Abstract
Pine wilt disease (PWD) caused by the pine wood nematode (PWN), Bursaphelenchus xylophilus, is responsible for devastating epidemics in pine trees in Asia and Europe. Recent studies showed that bacteria carried by the PWN might be involved in PWD. However, the molecular mechanism of the interaction between bacteria and the PWN remained unclear. Now that the whole genome of B. xylophilus (Bursaphelenchus xylophilus) is published, transcriptome analysis is a unique method to study the role played by bacteria in PWN. In this study, the transcriptome of aseptic B. xylophilus, B. xylophilus treated with endobacterium (Stenotrophomonas maltophilia NSPmBx03) and fungus B. xylophilus were sequenced. We found that 61 genes were up-regulated and 830 were down-regulated in B. xylophilus after treatment with the endobacterium; 178 genes were up-regulated and 1122 were down-regulated in fungus B. xylophilus compared with aseptic B. xylophilus. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses were used to study the significantly changed biological functions and pathways for these differentially expressed genes. Many pathogenesis-related genes, including glutathinone S-transferase, pectate lyase, ATP-binding cassette transporter and cytochrome P450, were up-regulated after B. xylophilus were treated with the endobacterium. In addition, we found that bacteria enhanced the virulence of PWN. These findings indicate that endobacteria might play an important role in the development and virulence of PWN and will improve our understanding of the regulatory mechanisms involved in the interaction between bacteria and the PWN.
Keywords: transcriptome, differentially expressed genes, bacteria, pine, Bursaphelenchus xylophilus
1. Introduction
Pine wilt disease (PWD) is a worldwide forest disease affecting several species of pine trees (Pinus spp.), and it has been epidemic in Asia (Japan, China, and Korea) and in Portugal [1,2,3,4]. In these countries, PWD has killed a large number of pine trees and caused serious economic losses and ecological damages [5]. The pine wood nematode (PWN), Bursaphelenchus xylophilus [6], is considered the causal pathogenic agent of PWD and is designated an important quarantine species [7]. The PWN invades healthy pines via sawyer beetles (Monochamus spp.), particularly Monochamus alternatus during feeding or oviposition [8,9]. After PWNs invade the trees, they move inside the host rapidly via cambium, axial and radical parenchyma, and feeding upon living tissues, which ultimately results in complete wilting of pine trees.
Recent studies reported that the PWN carries diverse bacterial communities and bacteria may play a crucial role in PWD development [10,11,12,13]. Some bacteria isolated from B. xylophilus could produce toxins, which were suggested to cause pine tree wilting [10,14]. Several studies revealed that bacteria associated with B. xylophilus can also secrete a cellulose degradation enzyme, which is extremely important for PWN colonization [15,16]. The main bacterial genera associated with PWN are Bacillus, Brevibacteria, Burkholderia, Pseudomonas, Serratia, and Stenotrophomonas [17,18,19,20]. The PWN-associated bacteria are primarily on the body surface of nematodes. Additionally, PWNs from different countries and regions carry distinct genera of bacteria; the nematode could acquire and carry random bacteria from soil, pines, and vectors [17,18,19,20]. Many details of the relationship between bacteria and PWNs are still uncertain due to the complexity of the PWN surface coat.
Wu et al. [13] isolated 15 species of endobacteria from ten different strains of the PWN from Anhui, Jiangsu, Zhejiang, Guangdong, Yunnan, and Hubei Provinces, China. The dominant genera of endobacteria isolated from PWNs were Stenotrophomonas, Achromobacter, Ewingella, Leifsonia, Rhizobium, and Pseudomonas [13]. A recent study showed that endobacteria might play a role in the development and virulence of the PWN, i.e., Achromobacter xylosoxidans ss. xylosoxidans NSBx.22 from PWN AA3 with low virulence could slow the nematode growth on Botrytis cinerea and enhanced the virulence of PWN AA3 after pine seedlings were infected [21]. It was also found that the community structure of endobacteria from B. xylophilus was very similar among high virulence B. xylophilus, low virulence B. xylophilus, and B. mucronatus [22]. Xiang et al. [23] found that Stenotrophomonas, Pseudomonadaceae (unclassified) or Rhizobiaceae (unclassified) were predominant in the bacteria communities associated with virulent B. xylophilus and non-virulent B. mucronatus. It would be beneficial to study the mechanism of interaction between endobacteria and the PWN and the effect of bacteria on gene expression of B. xylophilus.
The plant cell wall comprises mainly pectin, cellulose, and hemicellulose, which are the primary barriers to pathogens and parasites [24]. Researchers cloned cellulose and pectin lyase genes from the PWN, and speculated that these hydrolase genes were closely related to pathogenicity of the PWN [25]. Pine trees generate numerous secondary metabolites to combat invasion by the PWN. Therefore, degrading these secondary metabolites has become an important factor in successful infection of the host by the PWN. The mechanism of the bacteria assisting PWNs to parasitize hosts has become a research focus for understanding the role of bacteria in PWD. RNA sequencing (RNA-seq) technology provides an effective method to investigate the expression level of messenger RNA (mRNA) under different ecological conditions and at different developmental stages. Daurelioet et al. [26] found that 316 transcript-derived fragments showed differential expression in tobacco after infection with Xanthomonas axonopodis pv. citri by transcriptome analysis. Kikuchi et al. [27] first published the whole genome of B. xylophilus. These previous results provided a good basis for analyzing the transcriptome of the PWN.
The objective of the present study is to elucidate the effects of bacteria on expression of genes related to the pathogenicity of the PWN using transcriptome analysis. These results will help us to further understand the nature of the relationship between bacteria and the PWN at the molecular level.
2. Results
2.1. Mapping of RNA-Seq Reads in the Genome of B. xylophilus
mRNA was sequenced from aseptic PWNs, PWNs treated with endobacterium (S. maltophilia NSPmBx03), and fungus PWN using paired end protocols on an Illumina HiSeqTM2000 system (Shenzhen, China). A total of 143,978,136 reads, 12.96 Gbp, and 17,286 genes were obtained from clean reads of three different treatments (Table S1). Approximately, 75.2% and 72.1% of our reads were mapped to the reference genome and PWN genes, respectively. Over 70% of our reads were uniquely mapped reads.
2.2. Differentially Expressed Genes in Transcriptome Sequence of PWNs Treated with Endobacterium and Fungus PWNs
Differentially expressed genes (DEGs) between aseptic PWNs and PWNs treated with S. maltophilia NSPmBx03 were examined. Compared to aseptic PWNs, a total of 891 significant DEGs were identified with at least a two-fold change at the expression level and false discovery rate (FDR) <0.001. Of these genes, 61 were up-regulated and 830 down-regulated. A total of 1300 genes were identified as DEGs between aseptic PWNs and fungus PWNs. Of these genes, 178 were up-regulated and 1122 down-regulated. The quantity of DEGs in PWNs treated with one endobacterial strain was less than that in fungus PWNs. The number of DEGs between PWNs treated with endobacterium and fungus PWNs was 625, which was less than that between aseptic PWNs and fungus PWNs (Figure S1). These results implied that bacteria affected expression of some genes of the PWN.
