Abstract
Immunotherapy for glioblastoma (GBM) provides a unique opportunity for targeted therapies for each patient, addressing individual variability in genes, tumor biomarkers and clinical profile. As immunotherapy has the potential to specifically target tumor cells with minimal risk to normal tissue, several immunotherapeutic strategies are currently being evaluated in clinical trials in GBM. With the Precision Medicine Initiative being announced in the President's State of the Union Address in 2016, GBM immunotherapy provides a useful platform for changing the landscape in treating patients with difficult disease.
KEYWORDS : glioblastoma, immunotherapy, precision medicine
Practice points.
Patients with glioblastoma (GBM), the most common primary malignant brain tumor of adulthood, have a median overall survival time of just 14–16 months despite optimized treatment including maximal safe resection followed by radiotherapy and chemotherapy.
Precision medicine in GBM immunotherapy provides a unique opportunity for tumor-specific targeted therapies for each patient.
Most therapeutic targets in GBM are only expressed in subsets of patients and, in many cases, rarely throughout the tumor.
Comprehensive molecular profiling of large patient cohorts will likely be required to identify patients that may benefit from targeted approaches.
In contrast to the implementation of precision medicine in other malignancies, GBM will require additional considerations for blood–brain barrier penetration for targeted agents and/or consideration of trafficking of antitumor immune responses to the CNS.
There are obstacles, yet potential solutions, in precision medicine implementation in GBM immunotherapy.
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults and has a median overall survival time of only 14–16 months despite optimal treatment including resection followed by radiotherapy and chemotherapy [1]. Unfortunately, GBM is an infiltrative tumor with vast heterogeneity, making a surgical cure impossible and treatment resistance frequent. The prognosis remains poor and recurrence is universal despite maximal treatment because the tumor carries mutations that may allow it to bypass drug-targeted pathways.
The overarching concept of precision medicine is personalized care that takes into account genetic variability, tumor biomarkers (including those that may correlate with immune therapeutic responses), and clinical profiles in order to provide targeted therapies for each individual patient. The ultimate goal of this strategy is to develop more specific therapeutics for effective and rational cancer treatment. Precision medicine is especially significant in cancer care where nonspecific, standardized chemotherapeutic treatments have the potential to induce significant toxicities. As such, this model has been implemented in the treatment of a variety of malignancies [2–6]. The USA has launched a Precision Medicine Initiative with an associated US$215 million investment, further indicating the importance of this treatment paradigm shift in human disease.
As our knowledge of glioma has advanced, including the designation of distinct molecular subtypes, identification of targetable molecular alterations and a better understanding of the tumor microenvironment, personalized GBM therapy based on specific tumor and patient factors is an increasingly viable therapeutic approach. As immunotherapy has the potential to specifically target tumor cells with minimal risk to normal tissue, several immunotherapeutic strategies are currently being evaluated in clinical trials. Immunotherapy is generally defined as therapy that centers on using the patient's own immune machinery to kill malignant cells. This treatment presents a unique opportunity for precision medicine in GBM, given that conventional therapy is nonspecific, leading to damage to surrounding normal brain tissue and systemic toxicity. There are certain components that are vital for an immunotherapeutic agent to be effective. First, there must be an appropriate therapeutic target. The ideal target would be specific to the tumor and have a high frequency of expression. Additionally, antigen expression would preferably be homogeneous so that potentially all cancer cells would be immunologically targeted and tied to the ‘driver’ activity of the tumor. Generation and maintenance of a robust immune response are also critical components of a successful immunotherapeutic. Agents should be able to activate the immune response, support infiltration of the tumor site and sustain immune effector function within the tumor microenvironment. In contrast to other malignancies, precision medicine in GBM requires additional considerations for blood–brain barrier penetration for targeted agents and/or consideration of trafficking of anti-tumor immune responses to the CNS. At this junction, there does not appear to be a monotherapeutic strategy that is capable of inducing all of these critical steps, and as such, ongoing efforts have been focused on the development of combining immune therapeutics with these various properties [7–10].
There are multiple reviews on GBM immunotherapy in the literature [11–15]; however, the goal of this particular review is to evaluate precision medicine strategies of selecting an appropriate immunotherapy based on a biomarker, thereby optimizing treatment regimens while minimizing ineffectual approaches for the patient.
Precision medicine in cancer
Precision medicine has played an increasingly significant role in the treatment of several malignancies via targeted therapies. A classic example is in the treatment of chronic myeloid leukemia (CML). The Philadelphia chromosome (translocation between the long arms of chromosomes 9 and 22) results in expression of a BCR–ABL fusion oncoprotein and is found in over 90% of CML cases [16]. This oncoprotein has constitutive tyrosine kinase activity promoting tumorigenesis [17]. Imatinib (Gleevec), a tyrosine kinase inhibitor, selectively targets this key oncogenic event, resulting in a complete and durable response in 69% of CML patients [18].
