Abstract
miR-126 has recently been implicated in modulating angiogenic factors in vascular development. Understandings its biological significance might enable development of therapeutic interventions for diseases like age-related macular degeneration (AMD). We aimed to determine the role of miR-126 in AMD using a laser-induced choroidal neovascularization (CNV) mouse model. CNV was induced by laser photocoagulation in C57BL/6 mice. The CNV mice were transfected with scrambled miR or miR-126 mimic. The expression of miR-126, vascular endothelial growth factor-A (VEGF-A), Kinase insert domain receptor (KDR) and Sprouty-related EVH1 domain-containing protein 1 (SPRED-1) in ocular tissues were analyzed by qPCR and Western blot. The overexpression effects of miR-126 were also proven on human microvascular endothelial cells (HMECs). miR-126 showed a significant decrease in CNV mice (p < 0.05). Both mRNA and protein levels of VEGF-A, KDR and SPRED-1 were upregulated with CNV; these changes were ameliorated by restoration of miR-126 (p < 0.05). CNV was reduced after miR-126 transfection. Transfection of miR-126 reduced the HMECs 2D-capillary-like tube formation (p < 0.01) and migration (p < 0.01). miR-126 has been shown to be a negative modulator of angiogenesis in the eye. All together these results high lights the therapeutic potential of miR-126 suggests that it may contribute as a putative therapeutic target for AMD in humans.
Keywords: miR-126, age-related macular degeneration, choroidal neovascularization, human microvascular endothelial cell (HMEC)
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in developed countries, affecting more than 50 million people over the age of 60 worldwide [1,2]. AMD can be categorized into two types: “dry type” or atrophic and “wet type” or neovascular. The “wet” form of AMD is associated with severe vision loss involving the progressive growth of abnormal, permeable blood vessels from the choroid into the subretinal space, known as Characterized by choroidal neovascularization (CNV) [3,4]. Excessive growth and secondary hemorrhage of these immature blood vessels lead to deterioration of the macula resident photoreceptors and if left untreated, permanent loss of vision can occur [5,6].
Organisms require an appropriate balance of stability and reversibility in gene transcription and translation to maintain cell identity or to enable responses to stimuli. This process is tightly regulated by proteinous and nonproteinous complexes such that, even slight dysregulation of this network results in the occurrence of several human diseases [7]. Ocular neovascularization is a very complicated pathophysiologic process, which is tightly regulated by the balance between the angiogenic stimulators such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF) and angiogenic inhibitors such as pigment epithelium derived factor (PEDF) [8]. CNV formation associated with “wet” AMD is the outcome of dysregulation of this finely tuned angiogenesis process [9]. The stimuli that lead to the pathogenesis of AMD are believed to be multifactorial, involving an intricate correlation between genetic, environmental, metabolic and inflammatory factors [6]. Anti-VEGF treatment has been used widely in treating neovascular AMD. However, the administration of anti-VEGF therapy has led to some severe side effects such as stroke [10]. Moreover, VEGF inhibitors have shown to be ineffective in a subset of AMD patients [11]. The opportunity exists for the development of new therapies.
miRNAs are short non-coding RNA molecules that consist of about 22 nucleotides. They play a vital role in regulatory mechanisms of complex physiological and pathological processes by post-transcriptionally modulating gene expression, generally by translational repression or degradation of mRNA [12,13]. Since their discovery, miRNAs have been implicated in the pathophysiological processes of various diseases such as cancer and cardiovascular diseases [14,15]. Recent studies have revealed that a number of miRNAs were involved in the process of angiogenesis [16,17,18], and several miRNAs have also been shown to be distinctly involved in new vascular development in the retina [19,20,21]. miR-31, miR-150 and miR-184 have shown to be downregulated in oxygen-induced retinopathy mice models [20]; miR-23~24~27 cluster was upregulated in laser induced CNV mice models [21]. These studies suggest a potential involvement of miRNAs in the development and progression of wet AMD [6,20], and further examination into the role of miRNAs may provide insight into potential treatments for this disease.
Among these miRNAs, miR-126 is a likely candidate for involvement in pathogenic neovascularization. miR-126 is an EC-specific miRNA encoded in the intron of EGF-like domain 7 and has been shown to be involved in tumor neovascularization [22,23]. VEGF plays a pivotal role in modulating endothelial cell function, such as blood vessel formation during embryonic development. Some studies indicated that miR-126 regulates tumor angiogenesis by targeting VEGF-A [24]. In endothelial cells, signaling through VEGF-receptor 2 (VEGFR2) promotes cell survival [25]. Kinase insert domain receptor (KDR), which encodes VEGFR2, also plays an important role to promote angiogenesis. Recently, Sprouty/Spred family proteins were identified as negative regulators for growth factor- and cytokine-induced ERK activation in angiogenesis [26,27,28,29]. In one study, miR-126 was found to regulate angiogenic signaling and vascular integrity by targeting Sprouty-related EVH1 doain-containing protein 1 (SPRED-1) and phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2) [19]. In ocular neovascular diseases, endothelial cells respond to various proteins and growth factors that regulate angiogenesis [9]. However, there have been contradicting reports on the roles of miR-126. Fish et al. and Wang et al. proved that miR-126 enhanced angiogenesis [19,30], while the decreased expression has been found in ischemia-induced retina [29,30]. The intention of this study was to validate the results from previous studies and investigate the role of miR-126 associated vascularization pathways in the laser-induced CNV mouse model, a well-established model which closely mimics the pathogenesis of AMD in human beings. The potential of miR-126 to be used as a therapy was also investigated by restoring miR-126 in vitro and in vivo.
2. Results
2.1. Expression of miR-126 in Laser-Induced Choroidal Neovascularization (CNV) Mouse Model
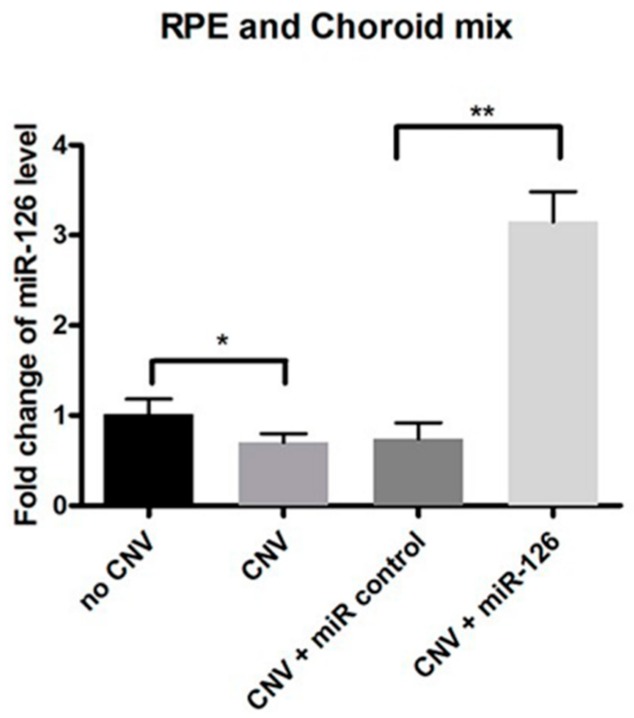
Fourteen days post transfection, the expression of miR-126 in mice eyes was measured by qPCR (RPE/choroid mix n = 12). miR-126 was significantly downregulated (p < 0.05) in the CNV eyes compared with the untreated eyes (Figure 1). CNV mice were transfected using a transfection agent coupled with either a miR-126 mimic or a negative control oligonucleotide (miR-control). Successful miR-126 transfection was confirmed using PCR analysis (n = 12, p < 0.01) after injection with miR-126 mimic (Figure 1). We demonstrated that the miR-126 remains effective even after 14 days of in vivo transfection.
