Abstract
The Phosphate Transporter1 (PHT1) family of genes plays pivotal roles in the uptake of inorganic phosphate from soils. However, there is no comprehensive report on the PHT1 family in Zea mays based on the whole genome. In the present study, a total of 13 putative PHT1 genes (ZmPHT1;1 to 13) were identified in the inbred line B73 genome by bioinformatics methods. Then, their function was investigated by a yeast PHO84 mutant complementary experiment and qRT-PCR. Thirteen ZmPHT1 genes distributed on six chromosomes (1, 2, 5, 7, 8 and 10) were divided into two paralogues (Class A and Class B). ZmPHT1;1/ZmPHT1;9 and ZmPHT1;9/ZmPHT1;13 are produced from recent segmental duplication events. ZmPHT1;1/ZmPHT1;13 and ZmPHT1;8/ZmPHT1;10 are produced from early segmental duplication events. All 13 putative ZmPHT1s can completely or partly complement the yeast Pi-uptake mutant, and they were obviously induced in maize under low Pi conditions, except for ZmPHT1;1 (p < 0.01), indicating that the overwhelming majority of ZmPHT1 genes can respond to a low Pi condition. ZmPHT1;2, ZmPHT1;4, ZmPHT1;6, ZmPHT1;7, ZmPHT1;9 and ZmPHT1;11 were up-regulated by arbuscular mycorrhizal fungi (AMF), implying that these genes might participate in mediating Pi absorption and/or transport. Analysis of the promoters revealed that the MYCS and P1BS element are widely distributed on the region of different AMF-inducible ZmPHT1 promoters. In light of the above results, five of 13 ZmPHT1 genes were newly-identified AMF-inducible high-affinity phosphate transporters in the maize genome. Our results will lay a foundation for better understanding the PHT1 family evolution and the molecular mechanisms of inorganic phosphate transport under AMF inoculation.
Keywords: Zea mays, phosphate transporters, evolution, expression, AMF-inducible, promoter
1. Introduction
Although phosphorus is an essential macro-elements for plant growth and development, levels of inorganic phosphate (Pi) in different types of soils are mostly insufficient for plant growth and vegetative productivity [1]. Plants have evolved multiple strategies to increase and assimilate available Pi from soil, including secretion of organic acids and phosphatases to solubilize Pi trapped in complexes, increasing the root-soil interface, forming a symbiotic association with arbuscular mycorrhizal fungi (AMF), etc. [1,2]. The symbiosis of plant roots with AMF is one of the important strategies. It is estimated that AMF form mycorrhizal with the roots of 80% of all terrestrial plant species and promote the growth of the host plant mainly by enhancing the phosphorus uptake [3]. The symbiosis with AMF is an important nutrition uptake method, which can contribute to up to 90% of plant phosphorus requirements [4,5].
Plants have two types of Pi uptake systems, i.e., a high-affinity Pi uptake system and a low-affinity Pi uptake system. Phosphate Transporter1 (PHT1) are high-affinity Pi transporters, which play pivotal roles in the uptake of phosphorus from soils under phosphorus-limited conditions [1]. Furthermore, a previous study suggested that dual affinity PHT1 transporters likely exist in Arabidopsis [6]. The majority of PHT1 genes are mainly expressed in root epidermal cells and outer cortex, implying their potential involvement in phosphate uptake from the soil [2]. Besides the direct uptake of phosphorus from soils around the roots, plants often acquire additional phosphorus from deep soils with the help of AMF fungal hyphae. During plant-AMF association, three types of PHT1 genes are involved in the absorption, i.e., fungal PHT1 genes, plant PHT1 genes and plant PHT1 genes induced by AMF [2]. The fungi first assimilate phosphorus by fungal PHT1 transporters and then transfer phosphorus to the plant via AMF-inducible plant PHT1 transporters [2,7].
Members of the plant PHT1 gene family share homologies with the yeast PHO84 Pi transporter and belong to the phosphate-H+ symporter family [8]. They have a similar structure, i.e., 12 putative transmembrane (TM) domains, hydrophilic N- and C-terminals and a hydrophilic loop between TM6 and TM7 [8]. The first crystal structure of high-affinity phosphate transporter (PiPT) from Piriformospora indica was solved recently. The structure shows exit pathways of protons and phosphate and suggests a phosphate transport mechanism called “rocker-switch” [9]. Several members of the PHT1 family in dicot and monocot plants, particularly from many economically-important plant species, have been identified [1,2]. The first plant PHT1 gene was isolated from Arabidopsis thaliana and exhibits similarities to PHO84 [10]. A large number of plant genomes have been sequenced with next generation sequencing technology [11,12,13]. All of the members of the PHT1 family have been identified in some species’ whole genome. For example, 9 members of the PHT1 family were identified in the Arabidopsis genome [14], 13 in Oryza sativa [15], 8 in Solanum lycopersicum [16], 10 in Solanum tuberosum [16], 6 in Astragalus sinicus [17], 14 in Glycine max [18], 13 in Brachypodium distachyon [19], 11 in Sorghum bicolor [17], 12 in Setaria italic [20], etc. A limited number of AMF-inducible Pi transporters in plants have been identified in Medicago truncatula [21], Astragalus sinicus [17], Oryza sativa (O. sativa) [15,22], Solanum tuberosum (S. tuberosum) [23], Solanum lycopersicum [24], Triticum aestivum [25], Brachypodium distachyon (B. distachyon) [19], Setaria italic (S. italic) [20] and Zea mays (Z. mays) [26].
As one of the most important crops in the world, maize is grown widely, but multiple environmental stresses limit its production, especially by different degrees of low Pi stress. Five genes encoding PHT1 phosphate transporters were identified from a commercial hybrid Maize cDNA library [26]; the expression level of ZEAma:PHT1;6 was much higher in plants colonized by AMF than that in non-mycorrhizal maize roots [27]. Compared to the investigation on PHT1 genes in whole genome sequenced plants, such as B. distachyon, S. bicolor and O. sativa, there is no comprehensive report on PHT1 family genes in the Z. mays whole genome level, particularly in inbred lines.
In this study, members of PHT1 family genes in Z. mays elite inbred line B73 genome were identified by bioinformatics and experimental investigations, and the characteristics of the genes were also investigated under a phosphorus-adverse environment, as well as the AMF inoculation condition. The detailed identification and functional analyses of Z. mays PHT1 genes might help us to find out some new approaches to improve the phosphate uptake efficiency of maize.
2. Results
2.1. Identification and Properties of Putative Z. mays PHT1 Genes
a total of 13 putative genes were identified in maize inbred line B73 genome and were denominated as ZmPHT1;1 to ZmPHT1;13 (Table 1). All 13 ZmPHT1 proteins contain a highly conserved Sugar_tr domain (PF00083) and encode proteins varying from 509 to 597 AA. The minimum and maximum theoretical molecular weights are 56.22 kDa (ZmPHT1;5) and 66.07 kDa (ZmPHT1;12), respectively. The amino acid number and molecular weight of these proteins have slight differences. Besides, all 13 genes are alkaline, with the isoelectric point ranging from 7.58 to 9.41. All ZmPHT1 proteins contain 12 putative membrane-spanning domains, as shown in Figure S1.
Table 1.