2.3. Functional Annotation and Classification for DEGs of the PWN
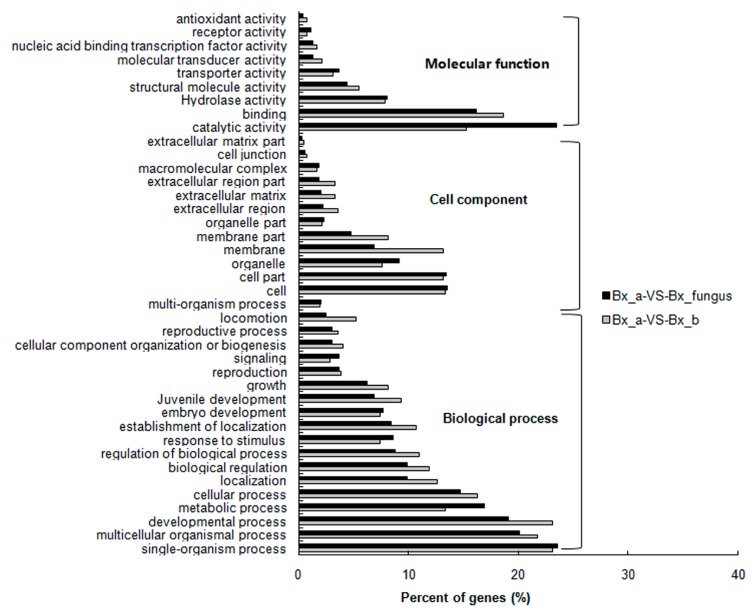
Gene Ontology (GO) analysis was used to search for significantly enriched GO terms of DEGs. The main GO categories included molecular function, cellular component, and biological process. Compared to aseptic PWNs, the “catalytic activity” (GO:0003824), “binding” (GO:0005488), “hydrolase activity” (GO:0016817), “structural molecule activity“ (GO:0005198), and “transporter activity” (GO:0005215) categories were among the top molecular function terms in both PWNs treated with NSPmBx03 and fungus PWNs (Figure 1). In the cell component category, most DEGs were involved in the “cell” (GO:0005623), “cell part” (GO:0044464), “organelle” (GO:0043266), “membrane” (GO:0016020), and “membrane part” (GO:0044425). In addition, “single-organism process” (GO:0044699), “developmental process” (GO:0003006), “multicellular organismal process” (GO:0032501), and “metabolic process” (GO:0008152) were the top categories enriched in the biological process. In general, the aforementioned GO terms accounted for the majority of the DEGs. Furthermore, some GO terms play an important role in PWN development, such as embryo development, juvenile development, regulation of growth rate and hydrolase activity, in response to external stimuli.
Figure 1.
Gene Ontology (GO) terms for the transcriptome sequences derived from differently treated B. xylophilus (p < 0.05). Gray bars indicate significantly enriched GO terms in endobacterium-treated PWN samples (Bx_b) compared to aseptic PWNs (Bx_a); black bars indicated significantly enriched GO terms in fungus PWNs (Bx_fungus) compared to aseptic PWNs (Bx_a).
2.4. Analysis of Cell Wall Degradation-Related Genes
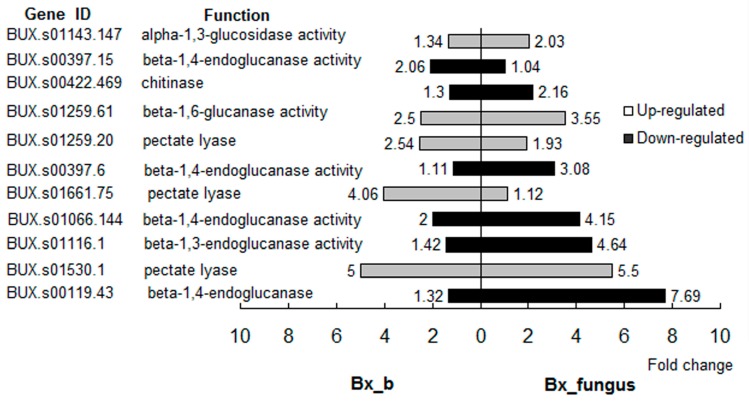
Enzymes of cell wall degradation are intrinsically related to nematode pathogenicity in host pines [25]. Compared with aseptic PWNs, the expression levels of six cell wall degradation-related genes changed significantly (over two-fold) in PWNs treated with S. maltophilia NSPmBx03. Of these DEGs, three pectate lyase genes (BUX.s01259.20, BUX.s01661.75, and BUX.s01530.1) and one cellulase gene (BUX.s01259.61) were up-regulated over two-fold (Figure 2). In fungus PWNs, eight cell wall degradation-related genes were DEGs (over two-fold). The expression of BUX.s01530.1 was up-regulated 5.5-fold. However, expression levels of cellulase genes were significantly down-regulated (Figure 2). These results indicated that endobacteria could affect the expression levels of cell wall degradation-related genes of PWNs and these genes changed much more evidently in fungus PWNs.
Figure 2.
Putative cell wall hydrolase genes in B. xylophilus treated with endobacterium and fungus B. xylophilus (more than two-fold change in B. xylophilus treated with endobacterium or wild-type B. xylophilus). Bx_b: B. xylophilus treated with endobacterium, Bx_fungus: fungus B. xylophilus.
2.5. Detoxification-Related Gene Analysis
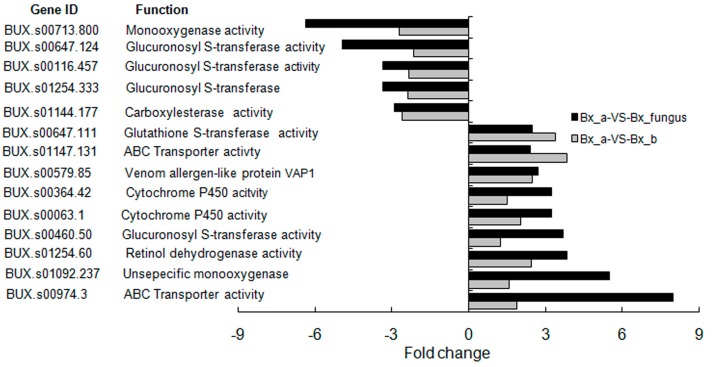
The capacity for detoxification is a key factor in PWN colonization of pines [27]. Compared with aseptic PWNs, the expression level of several detoxification-related genes changed significantly in PWNs treated with S. maltophilia NSPmBx03 and fungus PWNs, and their functions included glutathinone S-transferase (GST) activity, ATP-binding cassette (ABC) transporter, venom allergen-like protein VAP1, rethinol dehydrogenase, cytochrome P450 (CYP), and carboxylesterase (Figure 3). GST and CYP genes were primary DEGs related to detoxification. These results suggested that the endobacterium and other bacteria genera have an important role in regulation of detoxification-related gene expression, especially CYP and GST. The expression level of BUX.s01147.131 (ABC transporter) and BUX.s00647.111 (GST) were up-regulated over three-fold in PWNs treated with NSPmBx03. The expression of BUX.s00974.3 (ABC transporter) and BUX.s01092.237 (CYP) were up-regulated 8-fold and 5.5-fold in wild-type PWNs, respectively. In addition, the expression levels of BUX.s01147.131 and BUX.s00647.111 were more greatly up-regulated in PWN treated with NSPmBx03 than in fungus PWNs. These results indicated that the bacteria had an important function in the expression of these detoxification-related genes of PWN.