Figure 1.
miR-126 expression in choroidal neovascularization (CNV) mice. Fourteen days after transfection, the expression of miR-126 in CNV mice eyes in the following treatment groups: (1) No laser-induced CNV (no CNV): Control mice without treatment; (2) CNV: Mice exposed to CNV laser treatment only, without transfection; (3) CNV + miR-control: Mice exposed to CNV laser treatment and transfected with negative control through an intravitreal injection; and (4) CNV + miR-126: Mice exposed to CNV laser treatment and transfected with miR-126 mimic through an intravitreal injection. Data are presented as relative fold change of miR-126 level ± standard deviation (SD), n = 12, * p < 0.05, ** p < 0.01.
2.2. Changes of miR-126 Target Genes in the Eyes of CNV Mice
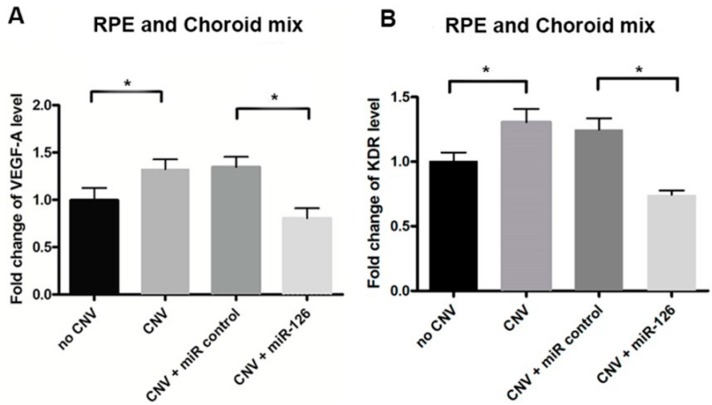
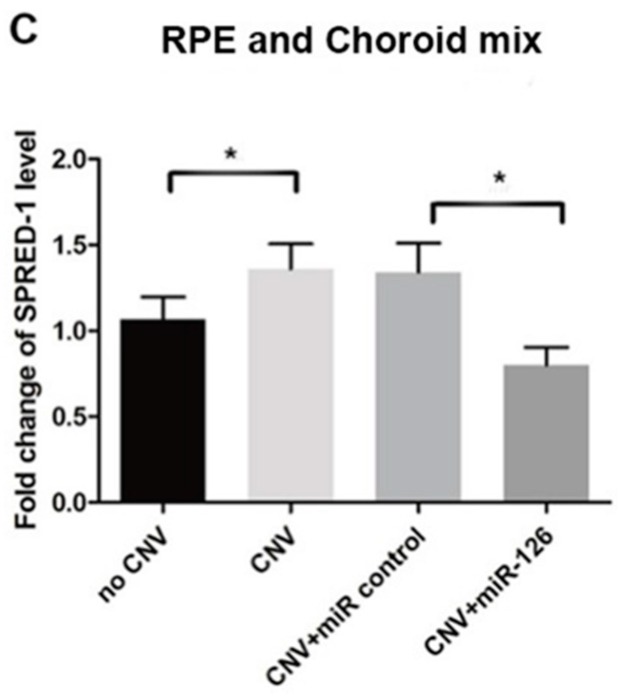
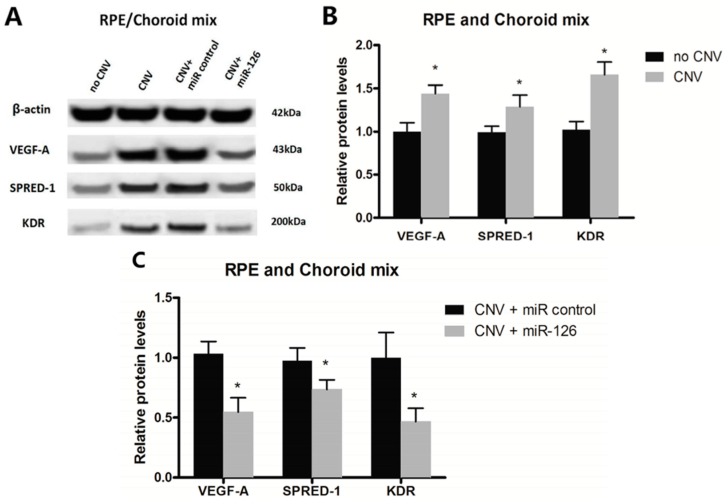
The downstream effect of reduced expression of miR-126 was assessed by qPCR and Western blot analysis. As shown, VEGF-A, KDR and SPRED-1 mRNA expression were upregulated in the CNV mice eyes compared with untreated controls (n = 12, p < 0.05, p < 0.01) (Figure 2). Similarly, an increase in these targets protein levels was observed in the CNV mice compared with untreated ones. To further explore the effects of miR-126 in vivo, the expression of miR-126 was altered in the CNV mice to determine if restoration of miRNA expression would result in modulation in the expression of predicted mRNA and protein targets. CNV mice with overexpressed miR-126 exhibited a significant decrease in mRNA and protein expression of VEGF-A, KDR and SPRED-1 (n = 12, p < 0.05), measured by qPCR and Western blot, respectively (Figure 2 and Figure 3). A clear functional impact of miR-126 regulated targets on CNV severity in vivo has been demonstrated.
Figure 2.
mRNA expression of miR-126 target genes in the eyes of CNV mice. VEGF-A, KDR and SPRED-1 mRNA expression in the eye tissue of control mice either transfected with a miR-control or a miR-126 mimic through an intravitreal injection: (A) relative VEGF-A mRNA expression; (B) relative KDR mRNA expression; and (C) relative SPRED-1 mRNA expression. Data are presented as mean fold change of mRNA levels ± SD, n = 12, * p < 0.01.
Figure 3.
Changes of miR-126 target genes protein level in the eyes of CNV mice: (A) Western blot expression pattern of VEGF-A, KDR and SPRED-1 in different groups of mice with or without CNV, and CNV mice transfected with miR-control or miR-126; (B) quantification of relative target genes protein expression in no CNV and CNV groups; and (C) relative expression of target genes protein in a different group of CNV mice after transfection. Data are expressed as relative fold change of protein level ± SD, n = 12, * p < 0.05.