Details of the PHT1 genes of maize.
| Gene Name | Transcript ID | Accession Number | Gene Size (bp) | CDS Size (bp) | Protein Length (AA) | Protein Molecular Mass (Da) | pI | Chromosome |
|---|---|---|---|---|---|---|---|---|
| ZmPHT1;1 | GRMZM2G326707_T01 | NM_001279426.1 | 2199 | 1617 | 539 | 58,485.66 | 7.65 | 5 |
| ZmPHT1;2 | GRMZM2G139639_T01 | NM_001156420.1 | 1835 | 1611 | 537 | 58,660.21 | 7.98 | 7 |
| ZmPHT1;3 | GRMZM2G112377_T01 | NM_001112347.2 | 2175 | 1629 | 543 | 57,899.24 | 8.11 | 1 |
| ZmPHT1;4 | GRMZM2G170208_T01 | NM_001279982.1 | 1809 | 1548 | 516 | 57,145.85 | 9.13 | 2 |
| ZmPHT1;5 | GRMZM2G041595_T01 | NM_001112348.1 | 1698 | 1527 | 509 | 56,222.71 | 8.93 | 7 |
| ZmPHT1;6 | GRMZM5G881088_T01 | NM_001112306.1 | 1971 | 1662 | 554 | 60,579.71 | 8.15 | 8 |
| ZmPHT1;7 | GRMZM2G075870_T01 | NM_001157730.1 | 1999 | 1761 | 587 | 63,148.92 | 8.34 | 1 |
| ZmPHT1;8 | GRMZM2G045473_T01 | NM_001139212.1 | 1870 | 1605 | 535 | 58,762.9 | 7.58 | 2 |
| ZmPHT1;9 | GRMZM2G154090_T01 | NM_001112346.1 | 2020 | 1623 | 541 | 58,752.98 | 7.63 | 1 |
| ZmPHT1;10 | GRMZM2G159075_T01 | XM_008664847.1 | 2172 | 1791 | 597 | 65,240.06 | 9.11 | 10 |
| ZmPHT1;11 | GRMZM2G009779_T01 | NM_001112348.1 | 1581 | 1551 | 517 | 57,538.36 | 9.18 | 2 |
| ZmPHT1;12 | GRMZM2G009800_T02 | NM_001112348.1 | 1846 | 1770 | 590 | 66,071.38 | 9.41 | 2 |
| ZmPHT1;13 | GRMZM2G070087_T01 | NM_001196972.1 | 1986 | 1572 | 524 | 57,450.92 | 8.37 | 1 |
CDS, coding sequence; pI, isoelectric point.
2.2. Phylogenetic Analysis of PHT1 Transporters in Z. mays
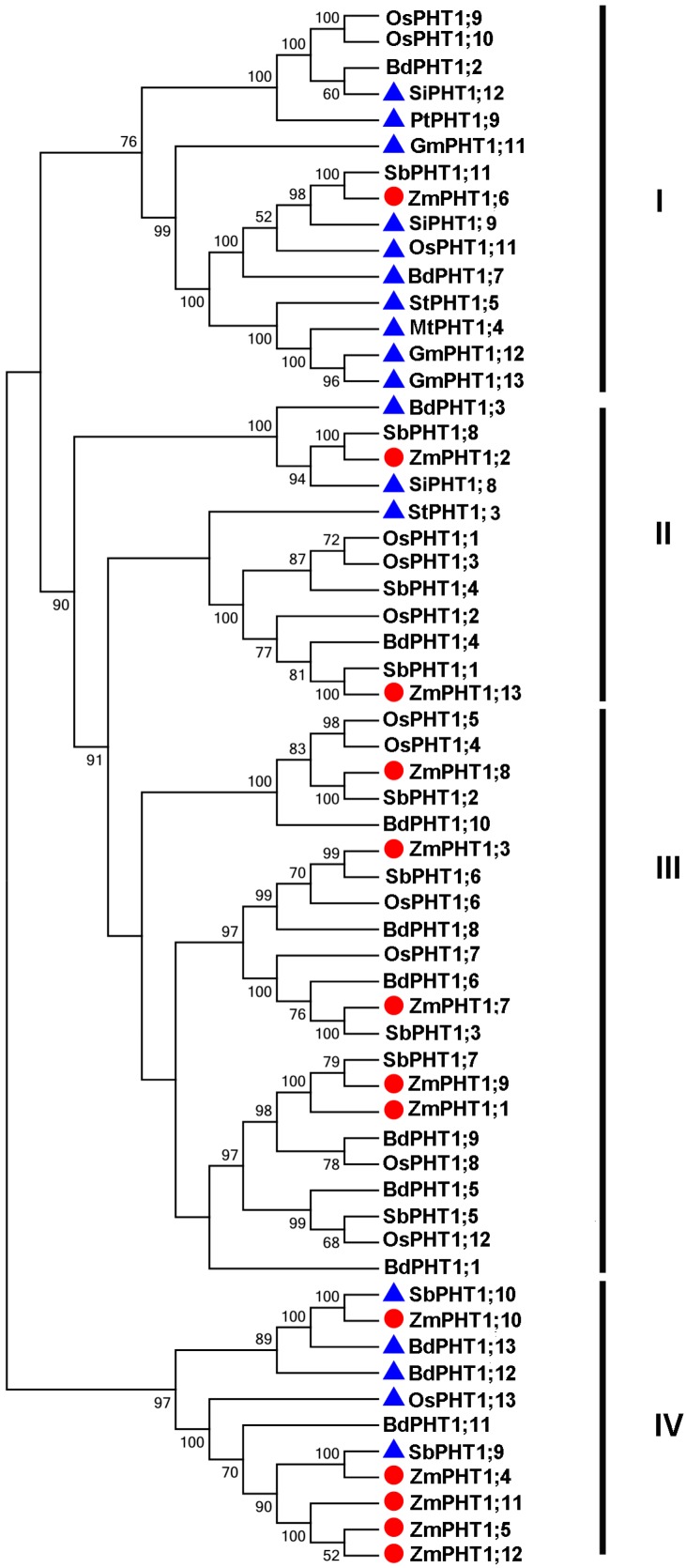
A phylogenetic tree of PHT1 transporters was constructed by multiple sequence alignment of proteins from maize, rice, sorghum Brachypodium and AM-induced PHT1 transporters from G. max, Medicago truncatula, P. trichocarpa, S. italic and S. tuberosum [20] (Figure 1). ZmPHT1 proteins were divided into four subfamilies, Classes I to IV. Class I was monocot and dicot AM-inducible PHT1s, only containing ZmPHT1;6. ZmPHT1;1, ZmPHT1;3, ZmPHT1;7 and ZmPHT1;9 are involved in monocot Class III without AM-inducible PHT1s. ZmPHT1;4, ZmPHT1;5, ZmPHT1;8, ZmPHT1;10, ZmPHT1;11 and ZmPHT1;12 together with other monocot AM-inducible proteins fall into Class IV. ZmPHT1;2 and ZmPHT1;13 are involved in Class II.
Figure 1.
Phylogenetic analysis of PHT1 transporters between maize and other plants. Roman numerals (I–IV) indicate the four PHT1 subfamilies. Transporters from maize (ZmPHT1;1 to ZmPHT1;13), rice (OsPHT1;1 to OsPHT1;13), sorghum (SbPHT1;1 to SbPHT1;11), Brachypodium (BdPHT1;1 to BdPHT1;13) and some recently-identified AM-inducible PHT1 transporters [20]. ZmPHT1s are indicated by filled red circles, and AMF-inducible PHT1s in other plants are indicated by filled blue triangles.
2.3. The Conserved Structure Analysis of Maize PHT1 Proteins
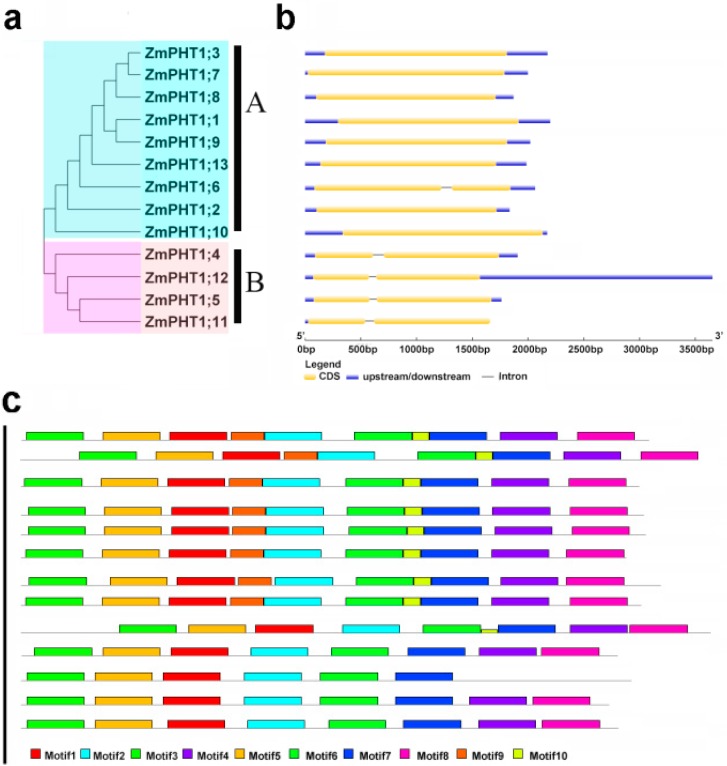
The phylogenetic tree of 13 ZmPHT1 transporters was divided into two subfamilies (Subfamily A and Subfamily B) (Figure 2). Ten conserved motifs were identified in ZmPHT1 proteins by MEME software. Proteins in Subfamily A contained two specific motifs (motifs 9 and 10) compared to Subfamily B, whereas the functions of the two motifs are unknown. Besides, motifs 1, 2, 4, 5 encoded Sugar_tr domains and motifs 3, 7, 8 encoded transmembrane domains (Figure S1). Based on the hydropathy plots in the homology modeling analysis, these genes commonly encode integral membrane proteins containing 12 hydrophilic loops between TM6 and TM7, forming a 6 + 6 configuration.
