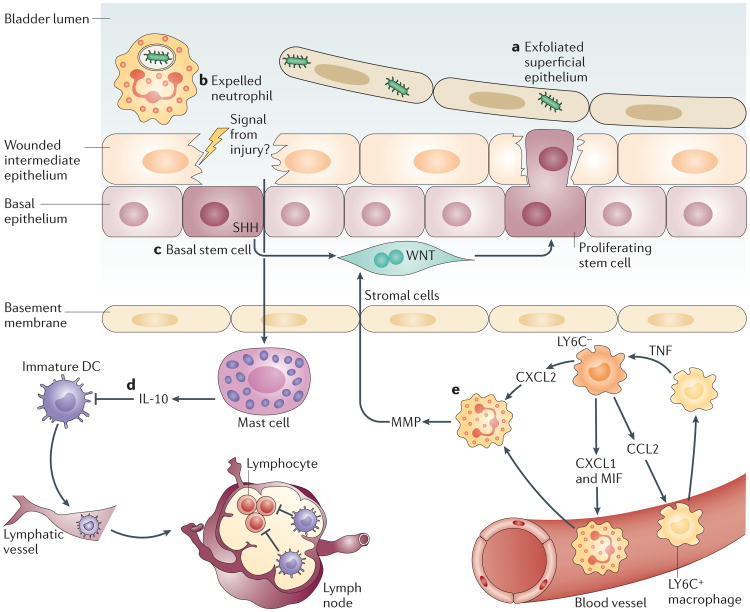
Figure 3. Mechanisms to curtail inflammatory responses in the bladder following infection.
Infections of the superficial epithelium can eventually lead to extensive exfoliation of bladder epithelial cells (part a), resulting in loss of the physical barrier. Each type of immune cell possesses specialized strategies to prevent excessive inflammation and to maintain the tissue integrity. Neutrophils are rapidly expelled into the urine, possibly to reduce the tissue damage from their toxic granules (part b). Local stem cells and stromal cells underneath the intermediate epithelium sense damage to the tissue and initiate a proliferation programme to regenerate the tissue barrier (part c). Local mast cells, which were previously active in mediating a pro-inflammatory immune response, sense the damaged epithelium and switch to mediating anti-inflammatory responses to facilitate regeneration of the epithelium (part d). This switch in activity is achieved by secreting large amount of interleukin-10 (IL-10). Local IL-10 suppresses the activation of dendritic cells (DCs) but not their migration to the iliac lymph nodes. Immature DCs are unable to induce substantial antibody responses in the lymph nodes. Intimate crosstalk between LY6C− and LY6C+ macrophages provides a ‘double-safe’ checkpoint to ensure precise initiation of neutrophil responses (part e): local LY6C− macrophages release CC-chemokine ligand 2 (CCL2), chemokine CXC-chemokine ligand 1 (CXCL1) and macrophage migration inhibitory factor (MIF) to recruit LY6C+ macrophages and neutrophils from the bloodstream. Upon sensing the infection, LY6C+ macrophages secrete tumour necrosis factor (TNF), which acts on local LY6C− macrophages to trigger their production of CXCL2, which induces neutrophils to cross the basal membrane. MMP, matrix metalloproteinase; SHH, sonic hedgehog.
Neutrophils
Neutrophils are the first immune cells to be recruited to the bladder following UTIs, and they have a predominant role in bacterial clearance33. They respond to CXC-chemokine ligand 1 (CXCL1) and other chemoattractants produced by superficial bladder epithelial cells, macrophages and mast cells after activation of PRRs by various bacterial products. In mouse models of UTI, neutrophils can be detected in urine as early as 2 hours post-infection, and their numbers reach a peak by 6 hours34. The number of neutrophils closely parallels the bacterial burden in the urinary tract, and as bacterial numbers decrease, so do the number of neutrophils35. Upon entry into urinary tract tissue, neutrophils control the infection via multiple mechanisms, and this is facilitated by various soluble factors in the urine, such as pentraxins26. However, owing to the release of reactive oxygen species and other cytotoxic products, activated neutrophils are also responsible for substantial toxicity to surrounding bladder tissue. In fact, excessive neutrophil responses — including the increased expression of cyclooxygenase 2 (COX2; also known as PTGS2) — cause inflammatory damage to the bladder tissue and predispose the bladder to persistent infections36. To reach bacteria in the bladder lumen, activated neutrophils cross multiple layers of epithelial cells, including the usually impermeable superficial epithelial cell layer33. Presumably, this activity could limit the cytotoxicity of neutrophils by removing them from the bladder tissue into the urine where they can be removed from the body during voiding.