2.3. Restoration of miR-126 Levels Reversed High mRNA and Protein Expression of Target Genes in Human Microvascular Endothelial Cell (HMEC)
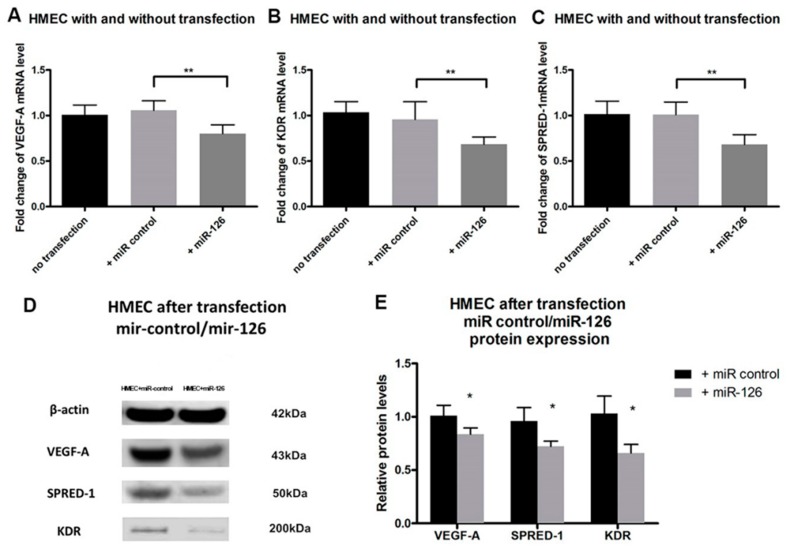
Overexpression of miR-126 in HMECs inhibited both mRNA and protein expression of VEGF-A, KDR and SPRED-1. Transfection of miR-126 in HMECs (Figure 4) resulted in decreased expression of VEGF-A and KDR mRNA (n = 4, p < 0.01) (Figure 5A,B), and SPRED-1 mRNA expression was also downregulated (n = 4, p < 0.01) (Figure 5C). The expression of the respective proteins was also significantly reduced (n = 4, p < 0.05) (Figure 5D,E).
Figure 4.
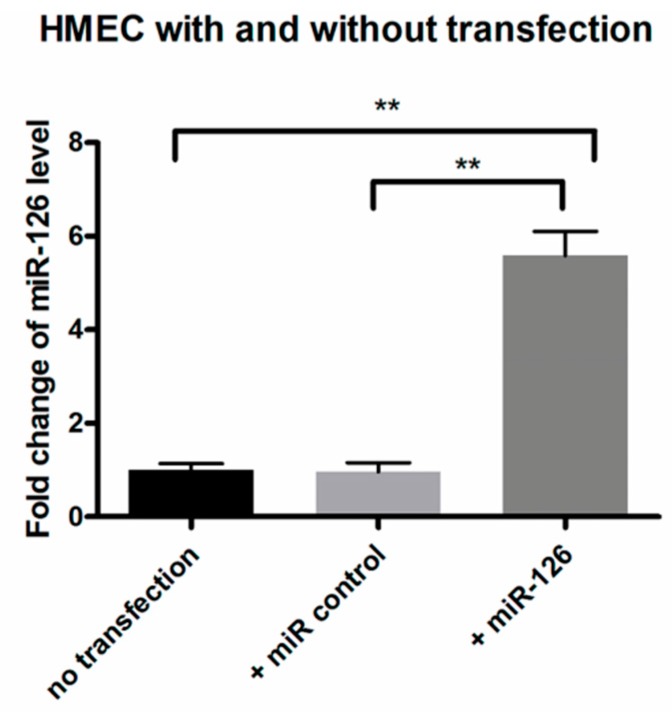
Expression of miR-126 in human microvascular endothelial cell (HMEC) after transfection. miR-126 expression in HMEC without transfection or transfected with either a negative control or miR-126. Data are expressed as relative fold change of miR-126 ± SD (n = 4), ** p < 0.01.
Figure 5.
miR-126 target genes expression in HMEC after transfection: (A) VEGF-A; (B) KDR; and (C) SPRED-1 mRNA expression in HMECs was determined by qPCR in the following treatment groups: No transfection: HMECs without transfection; +miR-control: HMECs transfected with miR-control; +miR-126: HMECs transfected with miR-126 mimic. Data are presented as mean fold change of mRNA levels ± SD (n = 4), ** p < 0.01; (D) Western blot images of miR-126 target genes protein in HMEC after transfection of either the negative control miR-126; and (E) Quantification of relative target genes protein expression in HMECs receiving miR-control or miR-126 transfection determined by Western blot. Data are presented as mean fold change of protein levels ± SD (n = 4), * p < 0.05.
2.4. Transfection of miR-126 Decreased Tube Formation in HMECs
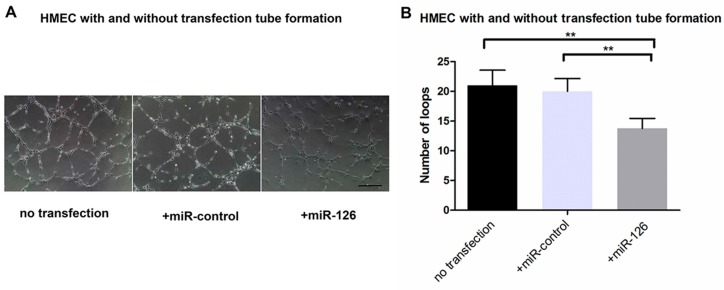
We performed tube formation after HMECs received scramble control miR or miR-126 mimic treatment. The number of loops in the tube formation assay showed a significant decrease (n = 4, p < 0.01) in the miR-126 transfected HMECs compared to both the nontransfected and miR-control groups (Figure 6A,B). It highlights that miR-126 can inhibit the formation of capillary tubes.
Figure 6.
HMECs tube formation after transfection: (A) representative images of HMEC tube formation, without transfection and after transfected with miR-control or miR-126 mimic; Scale bar = 20 μm and (B) analysis of capillary-like tube formation of HMECs in different groups with no transfection, transfected miR-control or miR-126 mimic. Data are present as mean number of loops formed ± SD (n = 4), ** p < 0.01.
2.5. Effect of miR-126 Overexpression on VEGF-Induced Migration of HMEC
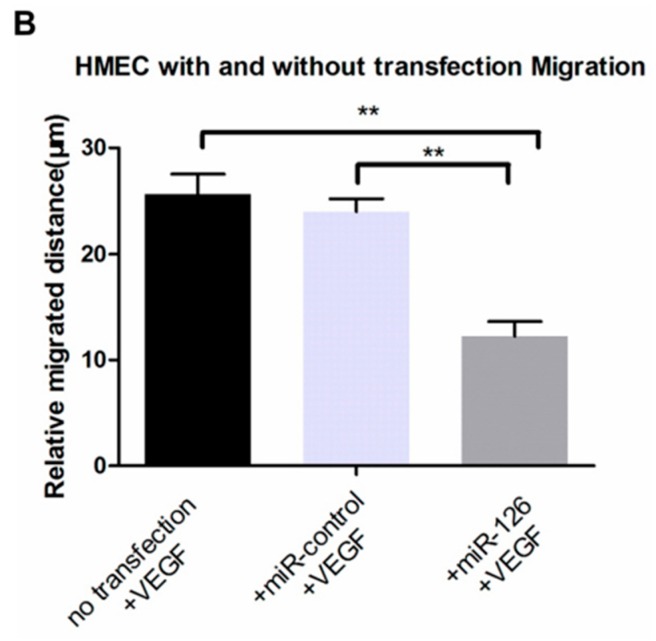
Migration of endothelial cells is an essential step in the process of blood vessel growth. In order to better clarify the role of miR-126 for HMEC migration, we used VEGF (50 ng/mL) as a stimulus. The distance of cells migration in scratch wound assay were significant decrease (n = 4, p < 0.01) in the miR-126 transfected HMECs compared to both the nontransfected and miR-control groups (Figure 7A,B).