Precision medicine has also had success in the treatment of multiple solid tumors. A well-known example is the establishment of HER2-neu and ER as effective therapeutic targets in breast cancer. HER2/neu is specifically overexpressed in the tumors of approximately 20–25% of breast cancer patients and plays an oncogenic role in cell proliferation, conferring a poorer prognosis [19–21]. Trastuzumab is a humanized monoclonal antibody that targets the protein encoded by the HER2/neu gene. A large study including 2091 patients with metastatic breast cancer showed that women with HER2/neu-positive disease who received trastuzumab had a 44% reduction in the risk of death compared with women with HER2/neu-negative disease (p < 0.0001) [21]. However, given the reported high incidences (over 30%) of trastuzumab-induced cardiotoxicity [22–25], patients must be closely monitored for cardiac effects of the drug.
Amplification of EGFR is a common genetic alternation in several malignancies, making it an attractive target for personalized therapy. Cetuximab, a monoclonal antibody against EGFR has proved effective in patients specifically with wild-type KRAS colon cancer, significantly increasing median overall (9.5 vs 4.8 months; p < 0.001) and progression-free survival times (3.7 vs 1.9 months; p < 0.001) in a study of 394 patients evaluated for KRAS tumor mutations [26]. Malignant melanoma is a devastating disease in which 60% of patients have a BRAF mutation that causes a decreased response to chemotherapy and increased disease severity [27,28]. A Phase III randomized clinical trial of 675 patients with previously untreated metastatic melanoma showed that vemurafenib (potent, selective inhibitor of mutated BRAF) resulted in a 63% reduction in the relative risk of death and a 74% reduction in the risk of either death or disease progression in patients with a BRAF mutation (p < 0.001) [29].
Cumulatively, these studies have demonstrated that the unique genetic features of a tumor can be exploited for therapeutic vulnerability. However, to date, no such therapeutic strategy has been successful in GBM, owing to a variety of factors including marked genetic diversity and heterogeneity and therapeutic delivery limitations produced by the blood–brain barrier.
GBM immunotherapy & precision medicine
Perhaps the most prototypical example of using immune therapy in the context of precision medicine in GBM has been the EGFRvIII peptide vaccine [30], which consists of a 14-mer peptide spanning the splice mutation site, GM-CSF and KLH. EGFRvIII is expressed in 30% of GBMs, and this mutant is ligand-independent and constitutively active, contributing to amplified cell proliferation [31–35]. Studied extensively in Phase II clinical trials in EGFRvIII-positive GBMs, the vaccine demonstrated a progression-free survival of 8.5 months from diagnosis, a median overall survival (OS) of 21.8 months and a 36-month overall survival of 26% [36]. Although not a randomized trial, these results fared favorably when compared with standard of care in which PFS is 6.8 months and OS is 14.6 months [1]. It is important to note that EGFRvIII expression does not impact median survival [34,37], as almost no GBM patients with EGFRvIII expression have historically survived more than 24 months. Further advancement of the EGFRvIII peptide approach has been halted due to recent Phase III results. Specifically, the control group, which included treatment with KLH, exceeded expectation (hazard ratio = 0.99; median overall survival: 20.4 months vs control 21.1 months) [38]. Since the GBM patients were selected based on tumor expression of EGFRvIII, an immunological target already exists for the immune system to be directed. Thus, additional systemic administration of an EGFRvIII peptide may not have been necessary. Viewed from this perspective, the Phase III clinical trial may have utilized immunological bio-equivalent strategies (i.e., a lymphodepleting temozolomide regimen to allow for expansion of clonotypic antigen-specific T cells with an immune activating agent such as KLH) in both arms. Since an antigenic target was already present in both cohorts, at least two criterion necessary for immunological clearance of a tumor were met. However, to date, there have been no published preclinical studies of therapeutic activity of KLH against EGFRvIII positive tumors within the CNS. Although restriction of EGFRvIII to GBM has made it an excellent target for immunotherapy from a safety and specificity perspective, treatment failure corresponds to the loss of the antigenic target [35]. This limitation of precision medicine is being increasingly recognized as a mechanism of treatment failure in other approaches that have targeted specific antigens.
Another peptide vaccine strategy targets the IDH1 mutation, specifically at the R132H site, which is found in the majority of WHO grade II and III gliomas and secondary GBM [39]. Although IDH mutations are drivers of tumor progression [40], patients with IDH-mutated gliomas exhibit improved prognosis compared with those with IDH wild-type [41,42]. Administration of this vaccine induces a specific antitumor immune response against IDH1(R132H)-mutated tumors [43], and significantly prolongs survival in an intracranial glioma murine model system [44]. The IDH1 peptide vaccine is currently being investigated in Phase I clinical trials in IDH1(R132H)-mutated grade III–IV gliomas (NCT02454634) and recurrent grade II gliomas (NCT02193347). Both trials are utilizing precision medicine in screening for the IDH1R132H-mutation to determine patient eligibility.
Considering the obstacle of antigenic loss, alternative approaches have included a multipeptide vaccine strategy, in which 10 to 15 tumor-associated peptides are combined in a single vaccine. This strategy has been studied in a Phase I trial in renal cell carcinoma [45], and is now being evaluated in GBM (NCT01920191). The development of a GBM multipeptide vaccine is based on a prior screen of 11 tumor-associated peptides found to be overexpressed in malignant glioma samples of 45–50 patients. In a Phase I trial of 45 GBM patients, 60% of the patients had an immunogenic response to one of the peptides and 35% had a response to two or more of the peptides [46]. Although this multipeptide vaccine is based on predetermined overexpressed antigens from a pooled cohort of GBM patients, this strategy provides the future possibility of screening an individual's primary tumor and creating a vaccine that specifically targets the patient's tumor based on the peptide screen.