Figure 2.
Phylogenetic tree, motif and gene structure of ZmPHT1 genes. (a) The phylogenetic tree contains only ZmPHT1s, which were divided into two groups (Class A and Class B); (b) exons are indicated by grey boxes, and introns are indicated by single lines; black lines represent untranslated regions (UTR); (c) motifs’ analysis; different colors represent different motifs.
2.4. Distribution and Genomic Duplications of Maize PHT1 Genes
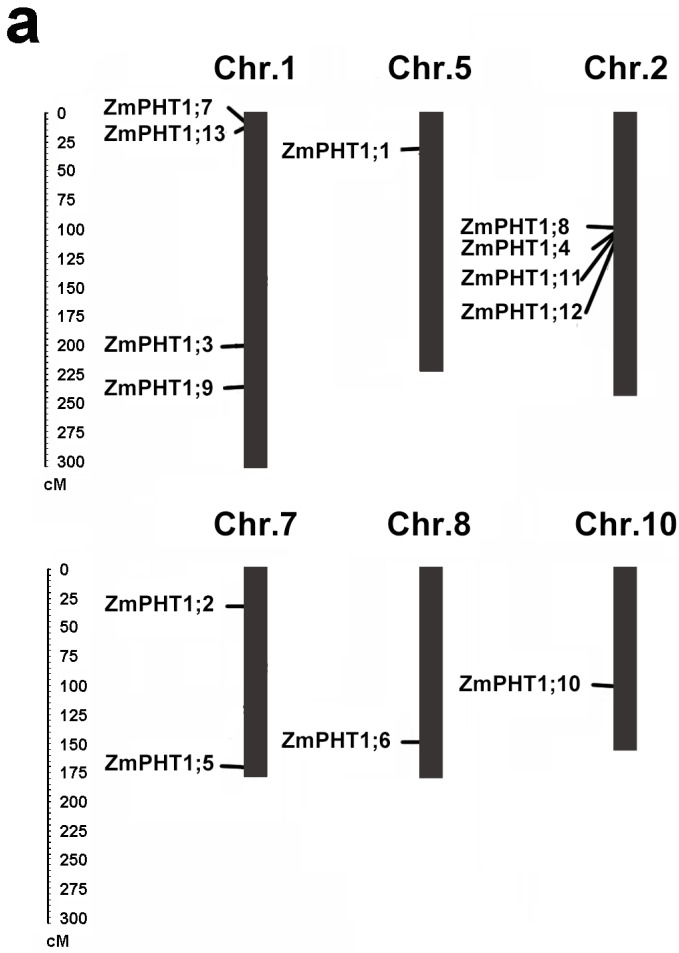
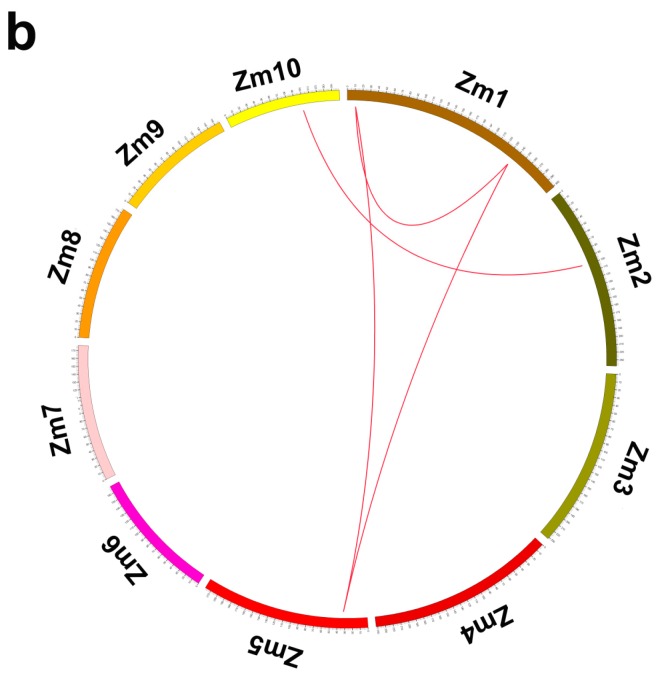
Compared to the whole maize genome, thirteen putative PHT1 genes are located on chromosomes 1, 2, 5, 7 and 10, respectively (Figure 3A). Four duplicated pairs of ZmPHT1 genes (ZmPHT1;1/ZmPHT1;9, ZmPHT1;9/ZmPHT1;13, ZmPHT1;1/ZmPHT1;13 and ZmPHT1;8/ZmPHT1;10) were identified on the synteny map (Figure 3B) and were a segmental duplication event. All of their Ka/Ks values (Table 2, Figure S2) were <1.0, except ZmPHT1;9/ZmPHT1;13 (2.76). Statistical analysis indicated that ZmPHT1;1/ZmPHT1;9, ZmPHT1;9/ZmPHT1;13, ZmPHT1;1/ZmPHT1;13 and ZmPHT1;8/ZmPHT1;10 were produced approximately 12.36, 7.91, 32.72 and 42.05 Mya (millions of years ago), respectively (Table 2). Further analysis revealed that amino acid sequence similarity between ZmPHT1;1/ZmPHT1;9, ZmPHT1;9/ZmPHT1;13, ZmPHT1;1/ZmPHT1;13 and ZmPHT1;8/ZmPHT1;10 was high (93.9%, 73.38%, 73.33% and 50.72%, respectively).
Figure 3.
Distribution and synteny of ZmPHT1 genes. (a) Distribution of ZmPHT1 genes on the maize chromosomes. On the top of each bar is the chromosome number; (b) synteny of ZmPHT1 genes. Zm1–10 represented maize chromosome 1–10, indicated by colored boxes. Chromosome box numbers represent sequence lengths in megabases. All of the syntenic genes were located in the map and linked by red lines.
Table 2.
Estimates of the dates for duplication events in maize PHT1 paralogs.
| Paralogous Pairs | Ks | Ka | Ka/Ks | Duplication Rate (Mya) | Duplication Type |
|---|---|---|---|---|---|
| ZmPHT1;1/ZmPHT1;9 | 0.16 | 0.03 | 0.19 | 12.36 | Segmental |
| ZmPHT1;1/ZmPHT1;13 | 0.43 | 0.18 | 0.41 | 32.72 | Segmental |
| ZmPHT1;8/ZmPHT1;10 | 0.55 | 0.36 | 0.66 | 42.05 | Segmental |
| ZmPHT1;9/ZmPHT1;13 | 0.10 | 0.28 | 2.76 | 7.91 | Segmental |
Mya, Millions of years ago; Ks, synonymous substitutions; Ka, nonsynonymous substitutions; Ka/Ks, nonsynonymous substitutions per synonymous site.
2.5. Tissue Specificity of ZmPHT1 Gene Expressions
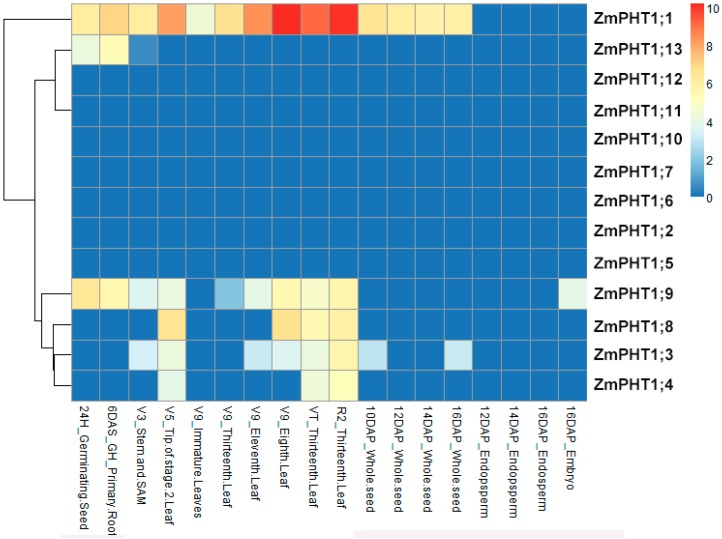
ZmPHT1 expression patterns were analyzed by HeapMap (Figure 4) [28]. The result showed that seven genes (ZmPHT1;2, ZmPHT1;5, ZmPHT1;6, ZmPHT1;7, ZmPHT1;10, ZmPHT1;11 and ZmPHT1;12) had no expression in any tissues, and ZmPHT1;1, ZmPHT1;3, ZmPHT1;4, ZmPHT1;8 and ZmPHT1;9 were expressed mainly in root and leaf, indicating that they play a significant role in Pi uptake and redistribution.