Figure 7.
Inhibition of VEGF-induced cell migration by miR-126 overexpression. (A) Representative images of HMEC migration in different groups at t = 0 h and t = 9 h; Scale bar = 20 μm and (B) analysis of migrated distance of HMECs with no transfection, transfected miR-control or miR-126 mimic group. Data are present as mean migrated distance ± SD (n = 4), ** p < 0.01.
2.6. Restoration of miR-126 in Laser-Induced CNV Lesions in Mice Eyes
CNV lesion size and severity were determined to confirm the effect of the overexpression of miR-126 on the CNV mice. Figure 8 shows that representative composite fundus images taken 1–2 weeks post treatment. In Figure 9, CNV mice transfection with the negative control miR showed no changes in CNV lesion area, but the mice treated with miR-126 mimic had significantly smaller CNV lesion areas. The findings of this study demonstrated that miR-126 successfully reduced angiogenesis in the mice eyes.
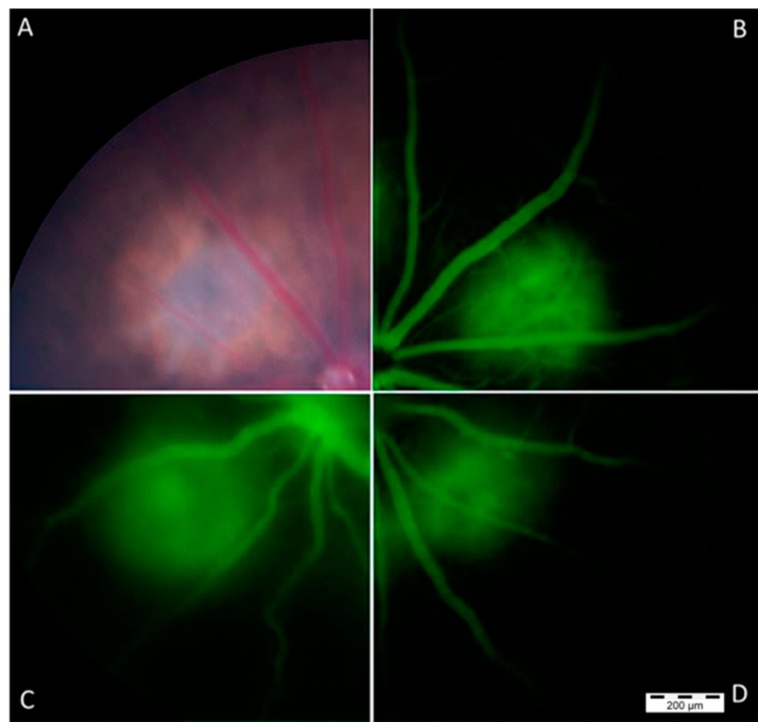
Figure 8.
Representative fundus images of the mouse retina were taken after laser treatment: (A) a representative color fundus image of the mouse after laser-induced CNV treatment; (B) fluorescein angiography (FFA) of CNV lesion of mouse receiving CNV generation only; (C) FFA of mouse receiving CNV-generating laser treatment and miR-control; and (D) FFA of mouse receiving CNV-generating laser treatment and miR-126 mimic. Scale bar = 200 μm.
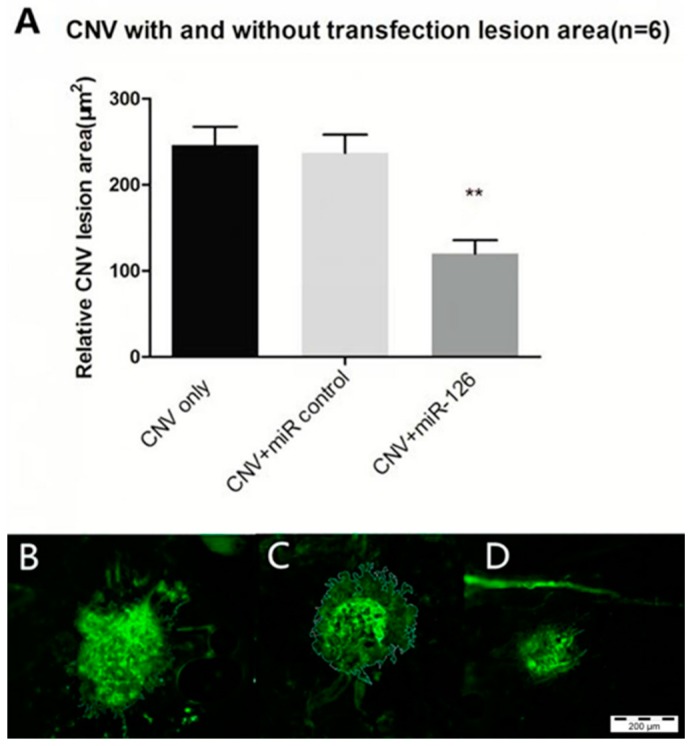
Figure 9.
Representative micrographs of the CNV lesions from choroidal flatmount in three groups: (A) Quantification of relative 2D lesion areas in the eyes of CNV mice after laser treatment in choroidal flatmount. Lesion areas were measured using the ImageJ software. Data are expressed as relative CNV lesion area ± SD, n = 6, ** p < 0.01; (B–D) Representative micrographs of CNV lesions of Isolectin GS-IB4 *AF488 stained choroidal flatmount, with manual border delineation in the ImageJ: (B) CNV-only (CNV mice without treatment); (C) CNV+ miR-control (CNV mice transfected with negative control); and (D) CNV+ miR-126 (CNV mice transfected with miR-126 mimic); Scale bar = 200 μm.
3. Discussion
The endothelial-specific microRNA miR-126 has been shown to play a role in regulating vascular integrity and angiogenesis as well as multiple disease processes [22,23]. However, the role of miR-126 in AMD still remains unknown at present.
In this study, we found that miR-126 was downregulated in the laser-induced CNV mice eyes. The decreased miR-126 expression is associated with the increased mRNA and protein levels of VEGF-A, KDR and SPRED-1. Restoration of miR-126 can reverse these increases in the CNV mouse model and inhibit CNV formation. HMECs transfected with miR-126 mimic also showed an inhibition in mRNA and proteins levels of VEGF-A, KDR and SPRED-1. Upregulation of miR-126 reduced endothelial cell tube formation and VEGF-induced migration. The results based on this study confirm the established knowledge of miR-126 involvement in angiogenesis and reveal a critical role for miR-126 in CNV.
Reported miR-126 levels in vascular pathogenesis were variable in the literature. Studies conducted by Fish et al. and Wang et al. showed that miR-126 normally promotes vessel formation and stability by “repressing the repressors” of VEGF signaling and that loss of miR-126 inhibited vascular growth in the embryonic stem cells and embryos of mice and zebrafish [19,31]. From those studies, it would appear that miR-126 enhanced angiogenesis. However, more recently, it has been found that miR-126 levels were decreased in ocular neovascularization diseases such as diabetic retinopathy or retinopathy of prematurity [32,33]. In our study, we found that miR-126 expression was substantially decreased in laser-induced CNV model—the well-established model mimicking the pathogenesis of neovascular AMD (Figure 1).