Figure 3.
Putative detoxification-related genes most differentially expressed, with higher levels in B. xylophilus treated with S. maltophilia NSPmBx03 and fungus B. xylophilus. Bx_a: aseptic B. xylophilus, Bx_b: B. xylophilus treated with S. maltophilia NSPmBx03, Bx_fungus: fungus B. xylophilus. The left of the vertical axis indicates down-regulated genes, and the right indicates up-regulated genes.
2.6. Reproduction-Related Gene Analysis
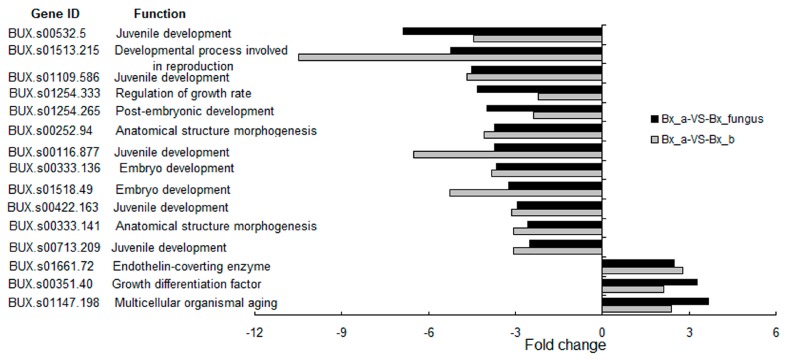
A number of reproduction-related genes were differentially expressed in PWNs treated with S. maltophilia NSPmBx03 and fungus PWNs compared with aseptic PWNs (Figure 4). The functions of reproduction-related genes were involved with juvenile development, embryo development, anatomical structure morphogenesis, endothelin-converting enzyme, growth differentiation factor, developmental process involved in reproduction, regulation of growth rate, and multicellular organismal aging. Most juvenile and embryo development-related genes were down-regulated. The expression level of BUX.s01513.215 (developmental process involved in reproduction) and BUX.s00116.877 (juvenile development) were down-regulated over six-fold in PWNs treated with NSPmBx03, and the expression of BUX.s00532.5 (juvenile development) was down-regulated over six-fold in fungus PWNs. These results suggested that PWN-associated bacteria could slow the growth of PWN.
Figure 4.
Putative reproduction-related genes in B. xylophilus treated with endobacterium and fungus B. xylophilus (fold-change > 2). Bx_a: aseptic B. xylophilus, Bx_b: B. xylophilus treated with S. maltophilia NSPmBx03, Bx_fungus: fungus B. xylophilus. The left of the vertical axis indicates down-regulated genes, and the right indicates up-regulated genes.
2.7. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
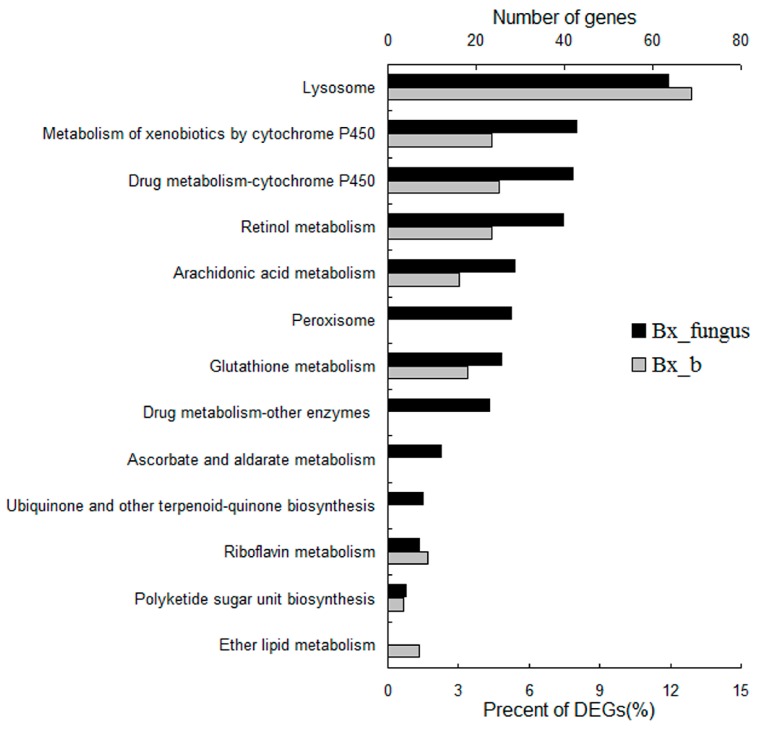
The pathway enrichment for DEGs was performed using KEGG. From the transcriptome of PWNs treated with S. maltophilia NSPmBx03, 75 DEGs were involved in detoxification pathways containing drug metabolism cytochrome P450, metabolism of xenobiotics by cytochrome P450, lysosome, and glutathione metabolism (p < 0.05) (Figure 5). Twenty-eight genes were enriched in turpentine-related metabolism including ether lipid metabolism, arachidonic acid metabolism, polyketide sugar unit biosynthesis, and retinol metabolism. These metabolic pathways may be important for the PWN to parasitize pines. In fungus PWNs, 238 genes (28.4%) were enriched in detoxification pathways containing lysosome, metabolism of xenobiotics by cytochrome P450, drug metabolism cytochrome P450, peroxisome, glutathione metabolism, drug metabolism-other enzymes, ascorbate and aldarate metabolism. Fifty-nine genes were involved in turpentine-related metabolism containing arachidonic acid metabolism, rethinol metabolism, ubiquinone and other terpenoid-quinone biosynthesis, and riboflavin metabolism. Amino acid metabolism and fat metabolism were the prominent pathways in both PWNs treated with NSPmBx03 and fungus PWNs. The results indicated that the endobacterium and other bacteria could affect detoxification-related metabolism. The bacteria of fungus PWNs affected gene expression to a greater extent than S. maltophilia NSPmBx03. In addition, endobacteria and other bacteria may help nutrition metabolism of PWNs.
Figure 5.
Relative pathways significantly enriched in differentially expressed genes (DEGs) of PWNs treated with endobacterium and fungus PWNs (p < 0.005). DEGs were enriched in detoxification pathways and turpentine-related metabolism pathway. Bx_b: B. xylophilus treated with S. maltophilia NSPmBx03, Bx_fungus: fungus B. xylophilus.