Figure 4.
Heat map of maize ZmPHT1 genes. The expression level of each ZmPHT1 genes can be estimated based on the scale to the right. Red indicates high expression level; yellow indicates medium expression level; and blue indicates low expression level. H, hours; DAS, days after sowing; GH, greenhouse; V, vegetative; DAP, days after pollination; VT, vegetative tasseling; R, reproductive.
2.6. Analysis of the Inorganic Phosphate (Pi) Transport Ability of ZmPHT1 Genes in a Pi-Uptake-Defective Yeast Strain
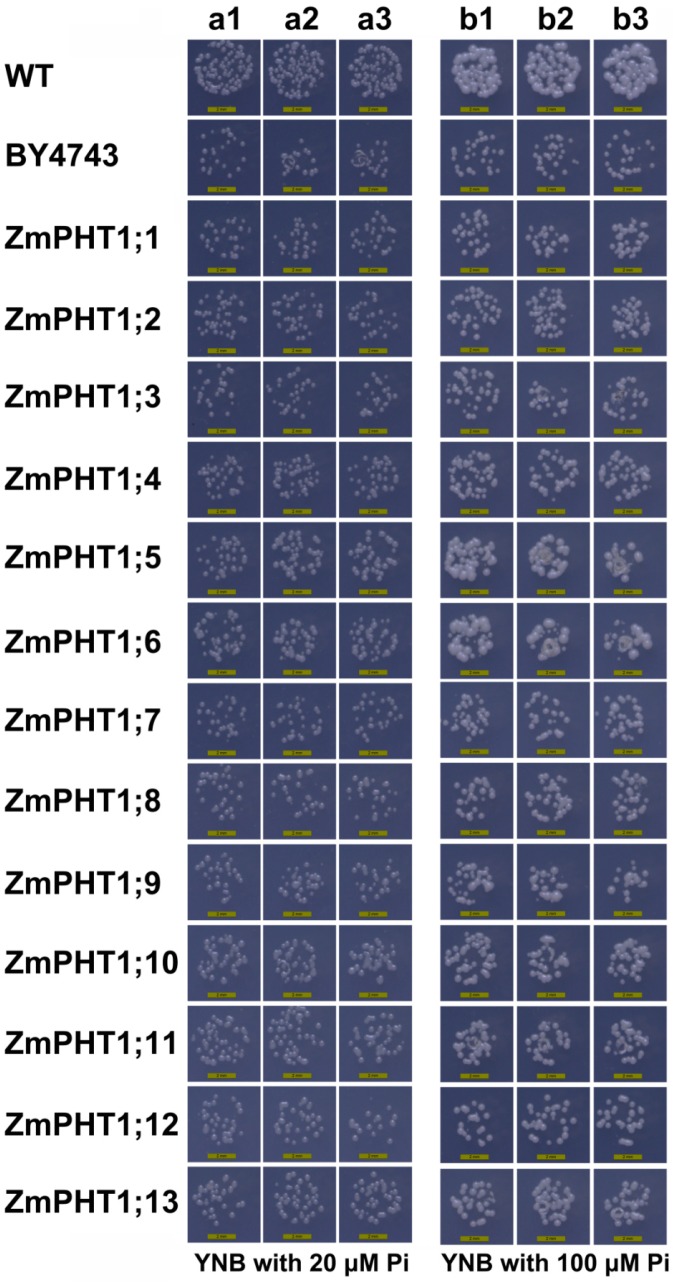
To analyze the Pi transport characteristics of 13 ZmPHT1 transporters, the whole open reading frames (ORF) were cloned into a yeast expression vector separately (pYES2). Each of these constructs was transformed into the yeast Pi transport mutant (BY4743) lacking the high-affinity Pi transporter gene PHO84. Transformed yeasts were cultured in YNB (Yeast Nitrogen Base) with low Pi condition (20 µM and 100 µM Pi). Results showed that the mutant cells of the yeast BY4743 strain grow poorly under low Pi conditions, while cells of ZmPHT1s transformants grow more or larger than that of the control, especially ZmPHT1;5, ZmPHT1;6, ZmPHT1;10, ZmPHT1;11 and ZmPHT1;13 (Figure 5), suggesting that all 13 putative ZmPHT1 proteins can completely or partly complement the yeast Pi transport mutant under low Pi statues.
Figure 5.
Functional characterizations of ZmPHT1s in a yeast inorganic phosphate (Pi) transport mutant. Under two Pi conditions three repeat experiments were set respectively. Repeat experiments were represented by a1, a2, a3 under 20 µM and b1, b2, b3 under 100 µM Pi conditions.
2.7. Influence of Pi Availability and AM Symbiosis on ZmPHT1 Gene Expression
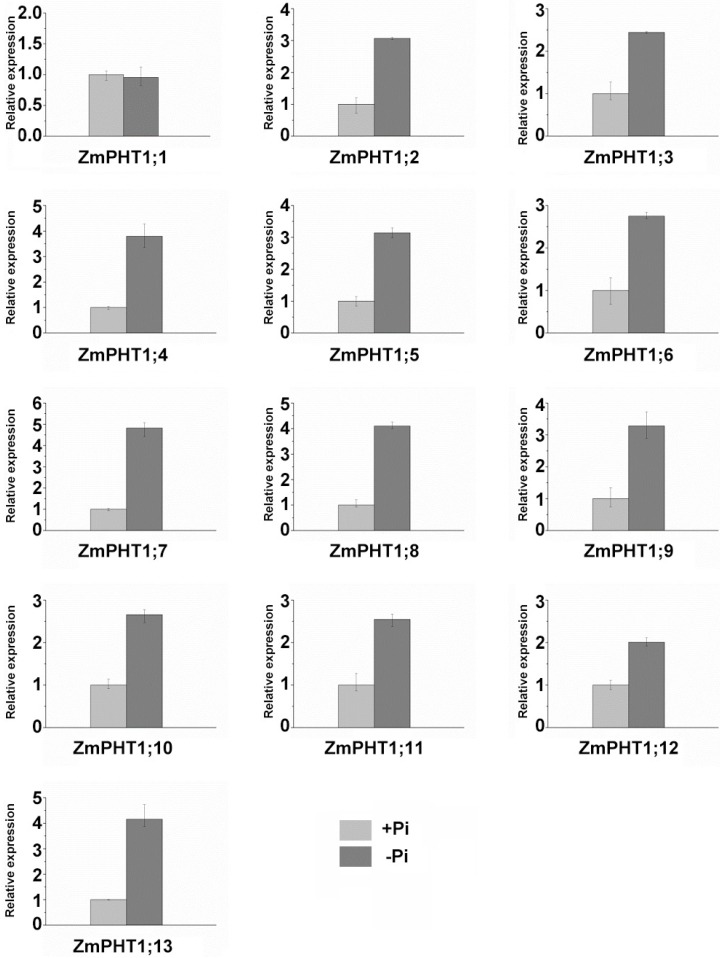
Transcripts levels of ZmPHT1 genes responding to Pi concentration were investigated using qRT-PCR under conditions of high Pi and low Pi (Figure 6). All of the ZmPHT1 genes were significantly induced under the low Pi condition except ZmPHT1;1 (p < 0.01), indicating that twelve of 13 ZmPHT1 genes can respond to low Pi.
Figure 6.
Expression pattern analyses of ZmPHT1 genes in root related to Pi availability. Maize was grown in nutrient solution containing 50 µM Pi and 5 mM Pi and sampled 40 days after treatment initiation. Data points are the means ± SD (n = 3 biological replicates of three plants each).