It is becoming evident that miRNAs expression and function are highly tissue-specific [34]. miRNAs regulate gene expression levels by several pre- and post-transcriptional mechanisms yielding diverse downstream effects in different cells based on their cellular environments and stages of life [15]. This may explain why there is such variability observed between studies utilizing different tissue samples, and disease models.
The different expression pattern of miR-126 in ocular neovascularization may be partly due to the anatomical characteristics of the eye. The physical barriers such as the blood–retinal barrier separate the ocular tissues from systemic circulation [35] and thus create a unique ocular microenvironment, which is important to protect the visual integrity. Ocular immune privilege allows for systemic immunological tolerance to antigens encountered into the eye and restricts the formation of potentially damaging immune responses. This may also restrict the influence of local factors and downstream effects of miRNA and explain the different expression of miR-126 in ocular diseases compared to other systemic disorders.
VEGF-A and its receptor KDR, are extensively involved in the stimulation of the MAPK/ERK cascade which is essential for cell proliferation and the growth of new blood vessels. In wet AMD patients, VEGF-A level is increased [36]. VEGF causes leaky, disorganized and uncontrolled growth of new blood vessels, which are prone to hemorrhaging and leakage of plasma into the subretinal space [37,38]. SPRED-1 has been known to inhibit the phosphorylation and the activation of Raf, one of the intermediate signaling components in the VEGF-dependent MAPK/ERK pathway involved in angiogenesis, thus negatively regulate VEGF signaling [19,39,40]. Our experiment shows that the low expression of miR-126 in CNV mice correlates with the increased mRNA and protein expression of VEGF-A, KDR and SPRED-1 and that restoration of miR-126 can inhibit these increases (Figure 2 and Figure 3).
Kamal et al. suggested that HMECs are an appropriate model for studies involving microvascular endothelial cells such as angiogenesis [41]. Costa et al. used HMECs to compare the multiple effects of bevacizumab and ranibizumab in ocular angiogenesis, they demonstrated that HMEC is probably a more accurate model to study the effects on the angiogenic process, given their capillary background, characteristic of angiogenic vessels [42]. HMECs were used here as the in vitro replication of the microvasculature resident in the eye. In cultured HMECs, we found that the decreased expression of VEGF-A, KDR and SPRED-1 after miR-126 transfection (Figure 5) were consistent with in vivo results. The changes in the target mRNA and protein levels suggest that miR-126 not only represses translation of these mRNAs, but also causes the degradation of mRNA [8,29].
Our results were also consistent with findings from other studies, suggesting that miR-126 can influence VEGF signaling to a certain extent, through SPRED-1 and PIK3R2 [39,43,44], or VEGF-A [31,32]. Although it has been suggested that miR-126 targets PIK3R2 as well [45], the concentration of PIK3R2 mRNA and proteins in our samples were below the detection limit. The decreased expression of KDR after miR-126 transfection suggested that KDR may be an indirect target of miR-126 in the eye; alternatively, the miR-126 mediated change in VEGF-A level may cause downstream changes in KDR expression (Figure 2 and Figure 3).
CNV development involves proliferation, differentiation, capillary formation and migration of endothelial cells. The significantly reduced tube formation and VEGF-induced migration were observed in miR-126 transfected HMECs, further reinforces the notion that the increase in miR-126 expression could inhibit and possibly reverse pathological angiogenesis in AMD (Figure 6 and Figure 7).
Based on the above findings, we postulate that restoring the miR-126 levels could inhibit the progression of CNV in the eye and then confirmed this hypothesis in the following experiments. miR-126 mimic was administered via a transfection reagent by intravitreal injection into the laser induced CNV eyes. After successful transfection, the mice treated with miR-126 mimic had significantly smaller CNV lesion areas compared to the untreated “CNV only” group (p < 0.01), while miR-control group showed no significant difference in CNV lesion size compared to the controls (Figure 9).
The primary pathogenesis in wet AMD occurs in the outer retina, including the RPE, Bruch’s membrane, and choroid [46,47,48,49,50]. In our study, the alteration of miR-126 expression by miR-126 mimc injection in the eye may target also RPE. RPE cells are important in secreting angioregulatory proteins, including VEGF [51,52] and PEDF [53,54,55]. The balance between VEGF-A and PEDF plays an important role in choroidal neovascularization [54,56,57,58]. Masayuki et al. cultured RPE under hypothermia that exhibit decreased VEGF-A and sustained PEDF expression [59]. In our study, decreased VEGF-A may induced the increased PEDF that is secreted from RPE. Even if it is not a large amount, may act as an antagonist for VEGF-A, it may play a role in preventing AMD development conjunction with miR-126 during this situation. Moreover, a study indicated that primary RPE cells were shown to secrete and respond to placental growth factor (PlGF), a member of VEGF family. There is evidence that the retinal expression of PlGF mRNA is increased during diabetic retinopathy [60]. PlGF facilitates endothelial cell proliferation and vascular permeability by potentiating the activity of VEGF [61,62,63]. Our study showed that miR-126 overexpression can inhibit CNV to some extent by regulating VEGF-A, and PlGF might be a potential target of miR-126 in AMD.
In this study, we found that expression of miR-126 was decreased in laser induced CNV mice; restoration of miR-126 mimic decreased VEGF-A levels and consequently influenced its downstream pathway and reduced CNV severity. miR-126 seems to be a beneficial alternative to conventional anti-VEGF antibody for the treatment of wet AMD. However the CNV was not fully inhibited by miR-126 transfection, indicating that there are other pathways involved in ocular neovascularization. Systemic delivery of engineered miRNA antagonists (antagomiRs) has been shown to effectively modulate angiogenesis with a long lived duration [64]. miR-122 as a therapeutic agent has demonstrated good efficacy and outstanding safety profile in participants in the first Phase I trial and progressed to Phase II trials [65]. The potential of miR-126 as a therapeutic target is promising and should be further explored for the treatment of AMD.
4. Experimental Section
4.1. Animals
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by Harbin Medical University and the Royal Victorian Eye and Ear Hospital Animal Research & Ethics Committee (11-233, 5 March 2014). All mice were euthanized by CO2 inhalation and all efforts were made to minimize suffering.
4.1.1. Establishment of Laser-Induced CNV Mouse Model
Adult C57BL/6 mice (9 weeks old) were maintained in a 12-h light/dark cycle with a room illuminance of 140–260 lux during the bright portion of the cycle. Animals were provided standard food and water ad libitum. Laser treatment was performed as described in a previous study [17]. CNV lesions were evaluated after laser treatment using Micron III, and choroidal flatmount. C57BL/6 mice were anesthetized through an intraperitoneal injection with a mixture of ketamine and xylazine (100 and 10 mg/kg, respectively) in a dosage of 0.1 mL/10 g of body weight. The pupils were dilated with a drop of 1% tropicamide solution (Alcon, Fort Worth, TX, USA), followed by 0.5% proxymetacaine hydrochloride (Alcon) as a topical anesthetic. Laser (50-µm spot size, 0.05 s duration, and 500 mW) was applied using a laser photocoagulator (532-nm wavelength) to the retina radially about the optic nerve with a coverslip as a contact lens. The formation of a small bubble at the laser spot indicated a successful rupture of the Bruch’s membrane and formation of CNV. Mice without the bubble formation or with vitreal hemorrhage obscuring the posterior segment were excluded. CNV lesions were evaluated after laser treatment using Micron III and histological staining of choroidal flatmount.