Macrophages
A substantial population of macrophages resides in the submucosa of the urinary tract, and more cells are recruited to these sites following infection37. Upon activation, these macrophages produce crucial cytokines and chemokines that modulate the activity of these and other immune cells in the vicinity, which markedly influences the timing and intensity of inflammatory responses during UTIs38,39.
Crosstalk between different subsets of macrophages in the bladder was recently shown to coordinate the precise recruitment and onset of neutrophil responses40. Resident macrophages in the bladder are mainly LY6C− and function as sentinels at this site. Following infection, these macrophages secrete the chemokines CXCL1 and macrophage migration inhibitory factor (MIF) to recruit neutrophils, and CC-chemokine ligand 2 (CCL2) to recruit LY6C+ macrophages40. Upon extravasation and reaching the epithelial region, recruited neutrophils need local tumour necrosis factor (TNF) signalling to be able to cross the basal membrane of the epithelium; this requirement was evident from a study showing that transepithelial migration of neutrophils was absent in TNF-deficient mice40. Interestingly, the recently recruited LY6C+ macrophages are the source of TNF, the effects of which are not directed at the neutrophils. Instead, TNF induces resident LY6C− macrophages to secrete a second wave of cytokines, mainly CXCL2, which triggers neutrophils to spontaneously produce matrix metalloproteinase 9 (MMP9) and initiate their transepithelial movement41. Thus, whereas the resident LY6C− macrophages function as the main pro-inflammatory cells, the recruited LY6C+ macrophages have a key role in keeping neutrophils in close proximity before targeting the pathogen. Furthermore, the recruited LY6C+ macrophages could also cause simultaneous activation of the surrounding microenvironment, as the locally generated TNF probably has a global activating effect. Paradoxically, studies have indicated that the infiltrating GR1hiLY6C+ monocytes are dispensable for bacterial clearance in the bladder, suggesting that our current understanding may be incomplete37. In any case, crosstalk between bladder macrophages leads to mobilization of neutrophils into the epithelium and their subsequent activation alongside neighbouring immune cells. These immune responses ensure efficient bacterial clearance and also minimize unnecessary and damaging inflammation.
Mast cells
Mast cells are another resident immune cell type located underneath the uroepithelium in close proximity to blood and lymphatic vessels that traverse the mucosal region (FIG. 2). In the bladder, mast cells are also found in high numbers in the detrusor muscle region42. Mast cells have a pivotal sentinel and key immunomodulatory role during UTIs, which is partly due to their ability to release many pre-stored pro-inflammatory mediators — such as TNF, histamine and several chemokines — upon activation43. These mediators are stored within cytoplasmic granules that gradually release their cargo after being released extracellularly. Substantial amounts of histamine are detectable in the urine as early as 30 minutes after bladder infection in mice44. It is unclear how bladder mast cells become activated when the epithelium is still intact, but it is possible that products of stressed epithelial cells — which are known mast cell activators and include ATP, LL-37 and IL-33 — could contribute to this activation. In response to UPEC infection, mast cell-deficient mice show impaired neutrophil responses and decreased bacterial clearance compared with wild-type mice, which indicates an important role for mast cells in the early recruitment of neutrophils44. The number of mast cells in the mucosal region of the bladder markedly increases during bladder infection, suggesting a very dynamic role for these cells at this site44.