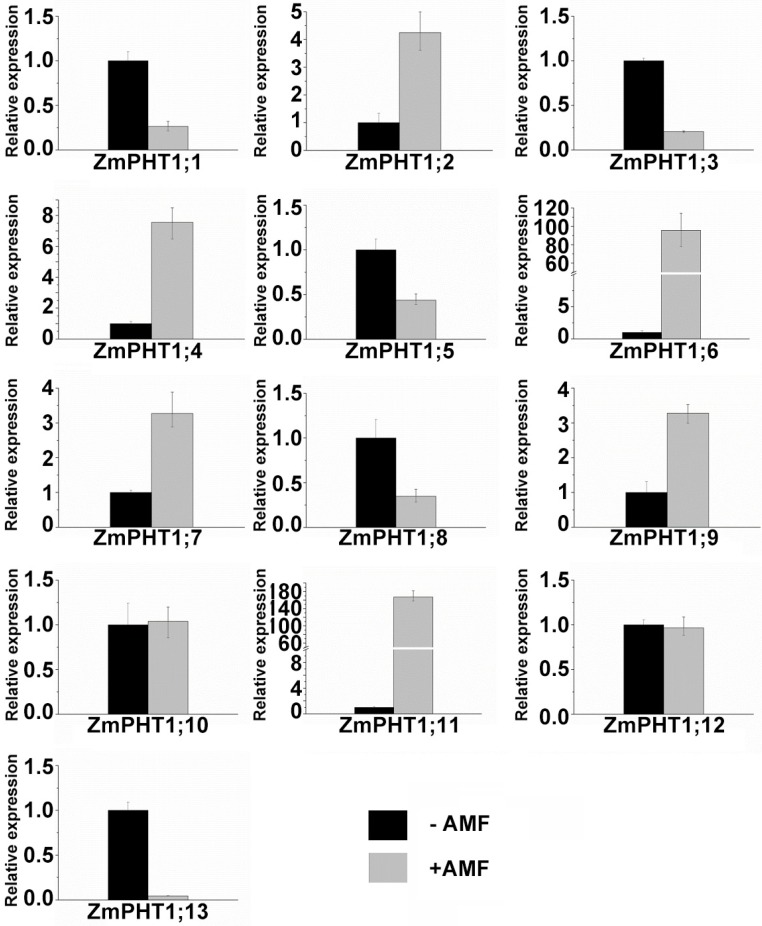
The expression of ZmPHT1 transporters in response to mycorrhizal colonization and non-inoculated roots was also assessed (Figure 7). ZmPHT1;2, ZmPHT1;4, ZmPHT1;6 and ZmPHT1;11 in the classes (I, II and IV) harboring AMF-inducible PHT1 transporters were induced in the AM fungal symbiont, except ZmPHT1;5 and ZmPHT1;8. In Class III, ZmPHT1;1, ZmPHT1;3 and ZmPHT1;13 were downregulated in inoculated profiles, whereas ZmPHT1;7 and ZmPHT1;9 were significantly upregulated (>3-fold). Conspicuously, the relative expression of the ZmPHT1;6 had a much higher transcript level in AM-induced roots than non-inoculated roots.
Figure 7.
qRT-PCR analysis of ZmPHT1 genes in root samples in response to AMF inoculation under low Pi supply. Maize were grown in a low Pi (50 µM) condition and an inoculation with autoclaved and active Glomus etunicatum, respectively. Data points are the means ± SD (n = 3 biological replicates of three plants each).
2.8. Identification of the Regulatory Cis-Elements
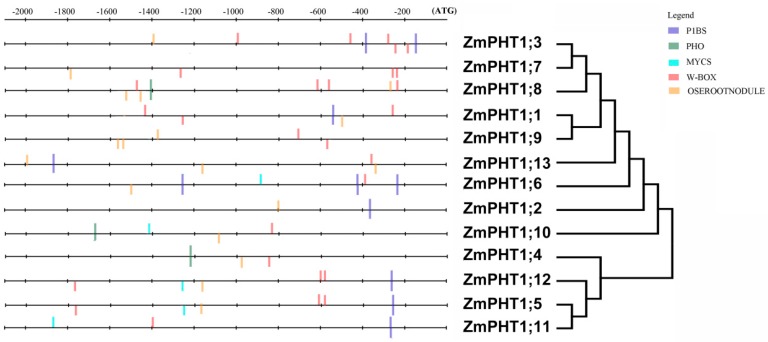
Regulatory elements in promoters of each ZmPHT1 were further analyzed. Elements involved in Pi starvation and AMF induction could be detected in 2.1-kb upstream regions of PHT1 genes (Figure 8). The number of AM-responsive elements (MYCS) and two Pi-regulated (P1BS and W-box) elements were differently distributed in the promoter regions of the 13 genes.
Figure 8.
Analysis of regulatory elements in the promoters of ZmPHT1 genes. Three previously reported Pi-responsive motifs (P1BS, PHO and W-box) and two AM-activated motif (MYCS and OSEROOTNODULE) were searched by DNA-pattern matching arithmetic. PHO (CACGTG), P1BS (GNATATNC), W-BOX (TTGACY), MYCS (TTTCTTGTTCT, or with one nucleotide variation), OSEROOTNODULE (AAAGAT).
P1BS (GNATATNC), playing a key role in Pi starvation response [29], exists in eight ZmPHT1s. It could be seen that one copy of P1BS was detected in the promoter of ZmPHT1;1, ZmPHT1;2, ZmPHT1;5, ZmPHT1;11, ZmPHT1;12 and ZmPHT1;13, while two copies in ZmPHT1;3 and three copies in ZmPHT1;6. No PIBS element was found in the promoter of ZmPHT1;4, ZmPHT1;7, ZmPHT1;8, ZmPHT1;9 and ZmPHT1;10. All of the 13 ZmPHT1s harbored another motif, OSEROOTNODULE (AAAGAT), which was a conserved AMF-inducible element. The MYCS (TTCTTGTTC) motif is only present in putative promoter regions of the five ZmPHT1s (ZmPHT1;5, ZmPHT1;6, ZmPHT1;10, ZmPHT1;11 and ZmPHT1;12); most of them could be upregulated by AM fungi (Figure 6) [29]. However, no MYCS (TTCTTGTTC) motif was detected in ZmPHT1;2 and ZmPHT1;4, implying that new AM-responsive elements should exist in the promoter of the two genes.
3. Discussion
3.1. Identification of ZmPHT1 Genes
In recent studies, many PHT1 genes have been identified from the genomes of several plants [15,16,17,18]. Although maize is an important crop species, only five high-affinity Pi transporters were isolated from the cDNA libraries of a commercial hybrid [18]. As one of the widely used and investigated elite maize inbred lines, B73, whose genome has been sequenced, however, no systematic work was reported on PHT1 family genes in the maize genome. In our study, a total of 13 PHT1 genes were predicted and identified from the B73 maize genome. The sequences of two PHT1 genes from the commercial hybrid were identical to the two corresponding sequences in inbred line B73 (Figure S3, Table S4). Of the 13 maize B73 PHT1 genes, eight of them were newly identified in this study. Similar to the PHT1 family genes in other species, proteins of the maize PHT1 family exhibited a high degree of identity and similar hydrophobic domains. The protein properties of putative ZmPHT1s, e.g., numbers of amino acid, theoretical molecular weight, putative membrane-spanning domain, etc., are also similar to that of other plants [10,15,16,17,18].
Yeast mutants losing the function in Pi transporters are used widely among three systems to characterize plant Pi transporters’ function (complementation of yeast mutants defective in Pi transports, monitoring Pi uptake using Xenopus laevis oocytes and ectopic expression of Pi transporters in plant suspension cells) [18,30]. In the present study, the yeast Pi transport mutant BY4743, which lacks the high-affinity Pi transport gene PHO84, was used to verify the complementary of Pi transport abilities of each putative ZmPHT1s. Our results indicated that all of 13 tested ZmPHT1s could completely or partly complement the yeast Pi-uptake mutant under a low Pi condition, implying that all of the putative ZmPHT1s functions have the function of Pi uptake and transportation under a low Pi condition [18].