4.1.2. Micron III Imaging
Anesthetized mice pupils were dilated with a drop of 1% tropicamide solution (Alcon). The mouse eyes were covered with a drop of ocular lubricating gel (Alcon), which served as an optical coupling medium, and the vascular network of the live CNV mice was imaged in vivo using the Micron III retinal imaging camera system (Phoenix Research Laboratories, Pleasanton, CA, USA). Fundus images of both eyes were obtained before a dose of 1 mL/10 g body weight of 1% fluorescein sodium was injected intraperitoneally into each mouse to obtain a fluorescence image [66].
4.1.3. Choroidal Flatmount
To analyze the area of the CNV lesion, mice eyes were enucleated and fixed in 4% paraformaldehyde for 60 min. Under a dissecting microscope, the anterior segment and neuroretina were removed. The sclera–choroid–retinal pigment epithelial complex was isolated and incubated in a blocking solution (0.5% bovine serum albumin, 0.5% triton-X) for 4 h at 4 °C and then for 16 h at 4 °C in Isolectin GS-IB4 conjugated with Alexa Fluor 488 (1:100 dilution, 500 μg/mL). Fluorescent micrographs of the choroidal flatmount were then taken using an Olympus DP-71 camera system attached to an Olympus BX61 microscope (Olympus, Tokyo, Japan). The area (µm2) was measured using the ImageJ software (2x 2.1.4.7, National Institute of Mental Health, Bethesda, MD, USA). The measurements obtained from multiple lesions from the eyes of each animal were averaged.
4.1.4. Intravitreal Injection of miRNA
Scrambled mirVana™ miRNA Mimic, Negative Control #1(Ambion, Foster City, CA, USA) or miR-126 mimic (5′-UCGUACCGUGAGUAAUAAUGCG-3′, Ambion) at a concentration of 5 µM was complexed with Invivofectamine 2.0 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. The CNV mice were randomly divided into three groups (n = 12/group, and 24 eyeballs/group). Each group received either no treatment or an intravitreal injection of 5 µL of either the scramble control miR or miR-126 mimic treatment on Day 0 (immediately after laser treatment) and readministered on Day 7. For the intravitreal injection, a hole was first made by inserting a 30-gauge needle posterior to the limbus of the mice eye. Then the respective compounds were injected through the initial hole using a 34-gauge Hamilton syringe. Insertion and infusion were performed and directly viewed through an operating microscope. Care was taken not to injure the lens or the retina [31,67]. Mice were sacrificed on day 14 for choroidal flatmount, real-time qPCR and Western blot analysis.
4.2. Cells
Human microvascular endothelial cells (HMECs) were obtained from Hitesh Peshavariya of Centre for Eye Research Australia. HMECs were grown in Endothelial Basal Medium (EBM, Lonza, Basel, Switzerland), supplemented with EGM-MV SingleQuot Kit Supplement & Growth Factors (Lonza) at 37 °C in a humidified chamber at 5% CO2.
4.2.1. In Vitro Transfection of HMECs
A 10 nM mimicking miR-126 (Pre-miR miRNA Precursor Molecule, Ambion) or nonspecific control miRNA (Pre-miR Negative Control #1, Ambion) was transfected into the HMEC cell lines using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions.
4.2.2. Tube Formation Assay and Scratch Wound Migration Assay
The ability of HMECs to form capillary-like tubes in culture was assessed by adding 5 × 104 cells to 24-well plate, which contained 200 μL of pre-gelled Matrigel (BD Biosciences, San Jose, CA, USA) in 1 mL of a complete medium. Tube formation in the HMEC culture was quantified at t = 9 h by counting all tube-like structures in the gel under an inverted phase contrast microscope (Olympus IX71, Tokyo, Japan) at × 40 magnification. The data was presented as the total number of loops formed [68]. The migration of endothelial cells was monitored by generating a “scratch” assay. After 48 h of transfection, 3 × 104 HMECs were plated in a 48-well plate previously labeled with lines to facilitate identification of the same regions at different time points. Cells were incubated for 48 h to reach confluence, and a scratch was made in the monolayer using a 200 µL pipette tip. Floating cells were removed by two gentle washes with phosphate buffered saline (PBS) and the growth medium was replaced with Endothelial Basal Medium only, with the addition of 50 ng/mL VEGF. Reference photographs were taken using a phase contrast microscope (Olympus IX71) and the photographed in the same areas at t = 0 h and 9 h. Endothelial cell migration was quantified as the difference in size between the denuded area immediately after the scratch and at t = 9 h.
4.3. RNA Preparation and qPCR
In mice, total mRNA was extracted using TRIzol (Invitrogen) from RPE/Choroids of both the control group and CNV group 14 days after the CNV laser procedure. In cells, the total mRNA and miRNA were isolated 48 h after transfection using the same method. The miR-126 first-strand cDNA was prepared from 1 μg of RNA from each sample via RT-PCR with random primers or with miR-126 and U6 primers using a TaqMan MicroRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative qPCR was performed using the TaqMan MicroRNA assay (Applied Biosystems), with U6 as the housekeeping gene. The qPCR analysis of mRNA was performed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and TaqMan gene expression master mix (Applied Biosystems) for VEGF-A, KDR and SPRED-1, with 18s as the housekeeping gene. Each PCR reaction was performed in triplicates in a 10 µL volume using 5 µL master mix (Applied Biosystems) for 2 min at 50 °C followed by 45 cycles of 95 °C for 10 min, 95 °C for 15 s and 60 °C for 60 s in a real-time PCR system (Applied Biosystems). The results were expressed as ΔCt, and fold changes in relative mRNA expression were calculated using the equation 2−ΔΔCt [69]. All assays were performed according to the manufacturer’s instructions.