Whereas mast cells function as pro-inflammatory immune cells during the early phase of infection, mouse models of UTIs have revealed that when the infection progresses to a later stage, typically 6–12 hours post-infection, mast cells start to produce anti-inflammatory cytokines such as IL-10 to suppress inflammatory responses45. This switch seems to occur in parallel with the breakdown of the epithelial barrier and could facilitate regeneration of the epithelium. There are typically two subsets of mast cells in the bladder (mucosal and connective tissue types), but it is unknown if this anti-inflammatory role is ascribable to one or both of these cell types. Nevertheless, mast cells in the bladder seem to have a dual role in immune regulation, presumably to balance the disparate needs of host defence and tissue homeostasis.
Other innate immune cells
Whereas natural killer (NK) cells are known to have a key role in clearing viral infections, their importance during bacterial infection is less well characterized. Recently, NK cell-depleted mice were reported to be particularly susceptible to UPEC infection46. Although the protective actions of NK cells were attributed to their production of TNF, the underlying basis of how this controls the UPEC infections remains unclear46. DCs have been shown to be highly active during UTIs47, but their specific contribution to innate immune responses in the urinary tract remains to be determined.
γδ T cells are an intriguing group of resident immune cells in the urinary tract. Mice deficient in γδ T cell receptors (TCRs) are markedly more susceptible to UTIs than wild-type mice48. A study on the susceptibility of IL-17-deficient mice to UTIs showed that γδ T cells were the source of IL-17 (REF. 49). Whether IL-17 production is the primary contribution of γδ T cells to host defence against UPEC is unclear. Innate lymphoid cells are increasingly being recognized for their contribution to mucosal immunity at various sites, but their importance in the urinary tract has not been defined.
Polymodal chemosensory cells located in the mammalian urethra represent another cell type that has recently been implicated in immune surveillance in the urinary tract50. Upon exposure to UPEC, these brush cells activate a signalling cascade composed of taste-specific G protein α-gustducin, phospholipase Cβ2 and the TRP cation channel melanostatin 5 (TRPM5)50. These cells are thought to sense putative bitter receptor-activating substances associated with UPEC and release acetylcholine to communicate with neighbouring cells50. The importance of this activity in relation to the overall immune responses of the urinary tract is currently unknown.
In summary, the innate immune cells in the urinary tract rapidly respond and evoke robust immune responses during UTIs. These responses are tightly controlled, but sometimes the innate responses of the bladder are prematurely terminated, which can have detrimental effects on the host, similar to what was recently shown for the development of inadequate adaptive immune responses to infection, as described below.
Adaptive immune responses
Whereas the wide-ranging innate immune responses of the urinary tract are highly responsive to infections, the adaptive immune responses, particularly in the bladder, tend to be limited. UTIs that progress to the kidneys can lead to the productions of antibodies specific for the infecting agent, but patients with infections limited to the bladder inexplicably fail to induce an antibody response51. This apparent defect in the antibody response of the bladder could be a major reason for the remarkable recurrence of UTIs, especially following bladder infection.
This clinical observation was also recently reproduced in mouse models in which UTIs that were strictly limited to the bladder induced little or no antibody response to the infecting bacteria, whereas a substantial antibody response was induced during infections of both the bladder and kidneys45. The underlying basis of the inability of the bladder to mount an adaptive response was linked to increased local IL-10 production, as IL-10-deficient mice showed substantial antibody responses to bladder infection45. As previously discussed, mast cells are a major source of IL-10 in the bladder following bacterial infection45. Although these secretory cells have an important role in initiating a vigorous immune response in the early phases of bladder infection, mast cells seem to reverse their activity approximately 6 hours post-infection by switching to IL-10 production to shut down this response45. Mast cell-generated IL-10 can prevent the expression of co-stimulatory molecules on DCs and thereby limit their capacity to function as effective antigen-presenting cells when they traffic to draining lymph nodes45. Hence, consistent with the role of mast cell-derived IL-10 in attenuating innate immune response, the inability of the bladder to mount an antibody response to bacterial infection could be a by-product of its attempt to prevent harmful adaptive immune responses to the contents of urine, as well as to facilitate the rapid regeneration of its epithelium following infection-induced damage.