2.8. Validation of DEGs by Real-Time Quantitative PCR
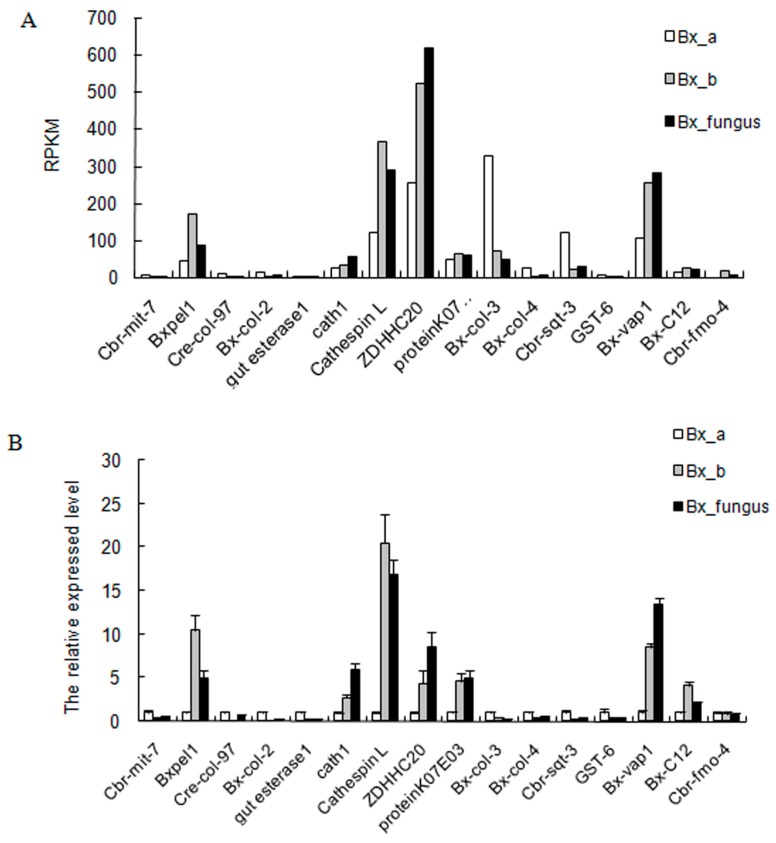
To validate the transcriptome results, 16 genes were selected at random from the DEGs and analyzed with qRT-PCR. We designed specific primers for seven up-regulated genes (Bxpel2, cath 1, cathespin L, ZDHHC20, protein KO7, Bx-vap1, and Bx-C12) and nine down-regulated genes (Cbr-mit-7, Cre-col-97, Bx-col-2, gut esterase1, Bx-col-3, Bx-col-4, Cbr-sqt-3, GST-6, and Cbr-fmo-4). The expression tendency of the 16 candidate genes for qRT-PCR was similar to expression tendency observed by RNA-seq, demonstrating reliability of transcriptome data (Figure 6).
Figure 6.
Relative gene expression. (A) Expression levels of 16 genes in sequencing results. RPKM, reads per kilobase per million reads; (B) qRT-PCR results of 16 differentially expressed genes under sterile, endobacterium treatment, and fungus conditions. White bars indicate expression levels of the genes in control samples (Bx_a); gray bars indicate expression levels of genes in endobacterium-treated PWN samples (Bx_b); black bars indicate expression levels of genes in fungus PWN samples (Bx_fungus). Data represent the means ± SD (n = 3). SD, standard deviations.
2.9. Symptoms of Pines Inoculated with PWNs
Pines inoculated with different PWNs showed differences in symptoms. The time of initial symptoms of fungus PWNs and PWNs treated with bacteria appeared 13 days after inoculation and 17 days for aseptic PWNs. After 21 days of inoculation, pines inoculated with fungus PWNs had an infection rate of 100% and a disease severity index (DSI) of 83.3, while S. maltophilia NSPmBx03 treatment infection rate was 66.7% and DSI was 58.3; aseptic PWN treatment infection rate was 33.3% and DSI was 33.3 (Table 1). After 26 days of inoculation, infection rates of all pines with different treated PWNs were 100%, but aseptic PWNs had the lowest DSI. After 30 days of inoculation, only one pine inoculated with aseptic PWNs was not completely wilted. After 35 days of inoculation, all pine needles were brown and wilting. Pines inoculated with S. maltophilia NSPmBx03 and sterile water remained healthy (Figure 7). These results indicated that the S. maltophilia NSPmBx03 enhanced the virulence of PWNs.
Table 1.
Symptoms of P. massoniana caused by aseptic B. xylophilus, B. xylophilus treated with S. maltophilia NSPmBx03, and fungus B. xylophilus. Bx_a: aseptic B. xylophilus, Bx_b: B. xylophilus treated with S. maltophilia NSPmBx03, Bx_fungus: fungus B. xylophilus.
| Time of Inoculation (d) | Bx_a | Bx_b | Bx_fungus | |||
|---|---|---|---|---|---|---|
| Infection Rate (%) | Disease Severity Index | Infection Rate (%) | Disease Severity Index | Infection Rate (%) | Disease Severity Index | |
| 13 | 0 | 0 | 33.3 | 25 | 66.7 | 50 |
| 21 | 33.3 | 33.3 | 66.7 | 58.3 | 100 | 83.3 |
| 26 | 100 | 83.3 | 100 | 91.7 | 100 | 100 |
| 30 | 100 | 91. 7 | 100 | 100 | 100 | 100 |
Figure 7.
Symptoms of P. massoniana inoculated with different PWN treatments. (A) Symptoms after 0 day of inoculation; (B) Symptoms after 21 days; (C) Symptoms after 26 days; (D) Symptoms after 30 days. Pines inoculated with: L1, fungus B. xylophilus; L2, B. xylophilus treated with S. maltophilia NS PmBx 03; L3, aseptic B. xylophilus; L4, S. maltophilia NSPmBx 03; and L5, sterile water.
3. Discussion
Symbiotic relationships between nematodes and bacteria are common in nature. Recent studies showed that bacteria carried by the PWN were likely to play a role in PWD [18,21,28]. Zhang et al. [29] reported that Stenotrophomonas was the most dominant group in the PWN. Wu et al. [13] isolated several endobacterial strains from PWNs, and S. maltophilia was a dominant species. The specific and functional diversities of endobacteria from various isolates of B. xylophilus have been studied [13]. Tian et al. [21] reported that endobacteria S. maltophilia NSBx.14 could decrease the reproduction of ZL1 in fungus-conditions. However, the molecular mechanisms between endobacteria and the PWN are poorly understood. In this study, we sequenced the transcriptome of B. xylophilus treated with S. maltophilia NSPmBx03 for the first time and found that NSPmBx03 affected the gene expression level of B. xylophilus (Table S3).