Similarly, the dendritic cell (DC) strategy provides a means of targeting multiple GBM antigens by utilizing tumor lysates, total tumor RNA, tumor peptides or products from cancer stem cells [47–49]. Autologous DCs manipulated ex vivo can then be administered to the patient. DC vaccination is safe and well tolerated [7,50–53], and is currently being investigated in several GBM clinical trials (NCT01204684, NCT01204684, NCT0004596, NCT01280552). Interestingly, infiltration of intratumoral cytotoxic T cells [54] as well as CD8+ immune responses [55] have been observed in some patients after vaccination with DCs. More importantly, DC vaccination has shown improved survival and tumor regression compared with historical or contemporary controls [7,51,54–56]. For example in a clinical study of 12 GBM patients, median overall survival was 23.4 months (p = 0.006) and median time to progression was 15.5 months (p = 0.028), compared with concurrent control patients who had an overall survival of 18.3 months. Two patients treated with DC vaccination were also long-term survivors (≥4 years) [54]. However, there are distinct limitations with DC strategies regarding the antigens (via tumor lysates, RNA, peptides, or cancer stem cell products) used to load them. Specifically, these antigens may induce nontumor-specific toxicities, fail to induce an immunological response, be limited by the immunosuppressive tumor microenvironment and target bystander cells that have no impact on the process of tumorigenesis, recurrence or resistance. Also, as approximately 65% of GBM patients are surgical candidates [57], a major limitation is that there must be sufficient tissue in order to implement this immune-based strategy.
Another way to potentially overcome screening for antigens and their limited frequency of expression is to target CMV, a herpes virus that leads to asymptomatic infection followed by viral persistence and latency. Although the role of CMV in GBM is not fully elucidated with conflicting data regarding the presence [58–60] or absence [61,62] of CMV in GBM, the association of CMV antigens with GBM is well established [63]. Adoptive transfer of CMV-specific effector T cells that have been collected from the patient and expanded ex vivo has been shown to be safe and to confer a median survival time of approximately 14 months [64]. A recent pivotal study by Mitchell et al. had extreme responders (>40 months survival) who received autologous dendritic cells pulsed with CMV mRNA phosphoprotein 65 (pp65) and underwent preconditioning of the vaccine site with tetanus/diphtheria (Td) toxoid, a potent recall antigen [7]. Another method of adoptive transfer therapy is the administration of cytotoxic T lymphocytes that have been collected from the patient, activated and amplified ex vivo. The tumor antigen-specific T cells then traffic to the malignant tumor cells. Preclinical studies demonstrate that administration of tumor antigen-specific T lymphocytes leads to rejection of brain tumors [65]. The applicability and feasibility of this treatment strategy in GBM have been evaluated in small Phase I trials and pilot studies [66–75]. For example, in a study of ten patients with recurrent or progressive malignant glioma, 6-month radiographic regression was observed in two patients with recurrent tumors, one patient demonstrated stable disease lasting more than 17 months and four patients remained alive more than 1 year after surgery for recurrent tumor [72]. Such adoptive cellular strategies are currently being evaluated in clinical trials (NCT02661282, NCT00693095, NCT00730613), but by strict definition cannot really be considered as precision medicine because the unique characteristics of the tumor are not, per se, being used to identify the applicable target patient population.
Another immunotherapeutic strategy that would lend itself to the precision medicine model is using chimeric antigen receptor (CAR) T cells, which are genetically modified to target surface tumor-associated antigens independently of major histocompatibility complex (MHC) presentation. CARs can be built with any tumor-specific or tumor-associated antigen of interest, and they can be fine-tuned to the level of antigen expression to distinguish tumor from nontumor cells [76]. Such fine-tuning is also seen in an EGFR-targeting probody, which remains inert in healthy tissues and active at the tumor site, minimizing on-target/off-tumor toxicities and improving the safety profile of antibody strategies [77]. An emerging treatment paradigm includes accessing the tumor, analyzing it for antigens and then selecting a CAR that is specific for that individual patient's tumor. Typically, it can take approximately 3–5 weeks to manufacture clinical-grade modified CAR T cells [78–80], depending on the genetic modification method used. The EGFRvIII and IL13Rα2 CAR T-cell therapies have shown efficacy in murine model systems of glioma and CNS melanoma [81–84], and these strategies, as well as HER2-CAR T cell therapy, are currently being investigated in GBM Phase I clinical trials (NCT02209376, NCT01454596, NCT02208362, NCT02442297, NCT01109095). However, this approach will require a portfolio of CARs and may also have treatment failures owing to antigenic loss/clonotypic selection. Upon recurrence, the tumor would require reprofiling (via surgical resection or biopsy) and would require alternative antigen-directed CAR therapeutics.