Immunomodulatory therapies
As the nature of the immune responses in the urinary tract has becomes clearer, this knowledge could be leveraged to develop novel and effective strategies for the prevention, treatment and/or management of UTIs. Several of these strategies are targeted at boosting innate immune responses, and some target adaptive immune responses. Adaptive immune responses targeted at various components of uropathogens have been found to be particularly protective in the urinary tract provided that they are induced at sites other than the bladder. However, the efficacy of these strategies is based almost exclusively on data from experimental mouse models.
Boosting bacterial expulsion from infected BECs
Recent studies have revealed that the innate capacity of BECs to expel intracellular UPEC present in RAB27b+ vesicles can be greatly accelerated by increasing intracellular cAMP levels27. For example, when UPEC-infected mice were treated systemically or intravesically with forskolin at various time points after infection, up to 90% of the bacterial burden was reduced compared with saline-treated mice27. Of note, forskolin treatment also reduced the secretion of the pro-inflammatory cytokine IL-6 (REF. 27) and ameliorated the pathology associated with UTIs52. There are currently many drugs approved by the US Food and Drug Administration (FDA) that regulate intracellular cAMP levels by inhibiting phosphodiesterase 4 activity (for example, roflumilast and ibudilast), and these could potentially be used in combination with current antibiotic treatments for UTIs to increase therapeutic efficacy27. As this type of treatment is directed at signalling events in BECs, it is unlikely that uropathogens will develop resistance to the treatment, as is the case with antibiotics.
Vaccination against bacterial virulence factors
As the adaptive immune responses naturally elicited by bladder infections are limited, inducing memory responses by administering vaccines could also be an approach to protect against recurrent UTIs. The bacterial virulence factor fimbrial adhesin FimH has been used as an effective vaccine antigen in mouse models53. However, studies examining the efficacy of a FimH vaccine in humans were not continued because of limited immunogenicity and the lack of a safe and effective adjuvant54. With the emergence of several safe and efficacious adjuvants for human use55, it may now be appropriate to revisit the use of FimH vaccines in clinical trials. Recently, iron-chelating factors of UPEC were shown to be effective vaccine antigens in mice, and therefore these could provide another vaccine target56. Of note, the current immunization regimen involving the use of a single vaccine antigen has never evoked complete protection, as re-infection upon challenge still occurred in immunized mice, albeit at a significantly lower level57. Thus, there is a growing consensus that a multivalent vaccine directed at multiple uropathogenic virulence factors may be more effective.
Exogenous regulation of hormones
As elderly women are particularly prone to recurrent UTIs, ageing-associated hormonal changes seem to be a predisposing factor. Furthermore, there is epidemiological evidence that oestrogen supplementation after menopause protects against UTIs58. Oestrogen supplementation can boost innate immunity by stimulating local production of AMPs and by strengthening the integrity of the urothelium58. Another recent study showed that administration of SR121463B — an antagonist of the vasopressin receptor V2, which is expressed in the kidney — stimulated local innate immune responses in the urinary tract in UPEC-infected mice. Thus, a hormone-based prophylactic strategy could be another effective strategy in elderly women who are prone to UTIs59.
Probiotics to exclude prospective uropathogens
With the success of probiotic therapy and faecal transplantation in the gut, there is keen interest in using similar approaches to manage UTIs. As UTIs are often preceded by the presence of unhealthy microbiota in the vagina and urethra60,61, a possible approach to prevent UTIs could be to normalize the vaginal and urethra microflora by direct administration of probiotics62. The use of strains that cause asymptomatic bacteriuria (ABU strains) — such as E. coli strain 83972 or VR11 (REF. 64) — to inoculate the urethra and even the bladder is a more provocative approach. ABU strains can potentially persist in the urinary tract for months or even years without evoking any destructive symptoms, while importantly excluding more virulent pathogens64,65. Furthermore, ABU strains can selectively suppress host cells from inducing pro-inflammatory responses, and as a consequence, the host remains asymptomatic during infection. The effectiveness of this approach was recently revealed in patients prone to UTIs, and administration of a prototype ABU strain into the urinary tract of susceptible individuals protected them from superinfections by virulent UPEC strains65.