Pectinases are the primary cell-degrading enzymes of the PWN secretome, intrinsically related to nematode pathogenicity in host pines [30,31]. Pectin is the most complex component of the pine cell wall polysaccharides, and plays a critical role in resisting invasion by PWN [32]. To invade the host successfully, PWN needs to break down this barrier. Therefore, pectinases are essential to allow PWN to infect its plant host. Qiu et al. found that pectin lyase genes of PWNs were highly expressed when PWNs infected P. thunbergii [33]. From the secretion of the PWN esophageal gland, DeBoer et al. [34] cloned Bxpel1 and Bxpel2, which enhanced the ability of PWNs to feed and migrate in the host pine. Our results showed that the endobacterium S. maltophilia NSPmBx03 affected expression of genes of carbohydrate active enzymes in the PWN. The expression of the pectate lyase gene BUX.s01530.1 was up-regulated 5.5-fold in fungus PWNs and 5-fold in PWN treated with endobacteria compared to control treatment (Figure 2). After P. massoniana was inoculated with aseptic PWNs, PWNs treated with endobacterium, and fungus PWNs, respectively, we found that the fastest group lead to pine trees wilting was fungus PWNs, followed by PWNs treated with endobacterium, and the last was the pines inoculated with aseptic PWNs. As previously reported, the bacteria species associated with B. xylophilus AmA3 were Stenotrophomonas, Rhizobiaceae (unclassified), Chitinophaga, Oxalobacteraceae (unclassified), and Ochrobactrum [23]. These results indicated that S. maltophilia NSPmBx03 and some other bacteria associated with the PWN could affect pectate lyase gene expression, which may play an important role in parasitizing pines.
Phytotoxins such as benzoic acid, 8-hydroxycarbotanacetone, and 10-hydroxyverbenone were identified and characterized from PWN-infested pines [35]. These toxic substances could defend against invasion and decrease the reproduction and migration rate of the PWN in host pines [36]. Futai et al. [37] found that tannic acid significantly accumulated in parenchymal cells of P. thunbergii after PWN invasion. Additionally, PWN inhabits the resin canals of host pines [38] and the resin is a complex mixture of compounds, including terpenoids and cyclic aromatic compounds [39]. These compounds are likely to have nematocidal activity. PWNs must detoxify these toxic compounds in order to invade the pines successfully. Our results showed that abundant derived enzymes were assigned to xenobiotic metabolism pathways in the transcriptome of PWNs treated with NSPmBx03 and fungus PWN. The major pathways were drug metabolism CYP, metabolism of xenobiotics by CYP and glutathione metabolism (Figure 5). CYP and GST were the primary DEGs of detoxification (Figure 3). These results were consistent with those of Yan et al., who reported that CYP and GST pathways were the primary detoxification metabolic pathways in PWN [40]. The detoxification process of PWN has been divided into three successive phases [27]. First, CYPs are the most important group of the first phase proteins, making molecules more suitable substrates for downstream. Second, the actual detoxification reaction occurs with GSTs and UDP-glucuronosyl transferases (UGTs) as essential enzymes. In the third phase, an ABC transporter is responsible for the efflux of detoxification molecules. Vicente et al. [41] reported that some PWN-associated bacteria could increase nematode survival under strong oxidative stress. Xu et al. [42] identified the function of CYP genes (BxCYP33C9, BxCYP33C4, and BxCYP3D3) in B. xylophilus, and revealed that these genes may affect their viability, proliferation, pesticide metabolism and pathogenicity. In our study, a number of detoxification-related genes, including GST, ABC transporter, venom allergen-like protein VAP1, retinol dehydrogenase, and CYP, were greatly up-regulated in the PWNs treated with S. maltophilia NSPmBx03 (Figure 3). These results indicated that NSPmBx03 played an important role in the xenobiotic detoxification of PWN, which may help PWN cope with a difficult environment.
A recent study showed that Pseudomonas spp. could promote the reproduction of B. xylophilus [43]. However, some other bacteria, such as Pantoea sp., Serratia marcescens, Buttiauxella agrestis and Enterobacter amnigenus had an inhibitory effect [43]. Tian et al. [21] reported that endobacteria isolated from highly virulent PWN could partially promote the development of nematodes, while endobacteria isolated from lowly virulent PWN may retard the growth of nematodes. Additionally, S. maltophilia NSBx.14, obtained from the highly virulent ZL1 strain of B. xylophilus, can prolong the development time of nematode embryos [22]. We found that the expression of juvenile and embryo development-related genes in PWNs treated with S. maltophilia NSPmBx03 and fungus PWNs were significantly down-regulated (Figure 4). As in previous results, we found that aseptic PWN had a higher reproduction rate than PWN treated with bacteria on B. cinerea, but completely opposite results were obtained for P. massoniana [44]. Cheng et al. [45] reported that Stenotrophomonas was the most common genera of symbiotic bacteria with PWN, and may play an important role in regulating ecological function. Qiu et al. [33] found that the expression levels of the pectate lyase, CYP, UGT, and ABC transporter genes, and reproduction-related genes of PWN associated with bacteria were up-regulated dramatically by growth on P. thunbergii compared with growth on B. cinerea. Therefore, we speculated that the effect of bacteria on PWN differed in fungus-conditions and parasitic conditions. In fungus-conditions, bacteria might be detrimental to nematodes to some extent. In parasitic conditions, the high-level expression of cell wall degradation-related and detoxification-related genes could help the PWN successfully parasitize pines and grow better in the host. These results will help us to perceive the underlying nature of the relationship between pine wood nematode and bacteria. However, the role of endobacteria in PWD requires further experimental verification.
4. Materials and Methods
4.1. The PWN and Its Endobacteria
PWNs (AmA3) were isolated from infected P. massoniana in Maanshan, Anhui, China [46]. An endobacterium S. maltophilia NSPmBx03 was initially isolated from inside the body of PWNs extracted from infected P. massoniana in Nanjing, Jiangsu, China. This bacterium was frequently isolated from PWN strains in our previous study [13]. The strains of the PWN and the endobacterium were provided by the Jiangsu Key Laboratory for Prevention and Management of Invasive Species.
4.2. Preparation of Bacteria-Free PWNs and Treatment of Aseptic PWNs with Endobacterium
A suspension of 6000 PWNs was used to inoculate six-year-old P. massoniana in the field (Nanjing Forestry University, Nanjing, China) [33]. The PWNs were isolated from dead P. massoniana using a Baermann funnel under aseptic conditions. Then, the PWNs were pipetted into a potato dextrose agar plate (6-cm diameter) containing B. cinerea. After growth at 25 °C for 7 days, the nematodes were collected in 10-mL centrifuge tubes and treated as fungus PWN (Bx_fungus).
The above-mentioned suspension (1 mL) of PWNs was poured onto a sterilized cover slip in a Petri dish and incubated at 25 °C for depositing eggs during 4–6 h, and the nematodes discarded after egg deposition [47]. The nematode eggs were collected and soaked in 15% H2O2 for 60 min at 25 °C. The eggs were rinsed three times with sterile water and placed on mycelia of B. cinerea grown on PDA medium at 25 °C in darkness. The hatched nematodes were collected in 10-mL centrifuge tubes under aseptic conditions and treated as aseptic PWNs (Bx_a). Aseptic PWNs was the control treatment.
The endobacterial strain of S. maltophilia NSPmBx03 maintained on slant nutrient agar medium in tubes was transferred to 50 mL of beef extract-peptone liquid medium and cultured on a shaker for 24 h. The concentration of S. maltophilia NSPmBx03 was adjusted to 8.0 × 108 CFU (Colony-Forming Units) and 200 µL of suspension was sprayed onto PDA plates containing B. cinerea [21]. Each plate was inoculated with 1000 bacteria-free nematodes (AmA3) and incubated for 7 days at 25 °C. Then PWNs were collected into 10-mL centrifuge tubes with a Baermann funnel under aseptic conditions. The nematodes (Bx_b) were washed three times with sterile water.