Approved by the US FDA in 2011, ipilimumab, an immune checkpoint inhibitor, became the first drug ever shown to extend survival of patients with metastatic melanoma in a large randomized Phase III trial [85]. The 25-year story of the development and implementation of ipilimumab, a CTLA-4 receptor blockade immunotherapeutic, has incited considerable efforts in cancer immunotherapy. CTLA-4 and PD-1 are immune checkpoint molecules that downregulate T-cell activation pathways, thereby hindering the immune response to cancer. Immune checkpoint inhibition, specifically by CTLA-4 and PD-1 blockade, has been implemented in cancers such as melanoma [86], renal cell carcinoma [87] and non-small-cell lung cancer [88], with significant clinical efficacy and survival benefit. In a Phase III study of 676 patients with unresectable stage III or IV melanoma, ipilimumab revealed improved overall median survival in patients with advanced melanoma (10 vs 6.4 months in controls; p < 0.001) [85]. Similarly, anti-PD1 therapy conferred 6-month disease stabilization in advanced melanoma, lung cancer and renal cancer [89,90]. Interestingly, patients shown by immunohistochemistry to have PD-L1-negative tumors did not have an objective response, implicating the need to further understand the influence of PD-1/PD-L1 expression on therapeutic response or failure and also the potential applicability of the immune checkpoints within the precision medicine initiative. In light of the clinical efficacy in the treatment of other cancers, there are an unprecedented number of clinical trials actively recruiting GBM patients for treatment with immune checkpoint blockade strategies (NCT02313272, NCT02530502, NCT02337686, NCT02658279, NCT02311582, NCT02529072, NCT02311920, NCT02017717, NCT02550249, NCT02526017, NCT02423343, NCT02327078). Moreover, overall mutational load, neoantigen load and expression of cytolytic markers in the immune microenvironment are all associated with clinical response to immune checkpoint inhibitors [91–93]. Thus, high mutational burden, as seen in other cancers, could also be considered as a ‘target’ in GBM. Such potential targets could be used to develop new immunotherapeutic agents and as selection biomarkers for patients who may benefit from this particular type of immune therapy. The field is rapidly heading toward moving many of the aforementioned approaches into combinatorial strategies; however, as we develop more precise approaches, the number of patients to whom they are applicable may become much more limited, as large-scale comprehensive profiling to identify those that will benefit is required.
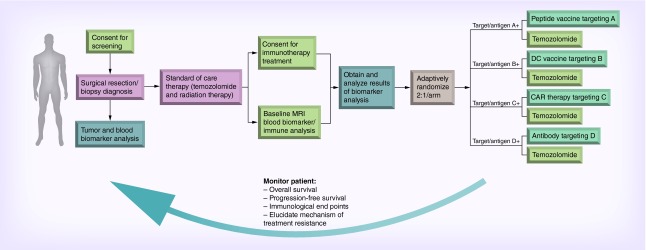
In an ideal clinical scenario, a patient would have surgical resection/biopsy for a definitive diagnosis of GBM. Individual tumor and blood samples then undergo personalized characterization, including genomic sequencing, immune evaluation, metabolic profiling and pathway analysis. Tumors would also be evaluated for expression of distinct immunoregulatory ligands and receptors. With the results of such testing and the selection of targeted therapies, patients would then receive biomarker-directed immunotherapy determined by the analysis of personalized tumor characterization. Bayesian statistical approaches can be used at this juncture to streamline and facilitate building complicated but maximally informative trials [94]. As the size and expense of current Phase II clinical trials in oncology continue to escalate, their success remains dismally low at 29% [95]. The use of adaptive clinical trial design has the distinct advantages of: identifying the appropriate patient population and therapeutic combinations; shortening the duration of drug development; and modeling longitudinal information, including immune monitoring assays. Such flexible clinical trials allow for stopping early if there is either superiority or futility, assigning doses to more efficiently assess the dose–outcome relationship, dropping arms or doses, allowing for seamless phases of drug development within the same trial, changing the proportion of patients randomized to each arm, homing in on an indication for a responder population, adding arms or doses, and changing accrual rate. Treatment response and side effects can then be monitored using imaging, tumor genome evolution, and immune monitoring to evaluate early progression and intervention (Figure 1). This algorithm provides an opportunity for combinatorial treatment strategies, in which T-cell-enhancing therapies and antigen-targeted approaches are tailored according to the patient's tumor profile. This strategy also provides an opportunity to treat patients with resistance to targeted therapies, which could be due to selective therapy pressure or activation of compensatory tumorigenic pathways.
Figure 1. . Precision immunotherapy schema for newly diagnosed glioblastoma patients.
Obstacles/solutions
Although the clinical potential of immunotherapy is clear, the delivery of personalized GBM therapy has many challenges. The heterogeneity of this disease due to accumulation of diverse genetic changes, redundant signaling pathways and the complex interaction between the tumor and the microenvironment make generating a global suitable therapeutic candidate difficult [96–98]. Administrative execution, cost and feasibility are all major obstacles. It is assumed that ultimately, personalized therapy will result in health/economic gains at the population level by streamlining treatment, and hence costs, by focusing on the most patient-specific, effective therapies [26]. However, developing a comprehensive precision medicine strategy for GBM will require a global effort, a large and diverse patient enrollment, an expansive database to maintain a robust portfolio of clinical data, the ability to do comprehensive and universal genomic screening and a way to systematically match patients with targeted treatment strategies. This requires the collaboration of multiple centers, a portal for storing clinical data and a large clinical and research team for data entry and maintenance. Additionally, genomic testing and sequencing of tumor blocks can be cost prohibitive, and only a limited number of centers have the ability to implement these tests. Moreover, the development of patient-specific therapies (i.e., adoptive cellular therapies) are more costly to produce than other treatment modalities (i.e., antibody approaches), due to complex cellular processing, labor intensive processes, availability of materials and technical demands.
