Abstract
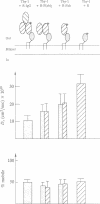
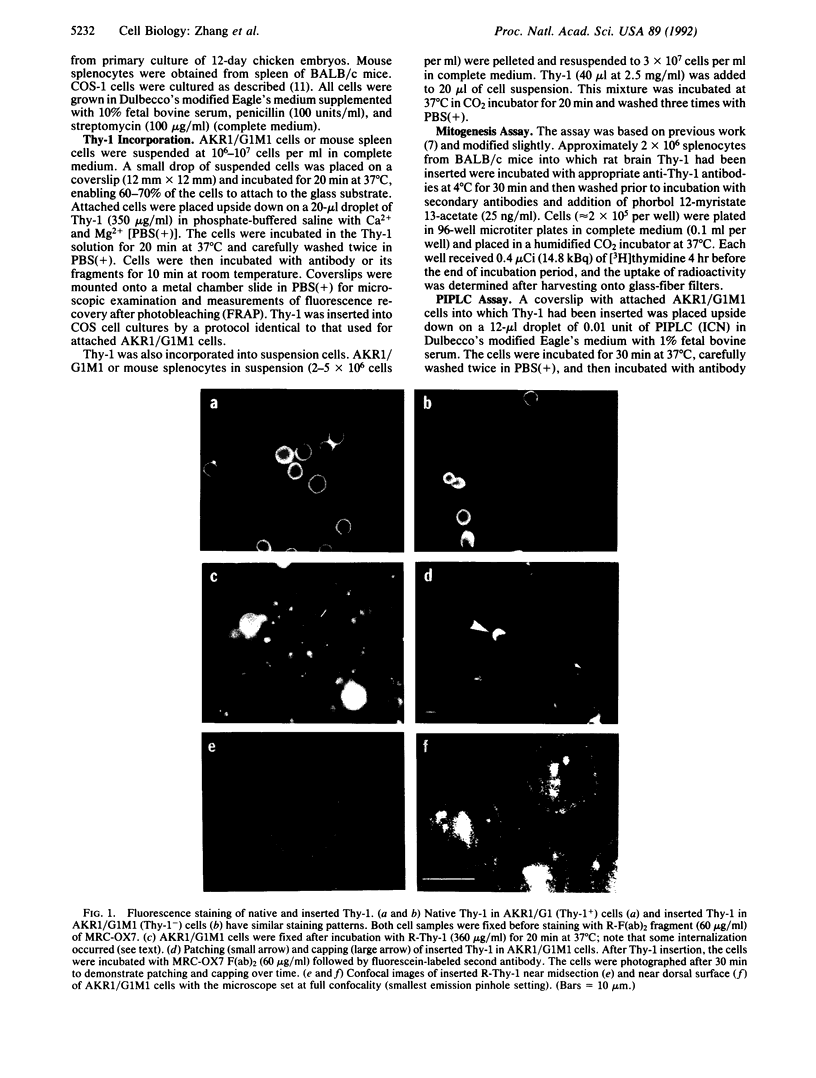
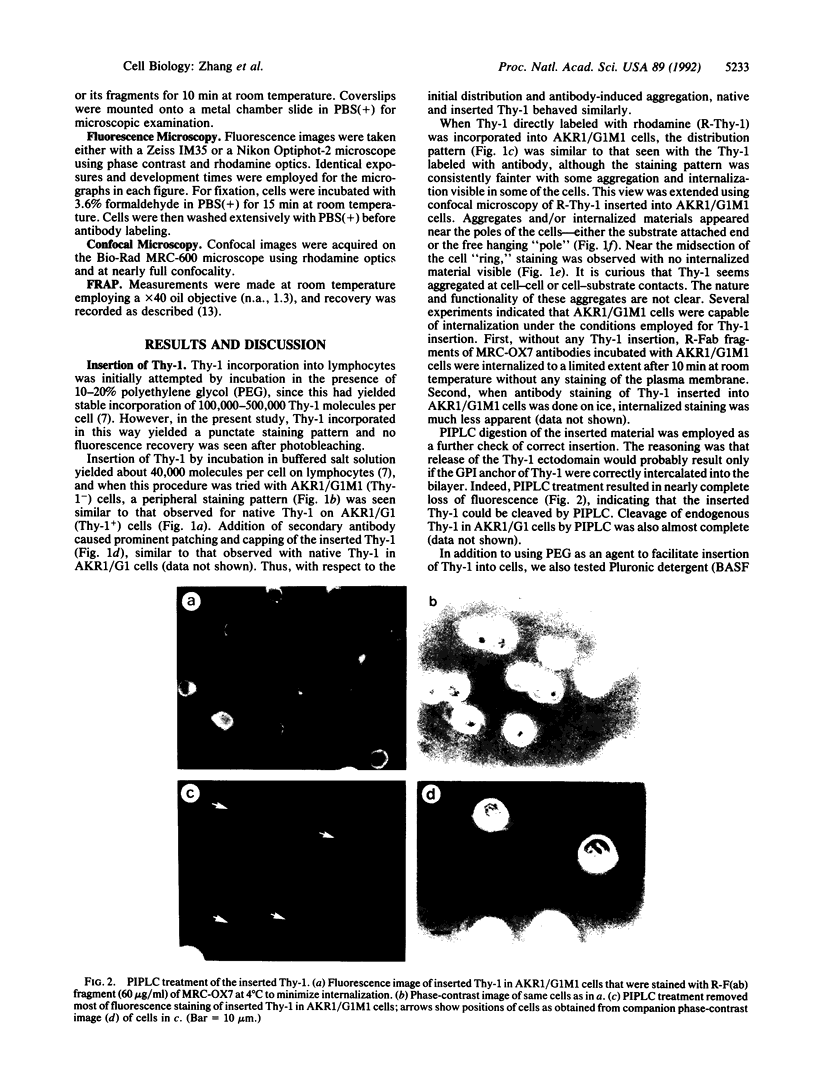
Thy-1 is a membrane protein that is attached to the plasma membrane by a glycosyl-phosphatidylinositol anchor. Purified rat brain Thy-1 could be reincorporated into the plasma membrane of murine Thy-1- cells directly from aqueous suspension and without the use of detergents. A peripheral staining pattern similar to that observed for endogenous Thy-1 was achieved. Treatment with phosphatidylinositol-specific phospholipase C removed nearly all antibody staining due to either endogenous or inserted Thy-1. Fluorescence recovery after photobleaching (FRAP) was used to compare the lateral mobility of endogenous and inserted Thy-1. Both forms exhibited large lateral diffusion coefficients, but with a substantial immobile fraction (approximately 50%) indicating that the immobile fraction was not due either to chemical differences between inserted and native Thy-1 or to some surface Thy-1 molecules having a protein anchor. However, the inserted Thy-1 failed to activate mouse T lymphocytes upon crosslinking as assayed by [3H]thymidine uptake. Since Thy-1 could be directly labeled with rhodamine, the effect of the size of the labeling ligand on the mobility obtained by the FRAP technique could be explored. Rhodamine-conjugated MRC-OX7 monoclonal antibody or its fragments [R-F(ab)2 or R-Fab] were compared with rhodamine as labels for Thy-1. The measured diffusion coefficients were 1.6 x 10(-9), 2.0 x 10(-9), and 3.2 x 10(-9) cm2/sec for Thy-1 labeled with R-F(ab)2, R-Fab, and rhodamine, respectively; mobile fractions were all in the 40-50% range. Thus, the size of the ligand affects the lateral mobility of this labeled membrane protein to a measurable extent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bülow R., Overath P., Davoust J. Rapid lateral diffusion of the variant surface glycoprotein in the coat of Trypanosoma brucei. Biochemistry. 1988 Apr 5;27(7):2384–2388. doi: 10.1021/bi00407a020. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. T., Naquet P., Caillol D., Pierres M. Thy-1 supports adhesion of mouse thymocytes to thymic epithelial cells through a Ca2(+)-independent mechanism. J Exp Med. 1991 Feb 1;173(2):515–518. doi: 10.1084/jem.173.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli D., Rungger-Brändle E. Association of specific cell-surface glycoproteins with a triton X-100-resistant complex of plasma membrane proteins isolated from T-lymphoma cells (P1798). Exp Cell Res. 1985 Jan;156(1):239–250. doi: 10.1016/0014-4827(85)90278-2. [DOI] [PubMed] [Google Scholar]
- Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W., Anand R., Williams A. F. Complete structure of the glycosyl phosphatidylinositol membrane anchor of rat brain Thy-1 glycoprotein. Nature. 1988 May 19;333(6170):269–272. doi: 10.1038/333269a0. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Hou Y., Jacobson K. The Thy-1 antigen exhibits rapid lateral diffusion in the plasma membrane of rodent lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1290–1293. doi: 10.1073/pnas.84.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Derzko Z., Wojcieszyn J., Organisciak D. Lipid lateral diffusion in the surface membrane of cells and in multibilayers formed from plasma membrane lipids. Biochemistry. 1981 Sep 1;20(18):5268–5275. doi: 10.1021/bi00521a027. [DOI] [PubMed] [Google Scholar]
- Kroczek R. A., Gunter K. C., Germain R. N., Shevach E. M. Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature. 1986 Jul 10;322(6075):181–184. doi: 10.1038/322181a0. [DOI] [PubMed] [Google Scholar]
- Kroczek R. A., Gunter K. C., Seligmann B., Shevach E. M. Induction of T cell activation by monoclonal anti-Thy-1 antibodies. J Immunol. 1986 Jun 15;136(12):4379–4384. [PubMed] [Google Scholar]
- Kuchel P. W., Campbell D. G., Barclay A. N., Williams A. F. Molecular weights of the Thy-1 glycoproteins from rat thymus and brain in the presence and absence of deoxycholate. Biochem J. 1978 Feb 1;169(2):411–417. doi: 10.1042/bj1690411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Tse A. G., Dwek R. A., Williams A. F., Rademacher T. W. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987 May;6(5):1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullion B. F., Hou Y., Puddington L., Rose J. K., Jacobson K. Effects of mutations in three domains of the vesicular stomatitis viral glycoprotein on its lateral diffusion in the plasma membrane. J Cell Biol. 1987 Jul;105(1):69–75. doi: 10.1083/jcb.105.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Tiveron M. C., Barboni E., Pliego Rivero F. B., Gormley A. M., Seeley P. J., Grosveld F., Morris R. Selective inhibition of neurite outgrowth on mature astrocytes by Thy-1 glycoprotein. Nature. 1992 Feb 20;355(6362):745–748. doi: 10.1038/355745a0. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Zhang F., Crise B., Su B., Hou Y., Rose J. K., Bothwell A., Jacobson K. Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol-linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J Cell Biol. 1991 Oct;115(1):75–84. doi: 10.1083/jcb.115.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brabander M., Nuydens R., Ishihara A., Holifield B., Jacobson K., Geerts H. Lateral diffusion and retrograde movements of individual cell surface components on single motile cells observed with Nanovid microscopy. J Cell Biol. 1991 Jan;112(1):111–124. doi: 10.1083/jcb.112.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]