Concluding remarks
The physical barrier and the antibacterial actions of urinary secretions and resident cells lining the urinary tract constitute an integrated multi-layered defence system that protects against most prospective pathogens. Paradoxically, the pathogens that typically cause UTIs are considered to be ‘opportunistic pathogens’ that are mostly of enteric origin, suggesting that these infections occur only because of a breakdown in one or more of the immune defences of the urinary tract. Although data for this notion are still limited, one major breakdown that we have highlighted here is the premature termination of innate immune responses to infection. Due to the overriding need to retain or restore the epithelial barrier, innate as well as adaptive immune responses in the bladder are often prematurely curtailed, predisposing individuals to either chronic or recurrent infections, often by the same bacterial strain. In addition, once infected, the architecture and cellular composition of the bladder is substantially affected, predisposing it to further infections66.
Several new but markedly different strategies to combat UTIs have emerged from studies carried out in experimental mouse models. With the sharp increase in multidrug resistance among UTIs, it is of paramount importance that these approaches are quickly advanced into the clinic for the treatment of UTIs in humans. However, determining which of these approaches is most appropriate for a particular patient may also depend on first identifying the specific defect in their immune system. Thus, in addition to isolating and characterizing the uropathogen, identifying the nature of the defect in the urinary immune system — for example, through screening for presence or absence of certain biomarkers and examining for single-nucleotide polymorphisms — may become a key requirement in the diagnosis of UTIs. This information should allow for the future tailoring of treatments for individual patients so that it is not only appropriate but also optimal.
Key points.
An overarching theme of the immune system in the bladder seems to be balancing the need to respond promptly to microbial challenge with the need to rapidly curtail inflammatory responses, as the structural integrity of the epithelial barrier is disrupted during prolonged immune responses.
Bladder epithelial cells not only alert the immune system during infection but also directly mediate bacterial clearance by secreting antimicrobial compounds into the urine and by expelling invading bacteria back into the bladder lumen to reduce intracellular load.
Crosstalk between different subsets of macrophages in the bladder coordinates the precise recruitment and onset of neutrophil responses, and thereby reduces harmful inflammatory reactions.
Mast cells seem to have a dual role in immune regulation in the urinary tract. They promote early mobilization of immune cells into the bladder and are central to terminating these pro-inflammatory responses presumably when the bladder epithelial barrier is disrupted. However, this homeostatic action often results in blunted adaptive immune responses.
Although neutrophils are the predominant immune cells mediating bacterial clearance in the bladder, excessive neutrophil responses can cause damage to the bladder tissue and predispose this organ to persistent infections.
Several unconventional, but potentially effective, strategies have been described that can boost immune defences of the bladder to contain or prevent urinary tract infections.
Acknowledgments
The authors' work is supported by the US National Institutes of Health (grants R01 AI96305, R01 AI35678, R01 DK077159, R01 AI50021, R37 DK50814 and R21 AI056101) and a block grant from Duke–National University of Singapore Graduate Medical School.
Glossary
- Uroplakins
Transmembranous tetraspanin-family proteins that form numerous plaques and cover the apical surface of the urothelium.
- Uromodulin
A highly mannosylated protein that integrates with mucin and, upon encountering bacteria, specifically adheres to the mannose-binding type 1 fimbriae on uropathogenic Escherichia coli.
- Neutrophil gelatinase-associated lipocalin (NGAL)
An iron-trafficking protein that binds to iron through its interaction with siderophores.