3.2. Evolutionary Expansion of ZmPHT1 Genes
Phylogenetic analysis of ZmPHT1s compared to other Pi transporters from rice, sorghum and Brachypodium indicated a similar pattern of evolutionary divergence, reflecting different biochemical and functional characters among different types of PHTs. ZmPHT1;4, ZmPHT1;5, ZmPHT1;6, ZmPHT1;11 and ZmPHT1;12 each has an intron, implying that they had arisen from a duplication event of a primordial gene [31]. Interestingly, all of the ZmPHT1s have no intron or only one intron in the coding regions, meaning that Pht1 genes are some of the fast responses genes related to abiotic or biotic conditions [32]. Ka/Ks values for ZmPHT1;1/ZmPHT1;9, ZmPHT1;1/ZmPHT1;13 and ZmPHT1;8/ZmPHT1;10 were <1.0, which indicated that the paralog ZmPHT1 gene pairs were undergoing purifying selection in the maize evolution and, thus, may have been subfunctionalized [31]. ZmPHT1;1/ZmPHT1;9 and ZmPHT1;9/ZmPHT1;13 were produced in a recent duplication event, while ZmPHT1;1/ZmPHT1;13 and ZmPHT1;8/ZmPHT1;10 were generated in an early duplication event, corresponding to the timing of a recent and early WGD (whole genome duplication) event of maize, which occurred 12 Mya and 70 Mya, respectively [33].
3.3. ZmPHT1s Response to Pi Availability and AMF Inoculation
Expression of ZmPHT1s under different tissues showed that ZmPHT1;1, ZmPHT1;3, ZmPHT1;4, ZmPHT1;8 and ZmPHT1;9 are expressed mainly in root and leaf, suggesting that these genes should not only be involved in Pi uptake from soil, but also in the relocation of Pi across different plant tissues. Nevertheless, none of the rest ZmPHT1s were expressed in any tissues (Figure 4). The specialized expressions of these genes reflect well the divergence of regulatory elements requiring for formation of AM symbiosis and controlling Pi uptake. PHT1 genes were investigated in several different plants and revealed a dominant expression in root of many PHT1 genes, suggesting that these genes have a potential role in Pi capture and uptake [18,34].
We tested the responses of 13 ZmPHT1s to Pi starvation by qRT-PCR further and observed that 12 ZmPHT1s were upregulated under low Pi conditions, whereas only ZmPHT1;1 was inhibited. Although the coding sequences of ZmPHT1;1 and ZmPHT1;9 are identical, the expression levels of two members were obviously different whether in the arbuscular mycorrhizal or low Pi supply condition. Such a discrepancy between them may be caused by the different numbers and distributions of promoter elements, such as P1BS (ZmPHT1;1 contained one, but ZmPHT1;9 did not) and OSEROOTNODULE (ZmPHT1;1 contained one, but ZmPHT1;9 contained three).
Compared to the expression patterns between AMF colonization and low Pi condition, ZmPHT1;3, ZmPHT1;5, ZmPHT1;8 and ZmPHT1;13 were enhanced in response to limited soil Pi availability without AMF, but downregulated in response to AMF. All of them displayed a typical characteristic of PHT1 proteins involved in the direct Pi uptake pathway of plants [1,2,23]. ZmPHT1;2, ZmPHT1;4, ZmPHT1;6, ZmPHT1;7, ZmPHT1;9 and ZmPHT1;11 were highly expressed in roots colonized by AMF, indicating that they are possibly involved in Pi uptake through a symbiotic pathway at the mycorrhizal interface [35]. ZmPHT1;2, ZmPHT1;4, ZmPHT1;6 and ZmPHT1;11, respectively clustering in a specific clade of AM-inducible Pi transporters from other plants, were induced by mycorrhizal and non-mycorrhizal roots under low Pi conditions, proposing the direct Pi uptake pathway shift to the mycorrhizal pathway possibly during the AM symbiosis establishment.
3.4. Regulatory Cis-Elements in Promoters of AMF-Inducible ZmPHT1s
A phylogenetic dendrogram containing mycorrhizal-specific genes between different species was constructed. With well-supported bootstrap values, ZmPHT1;2 clustered with Brachypodium mycorrhizal-specific BdPHT1;3 and S. italic mycorrhizal-specific SiPHT1;8, whereas ZmPHT1;4, ZmPHT1;5, ZmPHT1;11 and ZmPHT1;12 clustered with rice mycorrhizal-specific OsPHT1;13 and sorghum mycorrhizal-specific SbPHT1;9 and ZmPHT1;10 clustered with AM-inducible SbPHT1;10, BdPHT1;12 and BdPHT1;13, suggesting that these genes may be involved in AM response. Interestingly, ZmPHT1;6 clustered with AM-inducible PHTs in monocot (SiPHT1;9, OsPHT1;11 and BdPHT1;7) and dicot (StPHT1;5, MtPHT1;4, GmPHT1;12 and GmPHT1;13), indicating a specific AM-inducible PHT1 clade. Indeed, several plant species, such as Lycopersicon esculentum, O. sativa and S. tuberosum, possess multiple AM-induced PHT1 genes, which possibly result in a functional redundancy of Pi transport in AM symbiosis [22].
The expression pattern induced by AMF and the phylogenetic relationship of ZmPHT1s in this study are similar to the PHT1 genes of other plants [2,7,15,19]. However, ZmPHT1;5, ZmPHT1;8 and ZmPHT1;12 were repressed, indicating that the influence of AMF on ZmPHT1;s was not only enhanced, but also repressed, and ZmPHT1;5, ZmPHT1;8 or ZmPHT1;12 should be redundantly regulated for Pi uptake in maize AM symbiosis. In Class A, ZmPHT1;1, ZmPHT1;3 and ZmPHT1;13 with more than two P1BS elements were repressed in inoculated profiles, whereas ZmPHT1;7 (>3-fold) and ZmPHT1;9 (>3-fold) with no P1BS element were significantly induced, suggesting that P1BS possibly has some negative influence on the expression of the AM-induced gene. However, the relative expression of the ZmPHT1;6 genes with three P1BS, but one MYCS element, had much higher expression levels (~100-fold) in the AM-induced root, revealing that MYCS may be the main regulation factor for AM-induced gene expression compared to P1BS. It is interesting that some members of ZmPHT1s without the MYCS element, such as ZmPHT1;2, ZmPHT1;4, ZmPHT1;7 and ZmPHT1;9, still upregulated the expression, implying that new regulation element(s) might exist in the promoter regions of some maize PHT1 genes. Then, as a previous study had found that the CTTC motif is necessary and sufficient for plants responding to fungal colonization under Pi-limited conditions, a further study of CTTC motifs on ZmPHT1s was investigated [20,36]. The result showed that ZmPHT1;4, ZmPHT1;7 and ZmPHT1;9 harbored CTTC motifs in the putative promoter regions (Figure S4), which might be related to their upregulation in inoculated profiles. However, there is no such motif in the promoter of ZmPHT1;2 (Figure S4), despite its induction following AMF colonization. Thus, the elements in the ZmPHT1;2 promoter responsible for AMF induction remain unclear.
4. Materials and Methods
4.1. Bioinformatics Analyses
Previously reported maize, A. thaliana and O. sativa phosphate transporter proteins were used as queries against the maize genome database. After removal of overlapping sequences, the remaining genes were verified in the Pfam database (http://pfam.xfam.org/). The protein sequences were aligned by the ClustalX (Version 1.81) with default parameters. Then, the phylogenetic tree was constructed with the neighbor-joining method within MEGA 6, and the bootstrap was 1000 replicates in the mode of pairwise gap deletion [37]. The exon and intron structures of each ZmPHT1 gene were analyzed by GSDS (Gene Structure Display Server; http://gsds.cbi.pku.edu.cn/) through alignment of their CDS with corresponding genomic DNA sequences. Conserved motifs among the ZmPHT1 genes were examined via MEME software (Multiple Expectation Maximization for Motif Elicitation) [38], and the parameters were set as follows: the number of repetitions was choose any; the maximum number of motifs was 10; and the optimum motif width was set between 6 and 50 residues. Moreover, motif annotation was performed by the Pfam and SMART (http://smart.embl-heidelberg.de/) tools. Chromosome location was performed using the Map Inspect software [39]. The duplication pattern for each ZmPHT1 gene was analyzed by MCScanX software [40], and the synthesis map was drawn by Circos software (http://circos.ca/) [41]. Gene clusters that met the following specific clustering criteria were selected, i.e., genes arising from tandem duplication events on the same chromosome region, two or more homologous genes within a 200-kb region [42] and fragments derived from replication events [43]. The calculation of Ka, Ks was by DnaSPv5.0 [44]. The nonsynonymous substitution per synonymous site (Ka/Ks) ratio analysis was performed by sliding window analysis with the parameters as follows: window size, 150 bp; step size, 9 bp [42]. The gene expression data from Maize B73 transcriptomes were used to draw a heat map [28], including information from germinating seeds, primary roots, stems, SAMs (shoot apical meristem), leaves, endosperm, embryos and whole seeds. Promoters were analyzed by RSAT (http://floresta.eead.csic.es/rsat/) [45].