4.3. RNA Extraction, cDNA Preparation and Illumina Sequencing
Nine samples (each treatment had three replicates) were immediately frozen in liquid nitrogen and stored at −75 °C until use. Total RNA was respectively extracted from frozen nematode samples (Bx_a, Bx_b, and Bx_fungus), using a RNAprep Kit (Tiangen, Beijing, China) and purified using a RNAclean Kit (Tiangen) according to the manufacturer’s instructions. RNA integrity and quantity were determined with an Agilent 2100 Bioanalyzer (Agilent Technologies, Redwood City, CA, USA) before cDNA synthesis. One of the three RNA samples from each treatment PWN was used for RNA-seq. The mRNA was isolated from total RNA by Oligo (dT) magnetic beads. Isolated mRNA strands were fragmented and used as templates to synthesize cDNA using a cDNA Synthesis SystemKit (Roche Applied Science, Mannheim, Germany) [48]. The short fragments that were purified and resolved with EB buffer were subjected to end repair and addition of single nucleotide A (adenine). After that, the short fragments were connected with adapters. The qualified fragments of three cDNA samples were respectively selected for PCR amplification as templates. The quality of cDNA libraries was determined with an Agilent 2100 Bioanalyzer and ABI StepOnePlus Real-Time PCR System (Life Technologies, Grand Island, NY, USA). Each cDNA library was sequenced in a single lane of the Illumina HiSeqTM 2000 system using paired-end protocols according to the manufacturer’s instructions at BeiJing Genomics Institute (Shenzhen, China). The RNA-Seq experiments have three technical replicates. The raw data were deposited in the NCBI Short Read Archive (SAR) and are accessible through SAR accession numbers: SRR2336946, SRR2342509, and SRR2342510.
To obtain clean reads, adapter, reads in which unknown bases were more than 10%, low quality reads were removed from raw reads using the SeqPrep program (https://github.com/jstjohn/SeqPrep).
4.4. Read Mapping and DEG Analysis
The clean reads of three PWN samples with different treatment methods were mapped to the genome of B. xylophilus (http://www.ncbi.nlm.nih.gov/genome) using SOAP aligner/SOAP2 [49], allowing for maximum mismatch of 5 bp. Gene expression levels of three PWN samples were calculated by reads per kilobase transcriptome per million mapped reads (RPKM) method [50]. DEGs were defined as those with changes of at least two-fold between samples (Bx_a vs. Bx_b, and Bx_a vs. Bx_fungus) and at FDR ≤ 0.001 [51,52], excluding genes where the RPKM was 0 in either sample. Cluster analysis of gene expression patterns was conducted with cluster [53] and Java Treeview [54] software.
Finally, the DEGs were analyzed for enrichment of GO and KEGG pathway terms. All DEGs were mapped to GO terms in the database (http://www.geneontology.org). Then, gene numbers in every term were calculated, and hypergeometric test was used to detect the notably enriched GO terms [55]. The calculated p-value was subject to Bonferroni correction [56]. Set the corrected p-value ≤0.05 as a threshold. GO terms meeting this threshold were selected as notably enriched in DEGs. KEGG (www.genome.jp/kegg/) was used to perform metabolic pathways or signal transduction pathways enrichment analysis of DEGs. Significantly enriched pathways were identified in DEGs by comparing with the whole genome background. The method of calculation was used as that in GO analysis.
4.5. qRT-PCR Validation
To verify the RNA-seq results, nine extracted RNA samples of different treatment PWNs were used for qRT-PCR analysis. The first-strand cDNA was synthesized with Prime Script 1st strand cDNA synthesis Kit (TaKaRa, Shiga, Japan), and the cDNA samples were diluted to 20 ng/µL. Sixteen gene-specific primers (Table S2) were designed using Primer Premier 5.0 software. The Actin gene of B.xylophilus was used as internal control: Actin forward primer 5′-GCAACACGGAGTTCGTTGTA-3′ and reverse primer 5′-GTATCGTCACCAACTGGGAT-3′. The qRT-PCR was performed using the ABI 7500 system (Applied Biosystems, Waltham, MA, USA), with a 20-µL final reaction volume, which contained 2 µL of template, 10 µL of SYBR Premix Ex Tap, 0.4 µL of ROX Reference Dye II, 0.4 µL of forward primer, 0.4 µL of reverse primer, and 6.8 µL of ddH2O. The PCR amplification was performed as follows: 40 cycles of 95 °C for 30 s, and 60 °C for 34 s. The relative expressed levels in selected genes of three PWN samples were evaluated using the relative quantification method (2−ΔΔCt) [57]. These experiments were biological (n = 3) and technical (n = 3) repeated.
4.6. Observation of P. massoniana Symptoms for Different Treatments of PWNs
The experiment was conducted in a greenhouse. All P. massoniana (2 years old) were disinfected by spraying with 75% ethyl alcohol. After that, 0.5 mL of suspension (3000 nematodes) of different treatments of PWNs (Bx_a, Bx_b, and Bx_fungus) were respectively pipetted into cutting wounds (0.5 cm in length) in P. massoniana seedlings at about 20 cm above the soil level. Sterile water was used as control. The wounds on P. massoniana were sealed by Parafilm. Each treatment contained three replicates. The inoculated seedlings were placed in the greenhouse. PWD symptoms were evaluated and categorized as 0–4 [58]. The categories were as follows: 0 = all needles were green; 1 = 0%–25% needles discolored and turned yellow; 2 = 25%–50% needles turned yellow; 3 = 50%–75% needles turned yellow; and 4 = 75%–100% needles turned yellow. The infection rates and DSI were calculated with the formulae as follow:
5. Conclusions
The results demonstrated that the endobacterium S. maltophilia NSPmBx03 could affect expression levels of genes related to cell wall degradation, detoxification, and reproduction genes in PWNs and enhance the virulence of nematodes. This suggests that S. maltophilia NSPmBx03 played an important role in the gene expression of PWN. These results provide insights into the regulatory mechanisms involved in the interaction between nematodes and bacteria, which will facilitate a better understanding of the role of bacteria in PWD.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31270683) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are grateful to De-Wei Li, Connecticut Agricultural Experiment Station, USA and Yi Xu for reviewing the manuscript.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/6/778/s1.