The sequences of the primes used are list as following: miR-126: mature sequence, 5′-UCGUACCGUGAGUAAUAAUGCG-3′. U6: mature sequence, 5′-GTGCTCGCTTCGGCAGCACATATACTAAA-3′. VEGF-A: forward 5′-GATCATGCGGATCAAACCTC-3′; reverse 5′-CCTCCGGACCCAAAGTGCTC-3′ KDR: forward 5′-GCGAAAGAGCCGGCCTGTGA-3′; reverse 5′-TCCCTGCTTTTGCTGGGCACC-3′; SPRED-1: forward 5′-GGCCTAGACATTCAGAGCCG-3′; reverse 5′-TAGTCTCATCCCCACAGTGG-3′; 18S: forward 5′-GAAGGTGAAGGTCGGAGTC-3′; reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
4.4. Western Blotting Analysis
The ocular tissues (RPE and choroid mix) were isolated from enucleated eyes under dissecting microscope and homogenized in RIPA buffer containing a protease inhibitor cocktail (1:100 dilution, Sigma-Aldrich, St. Louis, MO, USA). Cells were collected and lysed as above using RIPA buffer. The lysates were cleared by centrifugation at 12,000× g for 20 min at 4 °C. The protein concentration of the lysate was determined using the bicinchoninic acid (BCA) assay. Equal amounts of proteins (30 µg in vivo and 20 µg in vitro) were separated by SDS-PAGE gels (Invitrogen) and transferred onto polyvinylidene fluoride (PVDF) membranes (GE Healthcare, Little Chalfont, UK). After blocking with 5% nonfat milk powder in Tris-buffered saline (pH 7.5), the membranes were incubated with anti-VEGFA (ab51745, Rabbit polyclonal to VEGF-A, Abcam Sapphire Bioscience, Cambridge, MA, USA), anti-SPRED-1 (ab77079, Rabbit polyclonal to SPRED-1, Abcam Sapphire Bioscience), anti-KDR (ab39256, Rabbit polyclonal to VEGF Receptor 2, Abcam Sapphire Bioscience) and anti-β-actin (ab3280, Mouse monoclonal to Actin, Abcam Sapphire Bioscience). After incubation with the horseradish peroxidase–conjugated secondary IgG (1:10,000, Sigma, USA), blots were developed and quantified with Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA).
4.5. Statistical Analysis
All experiments were repeated a minimum of three times, and the error bars on graphs represent the mean ± SD, unless otherwise stated. All data were analyzed using Prism 6.0 (GraphPad Software, San Diego, CA, USA). miR-126 and its target genes mRNA expression, CNV lesion area, HMECs tube formation and migration were determined by ANOVA, a p-value of <0.05 was considered as significant. The expression of miR-126 target genes protein was analyzed by two tailed Student’s t-test with p < 0.05 declared significant.
Acknowledgments
We would like to thank the staff of EMSU for their assistance with animal care.
Author Contributions
Conceived and designed the experiments: Hong Zhang and Ping Liu. Performed the experiments: Lei Wang and Amy Yi Wei Lee. Analyzed the data: Lei Wang. Contributed reagents/materials/analysis tools: Amy Yi Wei Lee, Jonathan P. Wigg, and Hitesh Peshavariya. Wrote the paper: Lei Wang, Amy Yi Wei Lee, and Hong Zhang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ohkuma Y., Hayashi T., Sakai T., Watanabe A., Yamada H., Akahori M., Itabashi T., Iwata T., Noda T., Tsuneoka H. Retinal angiomatous proliferation associated with risk alleles of ARMS2/HTRA1 gene polymorphisms in Japanese patients. Clin. Ophthalmol. 2014;8:143–148. doi: 10.2147/OPTH.S56483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Jager R.D., Mieler W.F., Miller J.W. Age-related macular degeneration. N. Engl. J. Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 4.Liu M.M., Chan C.-C., Tuo J. Epigenetics in Ocular Diseases. Curr. Genom. 2013;14:166–172. doi: 10.2174/1389202911314030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campochiaro P.A., Nguyen Q.D., Shah S.M., Klein M.L., Holz E., Frank R.N., Saperstein D.A., Gupta A., Stout T., Macko J., et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular: Results of a phase I clinical trial. Hum. Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 6.Wang S., Koster K.M., He Y., Zhou Q. miRNAs as potential therapeutic targets for age-related macular degeneration. Future Med. Chem. 2012;4:277–287. doi: 10.4155/fmc.11.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Nadal E., Ammerer G., Posas F. Controlling gene expression in response to stress. Nat. Rev. Genet. 2011;12:833–845. doi: 10.1038/nrg3055. [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro P.A. Ocular neovascularization. J. Mol. Med. 2013;91:311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neely K.A., Gardner T.W. Ocular neovascularization: Clarifying complex interactions. Am. J. Pathol. 1998;153:665–670. doi: 10.1016/S0002-9440(10)65607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlenker M.B., Thiruchelvam D., Redelmeier D.A. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am. J. Ophthalmol. 2015;160:569–580. doi: 10.1016/j.ajo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Van Romunde S.H., Polito A., Bertazzi L., Guerriero M., Pertile G. Long-Term Results of Full Macular Translocation for Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmology. 2015;122:1366–1374. doi: 10.1016/j.ophtha.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Zampetaki A., Kiechl S., Drozdov I., Willeit P., Mayr U., Prokopi M., Mayr A., Weger S., Oberhollenzer F., Bonora E., et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 14.Caporali A., Emanueli C. MicroRNAs in postischemic vascular repair. Cardiol. Res. Pract. 2012;2012:486702. doi: 10.1155/2012/486702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., Gorski D.H. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehbacher A., Urbich C., Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol. Sci. 2008;29:12–15. doi: 10.1016/j.tips.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Urbich C., Kuehbacher A., Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 19.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D., Ivey K.N., Bruneau B.G., Stainier D.Y.R., Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J., Yang X., Xie B., Chen Y., Swaim M., Hackett S.F., Campochiaro P. MicroRNAs regulate ocular neovascularization. Mol. Ther. 2008;16:1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q., Gallagher R., Ufret-Vincenty R., Li X., Olson E.N., Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23∼27∼24 clusters. Proc. Natl. Acad. Sci. USA. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fish J.E., Srivastava D. MicroRNAs: Opening a new vein in angiogenesis research. Sci. Signal. 2009;2 doi: 10.1126/scisignal.252pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson E., Fredlund Fuchs P., Heldin J., Barkefors I., Bondjers C., Genové G., Arrondel C., Gerwins P., Kurschat C., Schermer B., et al. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1:108. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Li L., Wang S., Lei Y., Ge Q., Lv N., Zhou X., Chen C. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget. 2014;5:11873–11885. doi: 10.18632/oncotarget.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casci T., Vinos J., Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell. 1999;96:655–665. doi: 10.1016/S0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- 27.Hacohen N., Kramer S., Sutherland D., Hiromi Y., Krasnow M.A. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/S0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 28.Kato R., Nonami A., Taketomi T., Wakioka T., Kuroiwa A., Matsuda Y., Yoshimura A. Molecular cloning of mammalian Spred-3 which suppresses tyrosine kinase-mediated Erk activation. Biochem. Biophys. Res. Commun. 2003;302:767–772. doi: 10.1016/S0006-291X(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 29.Wakioka T., Sasaki A., Kato R., Shouda T., Matsumoto A., Miyoshi K., Tsuneoka M., Komiya S., Baron R., Yoshimura A. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412:647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 30.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Olson E.N. AngiomiRs—Key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai Y., Bai X., Wang Z., Zhang X., Ruan C., Miao J. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp. Mol. Pathol. 2011;91:471–477. doi: 10.1016/j.yexmp.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Ye P., Liu J., He F., Xu W., Yao K. Hypoxia-induced deregulation of miR-126 and its regulative effect on VEGF and MMP-9 expression. Int. J. Med. Sci. 2014;11:17–23. doi: 10.7150/ijms.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh C.H., Rau C.S., Jeng S.F., Lin C.J., Chen Y.C., Wu C.J., Lu T.H., Lu C.H., Chang W.N. Identification of the potential target genes of microRNA-146a induced by PMA treatment in human microvascular endothelial cells. Exp. Cell Res. 2010;316:1119–1126. doi: 10.1016/j.yexcr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Kramer A., Behrens-Baumann W. Antiseptic Prophylaxis and Therapy in Ocular Infections: Principles, Clinical Practice and Infection Control. Volume 33. Medical and Scientific Publishers; Canton Basel-City, Switzerland: 2002. p. 8. [Google Scholar]