4.2. Plant Material and Cultivation Conditions
Maize B73 seeds germinated in a sterile environment with a photoperiod of 16 h light and 8 h dark at 28 °C. After germination, some seedlings were transferred to pots filled with sand-based culture for inoculation with AMF. Additionally, the rest of the seedlings were transferred to pots filled with sand-based culture for cultivation with 50 µM and 5 mM Pi supplied. The nutrient solution contained 50 µM/5 mM KH2PO4. The basal nutrient solution contained 2 mM Ca(NO3)2, 0.65 mM MgSO4, 25 μM FeEDTA, 5 μM MnSO4, 50 μM KCl, 2 μM ZnSO4, 0.5 μM CuSO4, 0.005 μM (NH4)6Mo7O24 and 25 mΜ H3BO4. In addition, K+ was supplied in the LP (Low Phosphate condition) solution in the form of 0.95 mM KCl [46]. Three seedlings were transplanted to a sterilized sand-based pot. The sand base contained Glomus etunicatum were used for colonization. Tissues (wild type and inoculated plants) were harvested at 40 days post-inoculation. Parts of secondary roots were used for the detection of mycorrhizal colonization, and the rest of the secondary roots were stored at −80 °C after being frozen in liquid nitrogen and were used for subsequent RNA isolation.
4.3. Detection of Mycorrhizal Colonization
Maize roots were firstly treated with 10% KOH and heated 1 h at 90 °C, further treated 5 min with 2% HCl solution. Then, the root samples were stained with Trypan Blue and then they were transparent with lactic acid and glycerin. Dyed roots were detected by a microscope (Figure S5).
4.4. Total RNA Extraction
Root total RNA was isolated using RNA Plus (Takara, Dalian, China) with the guanidine thiocyanate extraction method. Then, 1.2% agarose gel and the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) were used to evaluate and quantify RNA, respectively. DNase was used to eliminate the potential trace of genomic DNA in RNA samples.
4.5. qRT-PCR
The Roche reverse transcription kit was used to synthesize cDNA first. A 20-µL reaction solution contained 1 µg RNA approximately. Then, synthesized cDNAs were used as templates for qRT-PCR. The qRT-PCR was carried out on Applied Biosystems 7300 using the SYBGREEN kit (Roche, Basel, Switzerland). The qRT-PCR program was used as follows: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The expression level of the maize Actin 1 gene was used as an internal control. The relative transcript level was calculated as 2−ΔΔCt. The relative expression level in the normal plant without tress treatment was normalized to 1. The primer pairs for each of the ZmPHT1 genes are listed in Supplementary Table S2, and the melting curves are listed in Figure S6.
4.6. Yeast Manipulations
The yeast mutant BY4743, which defects PHO84, a high-affinity Pi transporter gene [18,47], and the expression vector pYES2 were used in yeast complement experiments. The full length coding sequences of ZmPHT1s were amplified from cDNAs using PrimeSTAR Max DNA Polymerase (Takara), which has high amplification efficiency, high sensitivity and high specificity characteristics. The PCR was performed in a 50-μL volume, which contained 25 μL of 2× PrimerSTAR Max Premix, 2 μL diluted cDNA template, 1.25 μL of each specific primer (10 μM of each primer) and 20.5 μL ddH2O. The PCR program was used as follows: 35 cycles at 98 °C for 10 s, 56 to 68 °C for 30 s, 72 °C for 1 min and at last, 72 °C for 10 min. Sequencing was used for all PCR products’ verity. Then, PCR productions were subcloned into pYES2 (preserved by our lab). These constructs were transformed into the yeast BY4743. Transformed yeast strains grew in YNB medium to the logarithmic phase, and then were harvested and washed with Pi-free YNB medium. Collected yeast was firstly suspended in Pi-free YNB medium and cultivated until the absorbance at 600 nm was 1.0. Then, 10-fold serial dilution with equal volumes was applied to solid YNB medium (the sample volume was 3 µL) supplied with 20 and 100 µM KH2PO4 and incubated at 30 °C for 2 days.
5. Conclusions
Eight of 13 putative PHT1 genes were newly identified in the maize elite inbred line B73 genome. All of the 13 genes were complementary verified in the yeast Pi-uptake mutant. The locations of the ZmPHT1s on maize chromosomes and duplication events were also investigated in detail. All of the ZmPHT1 genes were significantly induced under the low Pi supply condition, with the exception of ZmPHT1;1 (p < 0.01). ZmPHT1;2, ZmPHT1;4, ZmPHT1;6, ZmPHT1;7, ZmPHT1;9 and ZmPHT1;11 were upregulated in arbuscular mycorrhizal. The AM-responsive element (MYCS) and P1BS element can be observed in promoters of the majority of AMF-inducible ZmPHT1 genes.
Acknowledgments
This study was financially supported by the National Science Foundation of China (Project No. 31470465, 51309003 and 31301756), the biological science promotion project of Anhui Agricultural University (2014TSTD001) and the Doctoral Fund of Ministry of Education of China (Project No. 20133418120003).
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| NJ | Neighbor-joining |
| Ks | The rate of synonymous substitutions |
| Ka | The rate of nonsynonymous substitutions |
| MYCS | Mycorrhizal transcription factor binding sequence |
| P1BS | PHR1 binding site |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/6/930/s1.
Author Contributions
Fang Liu and Yunjian Xu designed and carried out the majority of the bioinformatics studies. Huanhuan Jiang implemented the software and participated in performing the experiments. Chaosheng Jiang and Yibin Du were involved in plant cultivation and experimental data analysis. Fang Liu and Yunjian Xu wrote the manuscript. Cheng Gong, Suwen Zhu and Wei Wang participated in the design of the study and helped to draft the manuscript. Beijiu Cheng and Guomin Han conceived of and directed the study. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lopez-Arredondo D.L., Leyva-González M.A., González-Morales S.I., López-Bucio J., Herrera-Estrella L. Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops. Annu. Rev. Plant Biol. 2014;65:95–123. doi: 10.1146/annurev-arplant-050213-035949. [DOI] [PubMed] [Google Scholar]
- 2.Javot H., Pumplin N., Harrison M.J. Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles. Plant Cell Environ. 2007;30:310–322. doi: 10.1111/j.1365-3040.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsuzuki S., Handa Y., Takeda N., Kawaguchi M. Strigolactone-Induced Putative Secreted Protein 1 Is Required for the Establishment of Symbiosis by the Arbuscular Mycorrhizal Fungus Rhizophagus Irregularis. Mol. Plant Microbe Interact. 2016;29:277–286. doi: 10.1094/MPMI-10-15-0234-R. [DOI] [PubMed] [Google Scholar]
- 4.Van Der Heijen M.G.A., Bardgett R., van Straalen N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008;11:291–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Heijden M.G.A., Streitwolf-Engel R., Riedl R., Siegrist S., Neudecker A., Ineichen K., Boller T., Wiemken A., Sanders I.R. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 2006;172:739–752. doi: 10.1111/j.1469-8137.2006.01862.x. [DOI] [PubMed] [Google Scholar]
- 6.Ayadi A., David P., Jean-François A., Chiarenza S., Marie-Christine T., Nussaume L., Marin E. Reducing the Genetic Redundancy of Arabidopsis PHOSPHATE TRANSPORTER1 Transporters to Study Phosphate Uptake and Signaling. Plant Physiol. 2015;167:1511–1526. doi: 10.1104/pp.114.252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walder F., Brule D., Koegel S., Wiemken A., Boller T., Pierre-Emmanuel C. Plant phosphorus acquisition in a common mycorrhizal network: Regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol. 2015;205:1632–1645. doi: 10.1111/nph.13292. [DOI] [PubMed] [Google Scholar]