Author Contributions
Long-Xi He and Xiao-Qin Wu conceived and designed the experiments; Long-Xi He and Qi Xue performed the experiments; Long-Xi He and Xiu-Wen Qiu analyzed the data; Long-Xi He and Xiao-Qin Wu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mamiya Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983;21:201–220. doi: 10.1146/annurev.py.21.090183.001221. [DOI] [PubMed] [Google Scholar]
- 2.Ceng H.R., Lin M.S., Ni W.Q., Fang Z.D. First report of pine wilt disease from Pinus thunbergii Parl in Nanjing. For. Pest Dis. 1983;4:1–5. [Google Scholar]
- 3.Mota M.M., Braasch H., Bravo M.A., Penas A.C., Burgermeister W., Metge K., Sousa E. First report of Bursaphelenchu sxylophilus in Portugal and in Europe. Nematology. 1999;1:727–734. doi: 10.1163/156854199508757. [DOI] [Google Scholar]
- 4.Yi C.K., Byun B., Park J., Yang S., Chang K. First finding of the pinewood nematode, Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle and its insect vector in Korea. Res. Rep. For. Res. Inst. 1989;38:141–149. [Google Scholar]
- 5.Zhao B.G., Futai K., Sutherland J.R., Takeuchi Y. Pine Wilt Disease. Springer; Tokyo, Japan: 2008. [Google Scholar]
- 6.Steiner G., Buhrer E.M. Aphelenchoides xylophilus n. sp., a nematode associated with blue-stain and other fungi in timber. J. Agric. Res. 1934;48:949–951. [Google Scholar]
- 7.Kiyohara T., Tokushige Y. Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J. Jpn. For. Sci. 1971;53:210–218. [Google Scholar]
- 8.Mamiya Y., Enda N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternates (Coleoptera: Cerambycidae) Nematologica. 1972;18:159–162. doi: 10.1163/187529272X00395. [DOI] [Google Scholar]
- 9.Morimoto K., Iwasaki A. Role of Monochamus alternates (Coleoptera: Cerambycidae) as a vector of Bursaphelenchusv lignicolus (Nematoda: Aphelenchoididae) J. Jpn. For. Soc. 1972;54:177–183. [Google Scholar]
- 10.Oku H., Shiraishi T., Kurozumi S. Pine wilt toxin, the metabolite of a bacterium associated with a nematode. Naturwissenschaften. 1980;67:198–199. doi: 10.1007/BF01086307. [DOI] [Google Scholar]
- 11.Han Z.M., Hong Y.D., Zhao B.G. A study on pathogenicity of bacteria carried by pine wood nematode. J. Phytopathol. 2003;151:683–689. doi: 10.1046/j.1439-0434.2003.00790.x. [DOI] [Google Scholar]
- 12.Zhao B.G., Lin F. Mutualistic symbiosis between Bursaphelenchus xylophilus and bacteria of the genus Pseudomonas. For. Pathol. 2005;35:339–345. doi: 10.1111/j.1439-0329.2005.00417.x. [DOI] [Google Scholar]
- 13.Wu X.Q., Yuan W.M., Tian X.J., Fan B., Fang X., Ye J.R., Ding X.L. Specific and functional diversity of endophytic bacteria from pine wood nematode Bursaphelenchus xylophilus with different virulence. Int. J. Biol. Sci. 2013;9:34–44. doi: 10.7150/ijbs.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawazu K., Yamishita H., Kanzaki H. Isolation of pine-wilting bacteria accompanying pinewood nematode, Bursaphelenchus xylophilus and their toxic metabolites. Sci. Rep. Fac. Agric. 1998;87:1–7. [Google Scholar]
- 15.Niu H., Zhao L., Lu M., Zhang S., Sun J. The ratio and concentration of two monoterpenes mediate fecundity of the pine wood nematode and growth of its associated fungi. PLoS ONE. 2012;7:778. doi: 10.1371/journal.pone.0031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicente C.S., Nascimento F., Espada M., Barbosa P., Mota M., Glick B.R., Oliveira S. Characterization of bacteria associated with pine wood nematode Bursaphelenchus xylophilus. PLoS ONE. 2012;7:778. doi: 10.1371/journal.pone.0046661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawazu K., Zhang H., Kanzaki H. Accumulation of benzoic acid in suspension cultured cells of Pinus thunbergii Parl. in response to phenylacetic acid administration. Biosci. Biotechnol. Biochem. 1996;60:1410–1412. doi: 10.1271/bbb.60.1410. [DOI] [PubMed] [Google Scholar]
- 18.Zhao B.G., Wang H.L., Han S.F., Han Z.M. Distribution and pathogenicity of bacteria species carried by Bursaphelenchus xylophilus in China. Nematology. 2003;5:899–906. doi: 10.1163/156854103773040817. [DOI] [Google Scholar]
- 19.Kwon H., Choi G., Choi Y., Jang K., Sung N., Kang M.S., Moon Y., Lee S.K., Kim J.C. Suppression of pine wilt disease by an antibacterial agent, oxolinic acid. Pest. Manag. Sci. 2010;66:634–639. doi: 10.1002/ps.1920. [DOI] [PubMed] [Google Scholar]
- 20.Proenca D.N., Francisco R., Santos C.V., Lopes A., Fonseca L., Abrantes I.M., Morais P.V. Diversity of bacteria associated with Bursaphelenchus xylophilus and other nematodes isolated from Pinus pinaster trees with pine wilt disease. PLoS ONE. 2010;5:778. doi: 10.1371/journal.pone.0015191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian X.J., Wu X.Q., Xiang Y., Fang X., Ye J.R. The effect of endobacteria on the development and virulence of the pine wood nematode, Bursaphelenchus xylophilus. Nematology. 2015;45:581–589. doi: 10.1163/15685411-00002892. [DOI] [Google Scholar]
- 22.Tian X.J. Master Thesis. Nanjing Forestry University; Nanjing, China: May 8, 2012. Realationship between Endophytic Bacteria of Bursaphlenchus xylophilus and B. mucronatus and Their Host. [Google Scholar]
- 23.Xiang Y., Wu X.Q., Zhou A.D. Bacterial diversity and community structure in the pine wood nematode Bursaphelenchus xylophilus and B. mucronatus with different virulence by high- throughput sequencing of the 16SrDNA. PLoS ONE. 2015;10:778. doi: 10.1371/journal.pone.0137386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert H.J. The biochemistry and structural biology of plant cell wall deconstruction. J. Plant Physiol. 2010;153:444–455. doi: 10.1104/pp.110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi T., Jones J.T., Aikawa T., Kosaka H., Ogura N. A family of glycosyl hydrolase family 45 cellulases from the pine wood nematode Bursaphelenchus xylophilus. FEBS Lett. 2004;572:201–205. doi: 10.1016/j.febslet.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Daurelio L.D., Petrocelli S., Blanco F., Holuigue L., Ottado J., Orellano E.G. Transcriptome analysisi reveals novel genes involved in nonhost response to bacterial infection in tobacco. J. Plant Physiol. 2011;168:382–391. doi: 10.1016/j.jplph.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi T., Cotton J.A., Dalzell J.J., Hasegawa K., Kanzaki N., McVeigh P., Takanashi T., Tsai I.J., Assefa S.A., Cock P.J.A., et al. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011;7:778. doi: 10.1371/journal.ppat.1002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proença D.N., Fonseca L., Powers T.O., Abrantes I.M.O., Morais P.V. Diversity of 495 bacteria carried by pine wood nematode in USA and phylogenetic comparison with isolates from other countries. PLoS ONE. 2014;9:778. doi: 10.1371/journal.pone.0105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q.L., Tian X.L., Tan Z.J., Chen G.H., Xie B.Y. Construction and analysis of the metagenomicfosmid library for the bacteria carried by the pine wood nematode. Acta Phytopathol. Sin. 2010;40:381–387. [Google Scholar]