- Siderophores
Iron-chelating compounds secreted by microorganisms growing under low iron conditions.
- Enterochelin
A high-affinity siderophore that is mainly secreted by Gram-negative bacteria to acquire iron.
- α-intercalated cells
Specialized cells that are located in the collecting duct of the kidney medulla and are responsible for regulating the electrolyte balance.
- Antimicrobial peptides (AMPs)
Short peptides that preferentially bind and insert into the outer leaflet of the bacterial membrane and form pores to damage the microbial membrane integrity.
- Pentraxins
Soluble pattern recognition receptors that specifically detect the lipopolysaccharides and outer membrane proteins of bacteria and promote their uptake by phagocytes.
- Fusiform vesicles
Specialized membrane vesicles that are found near the apical surface of the superficial epithelium of the bladder and are responsible for providing extra membrane during bladder expansion.
- Autophagy
An evolutionary conserved process in which acidic double-membraned vacuoles sequester intracellular contents (such as damaged organelles and intracellular pathogens) and target them for degradation through fusion to secondary lysosomes.
- Sonic hedgehog (SHH)
An essential intercellular signalling protein for pattern formation and tissue regeneration during development.
- Macrophage migration inhibitory factor (MIF)
A pro-inflammatory cytokine that regulates key functions of macrophages by inhibiting the anti-inflammatory effects of glucocorticoids.
- Matrix metalloproteinase 9 (MMP9)
An endopeptidase involved in the cleavage of a variety of substrates, including collagen and extracellular matrix components.
- Detrusor muscle region
A layer of bladder wall that is composed of smooth muscle.
- Forskolin
A plant extract and adenylyl cyclase activator with a potent capacity to increase intracellular cAMP levels.
- Fimbrial adhesin FimH
A highly conserved protein that is expressed by common uropathogens; it mediates bacterial adhesion of type 1 fimbriae by binding to host d-mannose.
Biographies
Soman N. Abraham received his Ph.D. from Newcastle University, Newcastle upon Tyne, UK. He did his postdoctoral training at the University of Tennessee, Memphis, USA. He then worked at Washington University, St. Louis, Missouri, USA, before moving to Duke University in Durham, North Carolina, USA, where he is now a professor in the Departments of Pathology, Immunology, and Molecular Genetics and Microbiology. He is also a professor in the Program of Emerging Infectious Diseases at Duke–National University of Singapore Graduate Medical School, Singapore. His research focuses on host–pathogen interactions in the urinary tract, as well as mast cell-mediated immune responses to infectious agents.
http://pathology.duke.edu/experimental-pathology/research-profiles/soman-abraham-phd
Yuxuan Miao received his B.S. from the Beijing Institute of Technology, Beijing, China. He recently received his Ph.D. in the Molecular Genetics and Microbiology programme at Duke University, North Carolina, USA, under the mentorship of Soman Abraham. His studies focus on cell-autonomous and innate immune responses to urinary tract infections.