The second major obstacle is time. From drug development, to preclinical testing, to clinical trial evaluation, to FDA approval, the path of getting a therapeutic to a patient can take several years. One reason for such an extensive time frame is that the traditional clinical trial framework has not changed since the early 20th century. However, innovative clinical trial designs (basket trials, adaptive Bayesian clinical trials, etc.) such as those seen in NCI-MATCH [99], FOCUS4 [100], I-SPY 2 [101] and the forthcoming GBM AGILE [102], represent the progress of biomarker, multiagent collaborative trials. The GBM AGILE trial, for example, will include multiple research arms and allocate patients based on Bayesian probability of treatment efficacy, thereby dropping treatments that are ineffective and accruing treatment arms that are successful. Such adaptive trial designs save time, cost and resources, with the goal of rapidly and dramatically reducing mortality in cancer.
In order for a proposed therapy to progress from the bench to the clinic, decisive clinical trials are required. Logistically, implementing an immunotherapy-based clinical trial is a feat. Patient selection based on individual genetic alterations is difficult, limiting the power of many immunotherapy clinical trials. An acceptable clinical protocol requires a standardized sample collection methodology, preparation and biomarker testing. Because GBMs are rare and sample numbers may be small due to limited tissue availability (needle biopsies, tumors in eloquent cortex decreasing the extent of resection), this issue becomes critical. As the availability and methods of testing vary from center to center, obtaining large-scale results focused on rare genomic alterations is difficult. Also, the current histopathological interpretation of GBM diagnosis can vary from pathologist to pathologist; thus, a tumor sample that is read as an anaplastic astrocytoma at one center may be classified as a GBM at another center. Indeed, as the field is moving toward genetic and molecular characterization of these tumors (e.g., the advent of microarray analysis, discovery of the IDH1 mutation, TCGA database, etc.), the classification system is bound to change as we continue to understand more about the biology of gliomas.
Another major challenge is utilizing proper, and preferably noninvasive, methods to monitor treatment response in patients who receive immunotherapeutic drugs for GBM. Immune monitoring of blood and tumor tissue is a method that has been used to predict clinical efficacy of immunotherapeutics and confirm immune responses [55,103–106]. For example, measuring tumor-specific immune responses via various assays for T-cell proliferation, CD4/CD8 cell phenotype, secretion of IFN-γ, cytokine responses of CD8+ T cells and downstream transcription markers have been used in GBM clinical trials [55,103–106]. Interpretation of immune-monitoring is primarily restricted to biomarkers that may be surrogate measures [107]. Also, the use of ‘liquid biopsies’, in which analysis of blood components can provide a real-time comprehensive picture of tumor-associated biomarkers, may have unique applications in tumor diagnostics and monitoring treatment responses [108]. Evaluated in a proof-of-principle study of various tumor types with a reported 96% accuracy, tumor-educated platelet RNA profiling appears as a unique platform for cancer diagnostics [109]. However, the clinical relevance, validation and applicability of these parameters have yet to be determined, as it is unclear if these assays truly recapitulate the genetic and immune composition of the tumor and the tumor microenvironment. The administration of steroids to suppress brain tumor symptoms from mass effect also suppresses the immune system, which may distort results. This is possibly due to several reasons, including reduced T-cell proliferation, disruption of the TCR complex after glucocorticoid-receptor-ligand binding [110] and suppression of immunomodulators resulting in fewer IFN-γ-producing T cells and increased IL-4-producing T cells [111].
Moreover, how does the field resolve the problem of distinguishing tumor progression from therapeutic immune response/inflammation as it pertains to clinical trial end points and current standard of care? The modified Response Assessment in Neuro-Oncology (RANO) criteria are now being considered for use in immunotherapeutic clinical trials to evaluate response and progression in malignant glioma [112] and to guide decision-making, preventing premature termination of immunotherapy [98]. The multimodal use of advanced brain tumor imaging, molecular imaging and magnetic resonance (MR) spectroscopy is potentially advantageous for noninvasively monitoring malignant glioma patients. For example, MR imaging inflammatory textural analysis, where volumetric and heterogeneity features are extracted from T1-post contrast MR and fluid-attenuated inversion recovery (FLAIR) images, can be used to build a classifier capable of discriminating inflammation status. Quantitative imaging tumor metrics and texture maps can then be used to assess the gene signatures of tumor cell apoptosis, tumor invasion and immune cell infiltration [113,114]. Advanced imaging not only provides potential in clinical trial design, correlating histological and immune functional data obtained directly from the tumor after surgical resection, but may also help with immunotherapeutic dose modification and treatment optimization.