- 30.Taisei K., Hajime S. Molecular and biochemical characterization of an endo-β-1,3-glucanase from the pine wood nematode Bursaphelenchus xylophilus acquired by horizontal gene transfer from bacteria. Biochem. J. Immed. Publ. 2005;23:1–40. doi: 10.1042/BJ20042042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi T., Shibuya H., Aikawa T., Jones J.T. Cloning and characterization of pectate lyases expressed in the esophageal gland of the pine wood nematode Bursaphelenchus xylophilus. Mol. Plant Microbe Interact. 2006;19:280–287. doi: 10.1094/MPMI-19-0280. [DOI] [PubMed] [Google Scholar]
- 32.Cheng X.Y., Dai S.M., Xiao L., Xie B.Y. Influence of cellulase gene knock down by dsRNA interference on the development and reproduction of the pine wood nematode, Bursaphelenchus xylophilus. Nematology. 2010;12:225–233. doi: 10.1163/138855409X12469541205044. [DOI] [Google Scholar]
- 33.Qiu X.W., Wu X.Q., Huang L., Tian M.Q., Ye J.R. Specifically expressed genes of the nematode Bursaphelenchus xylophilus involved with early interactions with pine trees. PLoS ONE. 2013;8:778. doi: 10.1371/journal.pone.0078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBoer J.M., Davis E.L., Hussey L.H., Popeijus H.E., Smant G., Baum T.J. Cloning of a putive pectate lyase gene expressed in the subventral esophagealglands of Heterodera glycines. Nematology. 2002;34:9–11. [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwazu K. Pathogenic toxin of in pine wood disease. Kagaku Sebutsu. 1998;36:120–124. [Google Scholar]
- 36.Futai K. Abnormal metabolites in pine wood nematode–inoculated Japanese black pine. Nematology. 2003;33:45–56. doi: 10.3725/jjn1993.33.2_45. [DOI] [Google Scholar]
- 37.Fukuda K. Physiological process of the symptom development and resistance mechanism in pine wilt disease. J. For. Res. 1997;2:171–181. doi: 10.1007/BF02348216. [DOI] [Google Scholar]
- 38.Ikeda T., Kiyohara T. Water relations, xylem embolism and histological features of Pinus thunbergii inoculated with virulent or avirulent pine wood nematode Bursaphelenchus xylophilus. J. Exp. Bot. 1995;46:441–449. doi: 10.1093/jxb/46.4.441. [DOI] [Google Scholar]
- 39.Kuroda K. Mechanism of cavitation development in the pine wilt disease. Eur. J. For. Pathol. 1991;121:82–89. doi: 10.1111/j.1439-0329.1991.tb00947.x. [DOI] [Google Scholar]
- 40.Yan X., Cheng X.Y., Wang Y.S., Luo J., Mao Z.C., Ferris V.R., Xie B.Y. Comparative transcriptomics of two pathogenic pine wood nematodes yields insights into parasitic adaptation to life on pine hosts. Gene. 2012;505:81–90. doi: 10.1016/j.gene.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 41.Vicente C.S.L., Ikuyo Y., Mota M., Hasegawa K. Pine wood nematode-associated bacteria contribute to oxidative stress resistance of Bursaphelenchus xylophilus. BMC Microbiol. 2013;13:778. doi: 10.1186/1471-2180-13-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X.L., Wu X.Q., Ye J.R., Huang L. Molecular characterization and functional analysis of three pathogenesis-related cytochrome P450 genes from Bursaphelenchus xylophilus (Tylenchida: Aphelenchoidoidea) Int. J. Mol. Sci. 2015;16:5216–5234. doi: 10.3390/ijms16035216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B.G., Liu Y., Lin F. Effects of bacteria associated with pine wood nematode (Bursaphelenchus xylophilus) on development and egg production of the nematode. J. Phytopathol. 2007;155:26–30. doi: 10.1111/j.1439-0434.2006.01188.x. [DOI] [Google Scholar]
- 44.He L.X., Xue Q., Wu X.Q. The effect of endobacteria on reproduction and virulence of Bursaphelenchus xylophilus. J. Nanjing For. Univ. 2016;40:47–51. [Google Scholar]
- 45.Cheng X.Y., Tian X.L., Wang Y.S., Lin R.M., Mao Z.C., Chen N.S., Xie B.Y. Metagenomic analysis of the pine wood nematode microbiome reveals a symbiotic relationship critical for xenobiotics degradation. Sci. Rep. 2013;3:1869. doi: 10.1038/srep01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang R.E. Doctoral Dissertation. Nanjing Forestry University; Nanjing, China: Jul 10, 2007. Studies on Aphelenchida from Pine Wood Infested with Pine Wood Nematode, Bursaphelenchus xylophilus. [Google Scholar]
- 47.Zhu L.H., Ye J.R., Negi S., Xu X.L., Wang Z.L., Ji J.Y. Pathogenicity of aseptic Bursaphelenchus xylophilus. PLoS ONE. 2012;7:778. doi: 10.1371/journal.pone.0038095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z.Q., Zhao M.Z., Li X.G., Lu Q.P., Li Y.H., Ge J.C., Pan J.L. Transcriptome profiling of the eyestalk of precocious juvenile Chinese mitten crab reveals putative neuro peptides and differentially expressed genes. Gene. 2014;569:280–286. doi: 10.1016/j.gene.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 49.Li R., Yu C., Li Y., Lam T.W., Yiu S.M., Kristiansen K. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- 50.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 51.Roy M., Kim N., Kim K., Chung W.H., Achawanantakun R., Sun Y.N., Wayne R. Analysis of the canine brain transcriptome with an emphasis on the hypothalamus and cerebral cortex. Mamm. Genome. 2013;24:484–499. doi: 10.1007/s00335-013-9480-0. [DOI] [PubMed] [Google Scholar]
- 52.Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29:1165–1188. [Google Scholar]
- 53.Eisen M.B., Spellman P.T., Brown P.O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 2001;29:1165–1188. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saldanha A.J. Java Treeview-extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 55.Reimand J., Arak T., Vilo J. g:Profiler-a web server for functional interpretation of gene lists (2011 update) Nucleic Acids Res. 2011;39:W307–W315. doi: 10.1093/nar/gkr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdi H. Bonferroni and Sidak corrections for multiple comparisons. In: Salkind N., editor. Encyclopedia of Measurement and Statistics. Sage; Thousand Oaks, CA, USA: 2007. [Google Scholar]
- 57.Mota M.M., Revel A.T., Talaat A.M., Norgard M.V. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu L.Z., Wu X.Q., Ye J.R., Zhang S.N., Wang C. NOS-like-mediated nitric oxide is involved in Pinus thunbergii response to the invasion of Bursaphelenchus xylophilus. Plant Cell Rep. 2012;31:1813–1821. doi: 10.1007/s00299-012-1294-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.