Footnotes
Competing interests statement: The authors declare no competing interests
References
- 1.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grist M, Chakraborty J. Identification of a mucin layer in the urinary bladder. Urology. 1994;44:26–33. doi: 10.1016/s0090-4295(94)80005-7. [DOI] [PubMed] [Google Scholar]
- 3.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(Suppl. 1A):14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer AJ. Recurrent urinary tract infections in women. Pathogenesis and management. Postgrad Med. 1987;81:51–58. doi: 10.1080/00325481.1987.11699724. [DOI] [PubMed] [Google Scholar]
- 6.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 7.Nicolle LE. Urinary tract pathogens in complicated infection and in elderly individuals. J Infect Dis. 2001;183(Suppl. 1):S5–S8. doi: 10.1086/318844. [DOI] [PubMed] [Google Scholar]
- 8.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. This is a comprehensive review describing the immune system in the kidney. [DOI] [PubMed] [Google Scholar]
- 9.Spencer JD, Schwaderer AL, Becknell B, Watson J, Hains DS. The innate immune response during urinary tract infection and pyelonephritis. Pediatr Nephrol. 2014;29:1139–1149. doi: 10.1007/s00467-013-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 11.Ingersoll MA, Albert ML. From infection to immunotherapy: host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 2013;6:1041–1053. doi: 10.1038/mi.2013.72. [DOI] [PubMed] [Google Scholar]
- 12.Ragnarsdottir B, Lutay N, Gronberg-Hernandez J, Koves B, Svanborg C. Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. 2011;8:449–468. doi: 10.1038/nrurol.2011.100. This review provides valuable information on the genetics of UTI susceptibility and also describes virulence factors of UPEC and complementary innate signalling events that occur in the urinary tract following UTI. [DOI] [PubMed] [Google Scholar]
- 13.Song J, et al. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagamatsu K, et al. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci USA. 2015;112:E871–E880. doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agace WW, Hedges SR, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates JM, et al. Tamm–Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 17.Mo L, et al. Ablation of the Tamm–Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–F802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 18.Saemann MD, et al. Tamm–Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest. 2005;115:468–475. doi: 10.1172/JCI22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flo TH, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 20.Goetz DH, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 21.Paragas N, et al. α-intercalated cells defend the urinary system from bacterial infection. J Clin Invest. 2014;124:2963–2976. doi: 10.1172/JCI71630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chromek M, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 23.Valore EV, et al. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. References 22 and 23 show the important role of secreted AMPs in the defence against UPEC infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer JD, et al. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011;80:174–180. doi: 10.1038/ki.2011.109. [DOI] [PubMed] [Google Scholar]
- 25.Danka ES, Hunstad DA. Cathelicidin augments epithelial receptivity and pathogenesis in experimental Escherichia coli cystitis. J Infect Dis. 2015;211:1164–1173. doi: 10.1093/infdis/jiu577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaillon S, et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Bishop BL, et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 28.Song J, et al. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci USA. 2009;106:14966–14971. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161:1306–1319. doi: 10.1016/j.cell.2015.05.009. References 27–29 investigate the molecular aspects of the powerful exocytic activities of BECs following invasion of UPEC. This activity seems to be a component of the cell-autonomous defence system and is an effective strategy to reduce intracellular bacterial load. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. This study describes how superficial BECs reduce bacterial load following UPEC infection by spontaneous exfoliation into the urine. [DOI] [PubMed] [Google Scholar]
- 31.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin K, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. References 31 and 32 reveal the existence of a highly efficient programme within the bladder epithelium to rapidly restore its barrier function after shedding of the superficial epithelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haraoka M, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 34.Agace WW, Patarroyo M, Svensson M, Carlemalm E, Svanborg C. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule-1-dependent mechanism. Infect Immun. 1995;63:4054–4062. doi: 10.1128/iai.63.10.4054-4062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahin RD, Engberg I, Hagberg L, Svanborg Eden C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local Gram-negative infection. J Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 36.Hannan TJ, et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine. 2014;1:46–57. doi: 10.1016/j.ebiom.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel DR, et al. CCR2 mediates homeostatic and inflammatory release of Gr1high monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J Immunol. 2008;181:5579–5586. doi: 10.4049/jimmunol.181.8.5579. [DOI] [PubMed] [Google Scholar]