Moreover, as the field is on the cusp of understanding GBM tumor biology and exploring effective therapeutic targets in this disease, we have yet to elucidate which combinatorial treatment strategies are actually beneficial with limited toxicity. For example, understanding chemoimmune interactions over time may shed some light on which patients may truly benefit from combinatorial approaches. Indeed, MGMT-methylated GBMs respond more favorably to temozolomide, a chemotherapy that has mutagenic properties [115–117] and can potentiate antitumor immune responses [103]. Therefore, theoretically, should an immunotherapy that targets increased ‘antigen load’ be used in combination with temozolomide in MGMT-positive GBM patients? Moreover, what is the best timing strategy for combinatorial therapy? How multiple immunotherapies can be safely combined and also be combined with other therapeutic modalities, such as small molecule inhibitors, tyrosine kinase inhibitors, viral-based strategies, antiangiogenic therapies and more, has yet to be determined. Additionally several preclinical studies are examining the best ways to combine therapeutic treatments for GBM [8,118,119]. Certainly there are ongoing and planned immunotherapy combinatorial clinical trials underway in GBM (NCT02423343, NCT02311920, NCT02529072, NCT02337491, NCT02017717, NCT02526017, NCT02423343, NCT02327078, NCT02327078, NCT02017717).
Conclusion & future perspective
A new era is emerging in precision medicine, as the field of GBM immunotherapy is rapidly progressing toward providing tumor-specific targeted therapies. However, there are challenges that must be resolved in order to address this unmet need in the field. With the development of immune-targeted drugs, progress in clinical trial design and a paradigm shift in the genetic and molecular characterization of gliomas, precision medicine in GBM immunotherapy provides a unique opportunity to change the landscape of how we treat cancer patients.
Acknowledgements
Special thanks to DM Wildrick and A Patrick for their editorial and administrative support.
Footnotes
Financial & competing interests disclosure
The Dr Marnie Rose Foundation, the Ben and Catherine Ivy Foundation, The University of Texas MD Anderson Cancer Center GBM Moonshot Program, and the NIH grants CA1208113, P50 CA127001 and P30 CA016672. AB Heimberger has received research grants from Merck, has been a paid consultant for Bristol Myers Squibb, and receives licensing and royalty fees from Celldex Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Kensler TW, Spira A, Garber JE, et al. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev. Res. (Phila) 2016;9:2–10. doi: 10.1158/1940-6207.CAPR-15-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnedos M, Vicier C, Loi S, et al. Precision medicine for metastatic breast cancer-limitations and solutions. Nat. Rev. Clin. Oncol. 2015;12:693–704. doi: 10.1038/nrclinonc.2015.123. [DOI] [PubMed] [Google Scholar]
- 4.Popper HH, Ryska A, Timar J, Olszewski W. Molecular testing in lung cancer in the era of precision medicine. Transl. Lung Cancer Res. 2014;3:291–300. doi: 10.3978/j.issn.2218-6751.2014.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantrill LA, Nagrial AM, Watson C, et al. Precision medicine for advanced pancreas cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin. Cancer Res. 2015;21:2029–2037. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
- 6.Politi K, Herbst RS. Lung cancer in the era of precision medicine. Clin. Cancer Res. 2015;21:2213–2220. doi: 10.1158/1078-0432.CCR-14-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong L, Wei J, Fuller GN, et al. Tipping a favorable CNS intratumoral immune response using immune stimulation combined with tumor-mediated immune suppression. Oncoimmunology. 2015 doi: 10.1080/2162402X.2015.1117739. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampson JH, Schmittling RJ, Archer GE, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE. 2012;7:e31046. doi: 10.1371/journal.pone.0031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Guo G, Niu XY, Liu J. Dendritic cell-based immunotherapy treatment for glioblastoma multiforme. Biomed. Res. Int. 2015;2015:717530. doi: 10.1155/2015/717530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin. Cancer Res. 2014;20:5620–5629. doi: 10.1158/1078-0432.CCR-14-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16:1441–1458. doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayegh ET, Oh T, Fakurnejad S, Bloch O, Parsa AT. Vaccine therapies for patients with glioblastoma. J. Neurooncol. 2014;119:531–546. doi: 10.1007/s11060-014-1502-6. [DOI] [PubMed] [Google Scholar]
- 15.Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J, Lim M. Immune checkpoint modulators: an emerging antiglioma armamentarium. J. Immunol. Res. 2016;2016:4683607. doi: 10.1155/2016/4683607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 17.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR–ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 18.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a Phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 20.Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J. Clin. Oncol. 1993;11:1936–1942. doi: 10.1200/JCO.1993.11.10.1936. [DOI] [PubMed] [Google Scholar]
- 21.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J. Clin. Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J. Clin. Oncol. 2007;25:3525–3233. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 23.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 24.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009;53:2231–2347. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Guglin M, Hartlage G, Reynolds C, Chen R, Patel V. Trastuzumab-induced cardiomyopathy: not as benign as it looks? A retrospective study. J. Card. Fail. 2009;15:651–657. doi: 10.1016/j.cardfail.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 27.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 29.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin. Cancer Res. 2003;9:4247–4254. [PubMed] [Google Scholar]
- 31.Moscatello DK, Holgado-Madruga M, Godwin AK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 32.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 33.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- 34.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin. Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 35.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster J, Lai RK, Recht LD, et al. A Phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17:854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 2007;25:2288–2294. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 38.Data Safety and Monitoring Board Recommends Celldex's Phase 3 Study of RINTEGA® (rindopepimut) in Newly Diagnosed Glioblastoma be Discontinued as it is Unlikely to Meet Primary Overall Survival Endpoint in Patients with Minimal Residual Disease. http://ir.celldex.com/releasedetail.cfm?ReleaseID=959021
