Abstract
Aim:
To correlate cerebral cavernous malformations (CCMs) disease aggressiveness with peripheral blood biomarkers hypothesized mechanistically.
Patients & methods:
A prospective case–control study enrolled 43 CCM patients, where 25-(OH) vitamin D, HDL and non-HDL cholesterol, CRP plasma levels and leukocyte ROCK activity were correlated with parameters of disease aggressiveness reflecting chronic and acute domains.
Results:
Patients with one or more features of chronically aggressive disease (early age at symptom onset, two or more symptomatic bleeds, high lesion burden) had significantly lower 25-(OH) vitamin D and non-HDL cholesterol levels in comparison to patients without these features.
Conclusion:
Validation of these biomarkers and their potential treatment modulation may influence the clinical care of patients with CCM disease.
Keywords: : aggressiveness, biomarker, cerebral cavernous malformation, inflammation, non-HDL cholesterol, vitamin D
The cerebral cavernous malformation (CCM) is a common cerebrovascular anomaly predisposing patients to a lifetime risk of hemorrhagic stroke, seizures and other neurological sequela [1]. CCM lesions consist of abnormal clusters of enlarged capillary vessels with aberrant angioarchitecture embedded in normal brain or spinal cord tissue found in 0.5–1% of the population [1]. Patients may harbor solitary lesions occurring sporadically and often associated with a developmental venous anomaly [2], or multifocal lesions, typically inherited as autosomal dominant traits at three known gene loci (CCM 1, 2 & 3). While the CCM3 genotype portends more aggressive disease, the clinical behavior of CCM cases, regardless of genotype, remains widely unpredictable [3]. Some patients remain asymptomatic, while others are disabled from recurrent bleeds and/or high lesion burden [4]. Early age of lesion onset, multiple hemorrhages and increased lesion burden have been correlated in various studies with chronic disease severity [5–9]. Features of acute disease aggressiveness include clinically overt hemorrhage, CCM lesion growth and the genesis of new lesions [10,11]. The molecular mechanisms influencing chronic or acute disease severity have not been elucidated, and there are to date no known peripheral blood biomarkers reflecting or predicting disease aggressiveness, nor any pharmaceutical treatment to alter the course of this disease.
Our group has previously demonstrated robust inflammatory cell infiltration, in situ antigen-driven B-cell clonal expansion, antibody production and local antibody-dependent activation of the complement pathway in CCM disease [12,13]. Another study indicated that proinflammatory genotypes [9] and low BMI [14] may be associated with greater aggressiveness of familial CCM cases harboring a common CCM1 mutation. Additionally, the inflammatory cells and their cytokines are modulated by vitamin D [15]. Indeed, cholecalciferol (vitamin D3), identified as potentially disease-modifying by using unbiased target-agnostic screening, was subsequently shown to decrease CCM lesion burden in a murine model of CCM, and to inhibit ROCK activity, known to affect CCM development [16]. Plasma vitamin D level has been associated with a favorable lipid profile related to inflammation and cardiovascular diseases [17], while an inverse association of vitamin D level with CRP has also been reported [15]. Thus, we hypothesize that these biomarkers would reflect the severity of CCM disease over time or with recent clinical behavior.
Patients & methods
Patient recruitment & categorization of disease
The prospective case–control study enrolled subjects with the diagnosis of CCM. Subjects were enrolled prior to having outpatient visits in conjunction with MRI involved in routine clinical care. From July 2014 to March 2015, 45 CCM patients were recruited for the plasma biomarker study. Two patients declined to be consented; thus, 43 CCM patients were included. One patient was excluded in the CRP analysis due to an insufficient amount of plasma sample collected.
As per currently accepted disease categorization [2,18,19], cases were classified as sporadic if they harbored a solitary lesion on the most sensitive susceptibility-weighted imaging (SWI) MRI sequences, or a cluster of lesions associated with a developmental venous anomaly. They were classified as familial if they harbored multifocal CCM lesions, a family history of CCM in a first degree blood relative, or a mutation genotyped at a CCM gene locus. Cases with prior cranial irradiation in association with CCM were excluded.
Extraction of clinical parameters, definitions & categorization of disease aggressiveness
Patients’ histories and previously adjudicated relevant disease parameters were obtained during their clinical visit and stored prospectively in a secure database for subsequent analysis [1,9,14].
A relevant disease biomarker might reflect chronic disease aggressiveness over the patient’s lifetime, or more acute clinical activity [3,9,14]. We considered validated features of chronic disease aggressiveness including a history of multiple adjudicated clinically overt hemorrhages, early age of clinical onset or high lesion burden in familial cases. Features of acute clinical aggressiveness included a demonstrated lesional growth or a symptomatic hemorrhage in the preceding year, or new lesion formation on comparable MRI image sequences in the preceding year in familial cases. Given the small numbers of cases with each disease aggressiveness feature, and the known correlation among the different features (i.e., patients with earlier symptom onset are more likely to experience greater lesion burden and multiple hemorrhages, patients with recent hemorrhage are more likely to experience recent lesional growth) [3], we subsequently considered the respective features of disease aggressiveness in chronic or acute domains for our primary hypothesis testing. Since CCM disease beginning in pediatric patients has been shown to be more severe than when disease onset is in adults [3,5–7], we used symptoms beginning at or before 18 years of age as a feature of chronic disease aggressiveness. We also considered more than 25 SWI lesions, more than five T2-weighted lesions and more than one hemorrhage as features of chronic disease aggressiveness because these same thresholds correlated with cerebral vascular permeability in patients with CCM [1]. We considered clinical hemorrhage, lesion growth or new lesion formation within the prior year as features of acute disease aggressiveness since most subjects had comparison imaging and clinical evaluation within the prior year. Thus, chronic disease aggressiveness was considered prospectively in cases harboring any of the following parameters: age of symptom onset prior or at 18 years, a history of two or more confirmed overt hemorrhages at any time, greater than 25 SWI lesions or greater than five T2-weighted lesions >4 mm in diameter [1]. Similarly, acute disease aggressiveness included any of the following events within the previous 12 months: lesion growth as demonstrated by T2-weighted MRI, new lesion formation or an overt lesional hemorrhage.