- 8.Karandashov V., Bucher M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005;10:22–29. doi: 10.1016/j.tplants.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen B.P., Kumar H., Waight A.B., Risenmay A.J., Roe-Zurz Z., Chau B.H., Schlessinger A., Bonomi M., Harries W., Sali A., et al. Crystal structure of a eukaryotic phosphate transporter. Nature. 2013;496:533–536. doi: 10.1038/nature12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muchhal U.S., Pardo J.M., Raghothama K.G. Phosphate transporters from the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1996;93:10519–10523. doi: 10.1073/pnas.93.19.10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodstein D.M., Shu S., Howson R., Neupane R., Hayes R.D., Fazo J., Mitros T., Dirks W., Hellsten U., Putnam N., et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham F., Amode M.R., Barrell D., Beal K., Billis K., Brent S., Carvalho-Silva D., Clapham P., Coates G., Fitzgerald S., et al. Ensembl 2015. Nucleic Acids Res. 2015;43:D662–D669. doi: 10.1093/nar/gku1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnable P.S. The B73 maize genome: Complexity, diversity, and dynamics (November, pg 1112, 2009) Science. 2012;337:1040. doi: 10.1126/science.1222458.. [DOI] [PubMed] [Google Scholar]
- 14.Misson J., Thibaud M.C., Bechtold N., Raghothama K., Nussaume L. Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol. Biol. 2004;55:727–741. doi: 10.1007/s11103-004-1965-5. [DOI] [PubMed] [Google Scholar]
- 15.Paszkowski U., Kroken S., Roux C., Briggs S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA. 2002;99:13324–13329. doi: 10.1073/pnas.202474599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen A., Chen X., Wang H., Liao D., Gu M., Qu H., Sun S., Xu G. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant Biol. 2014;14:930. doi: 10.1186/1471-2229-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie X., Huang W., Liu F., Tang N., Liu Y., Lin H., Zhao B. Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol. 2013;198:836–852. doi: 10.1111/nph.12188. [DOI] [PubMed] [Google Scholar]
- 18.Qin L., Guo Y., Chen L., Liang R., Gu M., Xu G., Zhao J., Walk T., Liao H. Functional characterization of 14 Pht1 family genes in yeast and their expressions in response to nutrient starvation in soybean. PLoS ONE. 2012;7:930. doi: 10.1371/journal.pone.0047726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong J.J., Park Y.S., Bravo A., Bhattarai K.K., Daniels D.A., Harrison M.J. Diversity of morphology and function in arbuscular mycorrhizal symbioses in Brachypodium distachyon. Planta. 2012;236:851–865. doi: 10.1007/s00425-012-1677-z. [DOI] [PubMed] [Google Scholar]
- 20.Ceasar S.A., Hodge A., Baker A., Baldwin S.A. Phosphate Concentration and Arbuscular Mycorrhizal Colonisation Influence the Growth, Yield and Expression of Twelve PHT1 Family Phosphate Transporters in Foxtail Millet (Setaria italica) PLoS ONE. 2014;9:930. doi: 10.1371/journal.pone.0108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison M.J., Dewbre G.R., Liu J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell. 2002;14:2413–2429. doi: 10.1105/tpc.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Güimil S., Chang H.S., Zhu T., Sesma A., Osbourn A., Roux C., Ioannidis V., Oakeley E.J., Docquier M., Descombes P., et al. Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc. Natl. Acad. Sci. USA. 2005;102:8066–8070. doi: 10.1073/pnas.0502999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rausch C., Daram P., Brunner S., Jansa J., Laloi M., Leggewie G., Amrhein N., Bucher M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature. 2001;414:462–470. doi: 10.1038/35106601. [DOI] [PubMed] [Google Scholar]
- 24.Nagy R., Karandashov V., Chague V., Kalinkevich K., Tamasloukht M., Xu G., Jakobsen I., Levy A.A., Amrhein N., Bucher M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005;42:236–250. doi: 10.1111/j.1365-313X.2005.02364.x. [DOI] [PubMed] [Google Scholar]
- 25.Glassop D., Smith S.E., Smith F.W. Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta. 2005;222:688–698. doi: 10.1007/s00425-005-0015-0. [DOI] [PubMed] [Google Scholar]
- 26.Nagy R., Vasconcelos M.J., Zhao S., McElver J., Bruce W., Amrhein N., Raghothama K.G., Bucher M. Differential regulation of five Pht1 phosphate transporters from maize (Zea mays L.) Plant Biol. 2006;8:186–197. doi: 10.1055/s-2005-873052. [DOI] [PubMed] [Google Scholar]
- 27.Tian H., Drijber R.A., Li X., Miller D.N., Wienhold B.J. Arbuscular mycorrhizal fungi differ in their ability to regulate the expression of phosphate transporters in maize (Zea mays L.) Mycorrhiza. 2013;23:507–514. doi: 10.1007/s00572-013-0491-1. [DOI] [PubMed] [Google Scholar]
- 28.Sekhon R.S., Briskine R., Hirsch C.N., Myers C.L., Springer N.M., Buell C.R., de Leon N., Kaeppler S.M. Maize Gene Atlas Developed by RNA Sequencing and Comparative Evaluation of Transcriptomes Based on RNA Sequencing and Microarrays. PLoS ONE. 2013;8:930. doi: 10.1371/journal.pone.0061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio V., Linhares F., Solano R., Martín A.C., Iglesias J., Leyva A., Paz-Ares J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001;15:2122–2133. doi: 10.1101/gad.204401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye Y., Yuan J., Chang X., Yang M., Zhang L., Lu K., Lian X. The Phosphate Transporter Gene OsPht1;4 Is Involved in Phosphate Homeostasis in Rice. PLoS ONE. 2015;10:930. doi: 10.1371/journal.pone.0126186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch M., Conery J.S. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Liu B., Li J., Zhang L., Wang Y., Zheng H., Lu M., Chen J. Hsf and Hsp gene families in Populus: Genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genom. 2015;16 doi: 10.1186/s12864-015-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei F., Coe E., Nelson W., Bharti A.L., Engler F., Butler E., Kim H., Goicoechea J.L., Chen M., Lee S., et al. Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genet. 2007;3:1254–1263. doi: 10.1371/journal.pgen.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F., Chang X.J., Ye Y., Xie W.B., Wu P., Lian X.M. Comprehensive Sequence and Whole-Life-Cycle Expression Profile Analysis of the Phosphate Transporter Gene Family in Rice. Mol. Plant. 2011;4:1105–1122. doi: 10.1093/mp/ssr058. [DOI] [PubMed] [Google Scholar]
- 35.Smith S.E., Jakobsen I., Grønlund M., Smith F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011;156:1050–1057. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lota F., Wegmüller S., Buer B., Sato S., Bräutigam A., Hanf B., Bucher M. The cis-acting CTTC-P1BS module is indicative for gene function of LjVTI12, a Qb-SNARE protein gene that is required for arbuscule formation in Lotus japonicus. Plant J. 2013;74:280–293. doi: 10.1111/tpj.12120. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Feng L., Zhu Y., Li Y., Yan H., Xiang Y. Comparative genomic analysis of the WRKY III gene family in Populus, Grape, Arabidopsis and Rice. Biol. Direct. 2015;10:1–27. doi: 10.1186/s13062-015-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., Lee T.H., Jin H., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holub E.B. The arms race is ancient history in Arabidopsis, the wildflower. Nat. Rev. Genet. 2001;2:516–527. doi: 10.1038/35080508. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y., Cheng Y., Jin J., Jin X., Jiang H., Yan H., Cheng B. Genome Duplication and Gene Loss Affect the Evolution of Heat Shock Transcription Factor Genes in Legumes. PLoS ONE. 2014;9:930. doi: 10.1371/journal.pone.0102825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 45.Medina-Rivera A., Defrance M., Sand O., Herrmann C., Castro-Mondragon J.A., Delerce J., Jaeger S., Blanchet C., Vincens P., Caron C., et al. RSAT 2015: Regulatory Sequence Analysis Tools. Nucleic Acids Res. 2015;43:W50–W56. doi: 10.1093/nar/gkv362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Bai J., Liu H., Sun Y., Shi X., Ren Z. Overexpression of a Maize Transcription Factor ZmPHR1 Improves Shoot Inorganic Phosphate Content and Growth of Arabidopsis under Low-Phosphate Conditions. Plant Mol. Biol. Rep. 2013;31:665–677. doi: 10.1007/s11105-012-0534-3. [DOI] [Google Scholar]
- 47.Winzeler E.A., Shoemaker D.D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J.D., Bussey H., et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.