- 36.Cross M.J., Claesson-Welsh L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001;22:201–207. doi: 10.1016/S0165-6147(00)01676-X. [DOI] [PubMed] [Google Scholar]
- 37.Adamis A.P., Berman A.J. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin. Immunopathol. 2008;30:65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 38.Ng Y.-S., Krilleke D., Shima D.T. VEGF function in vascular pathogenesis. Exp. Cell Res. 2006;312:527–537. doi: 10.1016/j.yexcr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Chung A.S., Ferrara N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Boil. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 40.Kamal K., Du W., Mills I., Sumpio B.E. Antiproliferative effect of elevated glucose in human microvascular endothelial cells. J. Cell. Biochem. 1998;71:491–501. doi: 10.1002/(SICI)1097-4644(19981215)71:4<491::AID-JCB4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Valencia-Sanchez M.A., Liu J., Hannon G.J., Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 42.Costa R., Carneiro A., Rocha A., Pirraco A., Falcão M., Vasques L., Soares R. Bevacizumab and ranibizumab on microvascular endothelial cells: A comparative study. J. Cell. Biochem. 2009;108:1410–1417. doi: 10.1002/jcb.22378. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi K., Kohno R., Ayada T., Kato R., Ichiyama K., Morisada T. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol. Cell. Biol. 2007;27:4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhnert F., Mancuso M.R., Hampton J., Stankunas K., Asano T., Chen C.Z. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 45.Zhu N., Zhang D., Xie H., Zhou Z., Chen H., Hu T. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol. Cell. Biochem. 2011;351:157–164. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda T., Sawa H., Koizumi K., Yasuhara T., Yamasaki T. Pars plana vitrectomy for regression of choroidal neovascularization with age-related macular degeneration. Acta Ophthalmol. Scand. 2000;78:460–464. doi: 10.1034/j.1600-0420.2000.078004460.x. [DOI] [PubMed] [Google Scholar]
- 47.Mojana F., Cheng L., Bartsch D.U., Silva G.A., Kozak I., Nigam N., Freeman W.R. The role of abnormal vitreomacular adhesion in age-related macular degeneration: Spectral optical coherence tomography and surgical results. Am. J. Ophthalmol. 2008;146:218–227. doi: 10.1016/j.ajo.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakamoto T., Sheu S.J., Arimura N., Sameshima S., Shimura M., Uemura A., Kawano H., Wu T.-T., Kubota T., Sohma R., et al. Vitrectomy for exudative age-related macular degeneration with vitreous hemorrhage. Retina. 2010;30:856–864. doi: 10.1097/IAE.0b013e3181c969cb. [DOI] [PubMed] [Google Scholar]
- 49.Roller A.B., Mahajan V.B., Boldt H.C., Abramoff M.D., Russell S.R., Folk J.C. Effects of vitrectomy on age-Related macular degeneration. Ophthalmology. 2010;117:1381–1386. doi: 10.1016/j.ophtha.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Schulze S., Neugebauer A., Kroll P. Appearance of age-related macular degeneration in vitrectomized and nonvitrectomized eyes: An intraindividual case study. Acta Ophthalmol. 2012;90:244–247. doi: 10.1111/j.1755-3768.2010.01929.x. [DOI] [PubMed] [Google Scholar]
- 51.Adamis A.P., Shima D.T., Yeo K.T., Yeo T.K., Brown L.F., Berse B., Berse B., Damore P.A., Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 1993;193:631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- 52.Ferrara N., Gerber H.P., Le Couter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 53.Bouck N. PEDF: Anti-Angiogenic guardian of ocular function. Trends Mol. Med. 2002;8:330–334. doi: 10.1016/S1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 54.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W., Bouck N.P. Pigment epithelium-Derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 55.Farnoodian M., Kinter J.B., Yadranji Aghdam S., Zaitoun I., Sorenson C.M., Sheibani N. Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells. Physiol. Rep. 2015;3:e12266. doi: 10.14814/phy2.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tombran-Tink J. PEDF in angiogenic eye diseases. Curr. Mol. Med. 2010;10:267–278. doi: 10.2174/156652410791065336. [DOI] [PubMed] [Google Scholar]
- 57.Ohno-Matsui K., Morita I., Tombran-Tink J., Mrazek D., Onodera M., Uetama T., Hayano M., Murota S., Mochizuki M. Novel mechanism for age-related macular degeneration: An equilibrium shift between the angiogenesis factors VEGF and PEDF. J. Cell. Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- 58.Barnstable C.J., Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: Molecular targets and therapeutic potential. Prog. Retin. Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Takeyama M., Yoneda M., Gosho M., Iwaki M., Zako M. Decreased VEGF-A and sustained PEDF expression in a human retinal pigment epithelium cell line cultured under hypothermia. Biol. Res. 2015;48:42. doi: 10.1186/s40659-015-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spirin K.S., Saghizadeh M., Lewin S.L., Zardi L., Kenney M.C., Ljubimov A.V. Basement membrane and growth factor gene expression in normal and diabetic human retinas. Curr. Eye Res. 1999;18:490–499. doi: 10.1076/ceyr.18.6.490.5267. [DOI] [PubMed] [Google Scholar]
- 61.Autiero M., Waltenberger J., Communi D., Kranz A., Moons L., Lambrechts D., Kroll J., Plaisance S., de Mol M., Bono F., et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat. Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 62.Dull R.O., Yuan J., Chang Y.S., Tarbell J., Jain R.K., Munn L.L. Kinetics of placenta growth factor/vascular endothelial growth factor synergy in endothelial hydraulic conductivity and proliferation. Microvasc. Res. 2011;61:203–210. doi: 10.1006/mvre.2000.2298. [DOI] [PubMed] [Google Scholar]
- 63.Park J.E., Chen H.H., Winer J., Houck K.A., Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 64.Bai Y., Ma J.X., Guo J., Wang J., Zhu M., Chen Y., Le Y.-Z. Müller cell—Derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 2009;219:446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 65.Anand S. A brief primer on microRNAs and their roles in angiogenesis. Vasc. Cell. 2013;5:2. doi: 10.1186/2045-824X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han Z., Guo J., Conley S.M., Naash M.I. Retinal angiogenesis in the Ins2Akita mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013;54:574–584. doi: 10.1167/iovs.12-10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambert V., Lecomte J., Hansen S., Blacher S., Gonzalez M.L., Struman I., Rozet E., de Tullio P., Michel Foidart J., Rakic J.-M., et al. Laser-Induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 68.Gilad S., Meiri E., Yogev Y., Benjamin S., Lebanony D., Yerushalmi N., Benjamin H., Kushnir M., Cholakh H., Melamed N., et al. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008;3:895. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caporali A., Meloni M., Völlenkle C., Bonci D., Sala-Newby G.B., Addis R., Spinetti G., Losa S., Masson R., Baker A.H., et al. Deregulation of microRNA-503 contributes to diabetes ellmitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]