- 38.Duell BL, Carey AJ, Dando SJ, Schembri MA, Ulett GC. Human bladder uroepithelial cells synergize with monocytes to promote IL-10 synthesis and other cytokine responses to uropathogenic Escherichia coli. PLoS ONE. 2013;8:e78013. doi: 10.1371/journal.pone.0078013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symington JW, et al. ATG16L1 deficiency in macrophages drives clearance of uropathogenic E. coli in an IL-1β dependent manner. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.7. http://dx.doi.org/10.1038/mi.2015.7. [DOI] [PMC free article] [PubMed]
- 40.Schiwon M, et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156:456–468. doi: 10.1016/j.cell.2014.01.006. This paper describes the crosstalk between different subsets of macrophages within the bladder mucosa that governs the recruitment and precise onset of neutrophil responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 42.Shelburne CP, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham S, Shin J, Malaviya R. Type 1 fimbriated Escherichia coli-mast cell interactions in cystitis. J Infect Dis. 2001;183(Suppl. 1):S51–S55. doi: 10.1086/318853. [DOI] [PubMed] [Google Scholar]
- 45.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. This study describes the homeostatic role of mast cells in terminating pro-inflammatory responses presumably after the bladder epithelial barrier is disrupted. However, this premature termination of inflammation also negatively affects the development of memory responses to the uropathogen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gur C, et al. Natural killer cell-mediated host defense against uropathogenic E. coli is counteracted by bacterial hemolysinA-dependent killing of NK cells. Cell Host Microbe. 2013;14:664–674. doi: 10.1016/j.chom.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engel D, et al. Tumor necrosis factor α- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones-Carson J, Balish E, Uehling DT. Susceptibility of immunodeficient gene-knockout mice to urinary tract infection. J Urol. 1999;161:338–341. [PubMed] [Google Scholar]
- 49.Sivick KE, Schaller MA, Smith SN, Mobley HL. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J Immunol. 2010;184:2065–2075. doi: 10.4049/jimmunol.0902386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deckmann K, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci USA. 2014;111:8287–8292. doi: 10.1073/pnas.1402436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ratner JJ, Thomas VL, Sanford BA, Forland M. Bacteria-specific antibody in the urine of patients with acute pyelonephritis and cystitis. J Infect Dis. 1981;143:404–412. doi: 10.1093/infdis/143.3.404. [DOI] [PubMed] [Google Scholar]
- 52.Wei Y, et al. Activation of endogenous anti-inflammatory mediator cyclic AMP attenuates acute pyelonephritis in mice induced by uropathogenic Escherichia coli. Am J Pathol. 2014;185:472–484. doi: 10.1016/j.ajpath.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langermann S, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 54.Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines. 2012;11:663–676. doi: 10.1586/erv.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLachlan JB, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 56.Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HL. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thankavel K, et al. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Invest. 1997;100:1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luthje P, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med. 2013;5:190ra80. doi: 10.1126/scitranslmed.3005574. [DOI] [PubMed] [Google Scholar]
- 59.Chassin C, et al. Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J Exp Med. 2007;204:2837–2852. doi: 10.1084/jem.20071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlager TA, Hendley JO, Wilson RA, Simon V, Whittam TS. Correlation of periurethral bacterial flora with bacteriuria and urinary tract infection in children with neurogenic bladder receiving intermittent catheterization. Clin Infect Dis. 1999;28:346–350. doi: 10.1086/515134. [DOI] [PubMed] [Google Scholar]
- 61.Bollgren I, Winberg J. The periurethral aerobic bacterial flora in healthy boys and girls. Acta Paediatr Scand. 1976;65:74–80. doi: 10.1111/j.1651-2227.1976.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 62.Reid G. Probiotic agents to protect the urogenital tract against infection. Am J Clin Nutr. 2001;73:437S–443S. doi: 10.1093/ajcn/73.2.437s. [DOI] [PubMed] [Google Scholar]
- 63.Abraham SN, et al. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun. 1985;48:625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrieres L, Hancock V, Klemm P. Biofilm exclusion of uropathogenic bacteria by selected asymptomatic bacteriuria Escherichia coli strains. Microbiology. 2007;153:1711–1719. doi: 10.1099/mic.0.2006/004721-0. [DOI] [PubMed] [Google Scholar]
- 65.Lutay N, et al. Bacterial control of host gene expression through RNA polymerase II. J Clin Invest. 2013;123:2366–2379. doi: 10.1172/JCI66451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nielubowicz GR, Mobley HL. Host–pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 68.Ulett GC, et al. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol. 2013;16:100–107. doi: 10.1016/j.mib.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Henderson JP, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009;5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta K, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]