- 39.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J. Clin. Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 42.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 43.Pellegatta S, Valletta L, Corbetta C, et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol. Commun. 2015;3:4. doi: 10.1186/s40478-014-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 45.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 46.Halford S, Rampling R, James A, et al. Final results from a cancer research UK first in man Phase I trial of IMA950 (a novel mulitipeptide vaccine) plus GM-CSF in patients with newly diagnosed glioblastoma. Ann. Oncol. 2014;25:iv361–iv72. [Google Scholar]
- 47.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 48.Zeppernick F, Ahmadi R, Campos B, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin. Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 49.Xu Q, Liu G, Yuan X, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunn MK, Bauer E, Wood CE, et al. Dendritic cell vaccination combined with temozolomide retreatment: results of a Phase I trial in patients with recurrent glioblastoma multiforme. J. Neurooncol. 2015;121:319–329. doi: 10.1007/s11060-014-1635-7. [DOI] [PubMed] [Google Scholar]
- 51.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ardon H, Van Gool S, Lopes IS, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J. Neurooncol. 2010;99:261–272. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 53.Ardon H, Van Gool SW, Verschuere T, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 Phase I/II trial. Cancer Immunol. Immunother. 2012;61(11):2033–2044. doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 55.Everson RG, Jin RM, Wang X, et al. Cytokine responsiveness of CD8(+) T cells is a reproducible biomarker for the clinical efficacy of dendritic cell vaccination in glioblastoma patients. J. Immunother. Cancer. 2014;2:10. doi: 10.1186/2051-1426-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 57.Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012;14:351–359. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cobbs C, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 59.Mitchell DA, Xie W, Schmittling R, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J. Virol. 2012;86:854–864. doi: 10.1128/JVI.06097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cimino PJ, Zhao G, Wang D, Sehn JK, Lewis JS, Jr, Duncavage EJ. Detection of viral pathogens in high grade gliomas from unmapped next-generation sequencing data. Exp. Mol. Pathol. 2014;96:310–315. doi: 10.1016/j.yexmp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Cosset E, Petty TJ, Dutoit V, et al. Comprehensive metagenomic analysis of glioblastoma reveals absence of known virus despite antiviral-like type I interferon gene response. Int. J. Cancer. 2014;135:1381–1389. doi: 10.1002/ijc.28670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dziurzynski K, Chang SM, Heimberger AB, et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012;14:246–255. doi: 10.1093/neuonc/nor227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuessler A, Smith C, Beagley L, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74:3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 65.Merchant RE, Baldwin NG, Rice CD, Bear HD. Adoptive immunotherapy of malignant glioma using tumor-sensitized T lymphocytes. Neurol. Res. 1997;19:145–152. doi: 10.1080/01616412.1997.11740788. [DOI] [PubMed] [Google Scholar]
- 66.Kitahara T, Watanabe O, Yamaura A, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J. Neurooncol. 1987;4:329–336. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- 67.Tsurushima H, Liu SQ, Tuboi K, et al. Reduction of end-stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J. Cancer Res. 1999;90:536–545. doi: 10.1111/j.1349-7006.1999.tb00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuboi K, Saijo K, Ishikawa E, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin. Cancer Res. 2003;9:3294–3302. [PubMed] [Google Scholar]
- 69.Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol. Immunother. 1997;45:77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quattrocchi KB, Miller CH, Cush S, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J. Neurooncol. 1999;45:141–157. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 71.Holladay FP, Heitz-Turner T, Bayer WL, Wood GW. Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. J. Neurooncol. 1996;27:179–189. doi: 10.1007/BF00177482. [DOI] [PubMed] [Google Scholar]
- 72.Plautz GE, Barnett GH, Miller DW, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J. Neurosurg. 1998;89:42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 73.Plautz GE, Miller DW, Barnett GH, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin. Cancer Res. 2000;6:2209–2218. [PubMed] [Google Scholar]
- 74.Wood GW, Holladay FP, Turner T, Wang YY, Chiga M. A pilot study of autologous cancer cell vaccination and cellular immunotherapy using anti-CD3 stimulated lymphocytes in patients with recurrent grade III/IV astrocytoma. J. Neurooncol. 2000;48:113–120. doi: 10.1023/a:1006456421177. [DOI] [PubMed] [Google Scholar]
- 75.Sloan AE, Dansey R, Zamorano L, et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg. Focus. 2000;9:e9. doi: 10.3171/foc.2000.9.6.10. [DOI] [PubMed] [Google Scholar]
- 76.Caruso HG, Hurton LV, Najjar A, et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desnoyers LR, Vasiljeva O, Richardson JH, et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl Med. 2013;5:207ra144. doi: 10.1126/scitranslmed.3006682. [DOI] [PubMed] [Google Scholar]
- 78.Singh H, Figliola MJ, Dawson MJ, et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh H, Huls H, Kebriaei P, Cooper LJ. A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunol. Rev. 2014;257:181–190. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maiti SN, Huls H, Singh H, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J. Immunother. 2013;36:112–123. doi: 10.1097/CJI.0b013e3182811ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi BD, Suryadevara CM, Gedeon PC, et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J. Clin. Neurosci. 2014;21:189–190. doi: 10.1016/j.jocn.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miao H, Choi BD, Suryadevara CM, et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS ONE. 2014;9:e94281. doi: 10.1371/journal.pone.0094281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 2015;7:275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohno M, Ohkuri T, Kosaka A, et al. Expression of miR-17–92 enhances anti-tumor activity of T-cells transduced with the anti-EGFRvIII chimeric antigen receptor in mice bearing human GBM xenografts. J. Immunother. Cancer. 2013;1:21. doi: 10.1186/2051-1426-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 87.Massari F, Santoni M, Ciccarese C, et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat. Rev. 2015;41:114–121. doi: 10.1016/j.ctrv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 88.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Champiat S, Ferte C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology. 2014;3:e27817. doi: 10.4161/onci.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodges TR, Ferguson SD, Caruso HG, et al. Prioritization schema for immunotherapy clinical trials in glioblastoma. Oncoimmunology. 2016 doi: 10.1080/2162402X.2016.1145332. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hay M, Rosenthal J, Thomas D, Craighead J. BIO CEO & Investor Conference. Bio/BioMedTracker clinical trial success rates study. 2011.
- 96.Rolle CE, Sengupta S, Lesniak MS. Challenges in clinical design of immunotherapy trials for malignant glioma. Neurosurg. Clin. N. Am. 2010;21:201–214. doi: 10.1016/j.nec.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin. Dev. Immunol. 2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weathers SP, Gilbert MR. Current challenges in designing GBM trials for immunotherapy. J. Neurooncol. 2015;123:331–337. doi: 10.1007/s11060-015-1716-2. [DOI] [PubMed] [Google Scholar]
- 99.McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J. Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv193. [DOI] [PubMed] [Google Scholar]
- 100.Kaplan R, Maughan T, Crook A, et al. Evaluating many treatments and biomarkers in oncology: a new design. J. Clin. Oncol. 2013;31:4562–4568. doi: 10.1200/JCO.2013.50.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin. Pharmacol. Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 102.GBM AGILE (An Adaptive, Global, Innovative Learning Environment) to Implement Unprecedented International Clinical Trial. National Biomarker Development Alliance. 2015. http://nbdabiomarkers.org/gbm-agile
- 103.Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanchez-Perez L, Choi BD, Reap EA, et al. BLyS levels correlate with vaccine-induced antibody titers in patients with glioblastoma lymphodepleted by therapeutic temozolomide. Cancer Immunol. Immunother. 2013;62:983–987. doi: 10.1007/s00262-013-1405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fadul CE, Fisher JL, Hampton TH, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J. Immunother. 2011;34:382–389. doi: 10.1097/CJI.0b013e318215e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin. Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lamano JB, Ampie L, Choy W, et al. Immunomonitoring in glioma immunotherapy: current status and future perspectives. J. Neurooncol. 2016;127(1):1–13. doi: 10.1007/s11060-015-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joosse SA, Pantel K. Tumor-educated platelets as liquid biopsy in cancer patients. Cancer Cell. 2015;28:552–554. doi: 10.1016/j.ccell.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 109.Best MG, Sol N, Kooi I, et al. RNA-eq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lowenberg M, Verhaar AP, van den Brink GR, Hommes DW. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol. Med. 2007;13:158–163. doi: 10.1016/j.molmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 111.Liao J, Wang X, Bi Y, et al. Dexamethasone potentiates myeloid-derived suppressor cell function in prolonging allograft survival through nitric oxide. J. Leukoc. Biol. 2014;96:675–684. doi: 10.1189/jlb.2HI1113-611RR. [DOI] [PubMed] [Google Scholar]
- 112.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 113.Naeini KM, Pope WB, Cloughesy TF, et al. Identifying the mesenchymal molecular subtype of glioblastoma using quantitative volumetric analysis of anatomic magnetic resonance images. Neuro Oncol. 2013;15:626–634. doi: 10.1093/neuonc/not008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang D, Rao G, Martinez J, Veeraraghavan A, Rao A. Evaluation of tumor-derived MRI-texture features for discrimination of molecular subtypes and prediction of 12-month survival status in glioblastoma. Med. Phys. 2015;42:6725–6735. doi: 10.1118/1.4934373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupte M, Tuck AN, Sharma VP, Williams KJ. Major differences between tumor and normal human cell fates after exposure to chemotherapeutic monofunctional alkylator. PLoS ONE. 2013;8:e74071. doi: 10.1371/journal.pone.0074071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin. Cancer Res. 2009;15:4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4–1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schrand B, Berezhnoy A, Brenneman R, et al. Targeting 4-1BB costimulation to the tumor stroma with bispecific aptamer conjugates enhances the therapeutic index of tumor immunotherapy. Cancer Immunol. Res. 2014;2:867–877. doi: 10.1158/2326-6066.CIR-14-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]