Experimental methods & statistical analyses
Methods of plasma preparation and leukocyte isolation, clinical laboratory measurements for 25-(OH) vitamin D, lipid panel and CRP and the leukocyte ROCK assay and statistical tests for analysis of data, including correlations of individual features of disease, between cohorts with and without disease aggressiveness and receiver operating characteristic (ROC) curves are presented in the Supplementary Methods section.
Results
Demographic characteristics
Demographic characteristics of the 43 subjects (20 with solitary/sporadic lesions and 23 with multifocal/familial CCMs) are summarized in Table 1, along with their clinical severity features. Overall, 26 patients had hemorrhage, seven patients had seizures and 11 of the 43 subjects had been asymptomatic or with nonspecific symptoms. Nine patients experienced at least two prior bleeding episodes during their lifetime. Eight cases among 23 familial subjects harbored more than 25 SWI lesions, and nine harbored more than five T2-weighted lesions 5 mm or larger. In the acute disease severity domain, 11 cases suffered a new bleed, five suffered lesional growth and four of the 23 familial cases had a documented new lesion formation on comparable imaging during the prior year. There was no difference in sex, age at time of enrollment, or in age at symptom onset between the sporadic and familial cohorts. However, in the sporadic cases, males were significantly younger (p = 0.02) at the time of study inclusion, and experienced earlier symptom onset (p = 0.005). One subject was receiving statin medication for cardiovascular indications unrelated to the CCM research. Correlations with disease aggressiveness were conducted with and without exclusion of this subject.
Table 1. . Lesion and clinical features of cerebral cavernous malformation subjects.
| Solitary/sporadic | Multifocal/familial | |||
|---|---|---|---|---|
| |
Male |
Female |
Male |
Female |
| Total subjects (n) |
7 |
13 |
7 |
16 |
| Mean age in years (SEM) |
31.1 (12.5) |
46.4 (12.6) |
41.2 (20.1) |
31.7 (21.2) |
| Mean age at first symptoms (SEM) |
19.7 (11.1) |
37.0 (9.2) |
31.1 (22.5) |
24.7 (21.7) |
| Mean # SWI lesions† per patient (SEM) |
1 (0.0) |
1 (0.0) |
24.0 (34.9) |
35.2 (42.2) |
| Mean # T2 lesions‡ >4 mm in size per patient (SEM) |
0.9 (0.4) |
0.8 (0.4) |
11.1 (22.5) |
9.4 (12.4) |
|
Symptoms | ||||
| Hemorrhage (n) |
4 |
9 |
3 |
12 |
| Seizures (n) |
0 |
5 |
1 |
1 |
| Asymptomatic/nonspecific (n) |
3 |
2 |
3 |
3 |
|
Aggressiveness parameters | ||||
| Symptoms onset ≤18 years (n) |
3 |
0 |
1 |
8 |
| >1 hemorrhages (n) |
1 |
3 |
0 |
5 |
| >25 SWI lesions (n) |
0 |
0 |
2 |
6 |
| >5 T2-weighted lesions (n) |
0 |
0 |
1 |
8 |
| Hemorrhage within 1 year (n) |
3 |
4 |
1 |
3 |
| Lesion growth within 1 year (n) |
0 |
1 |
0 |
4 |
| New lesion within 1 year (n) |
0 |
0 |
1 |
3 |
| Acute disease aggressiveness (n) |
3 |
4 |
2 |
6 |
| Chronic disease aggressiveness (n) | 4 | 3 | 3 | 12 |
†Mean # SWI lesions: mean number of susceptibility-weighted imaging lesions.
‡Mean # T2 lesions: mean number of T2-weighted lesions.
n: Number of subjects; SEM: Standard error of the means.
In all, 22 of the 43 subjects possessed one or more features of chronic disease aggressiveness (seven sporadic, 15 familial). Fifteen of the 43 subjects (seven sporadic, eight familial) had one or more feature of acute disease aggressiveness.
25-(OH) vitamin D & non-high-density lipoprotein cholesterol are biomarkers of chronic disease aggressiveness
We first tested the correlation between 25-(OH) vitamin D levels and individual parameters disease of chronic and acute disease aggressiveness. There was no statistical difference in 25-(OH) vitamin D levels between patients with and without the individual features of chronic or acute aggressiveness, except for age at first symptoms less than or at 18 years old in the sporadic cohort (Supplementary Table 1).
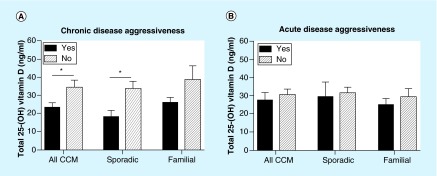
There were significantly lower total 25-(OH) vitamin D levels (mean difference: 11.06 ng/ml; 95%CI: [2.52–19.17]; p = 0.01) in CCM patients with one or more features of chronic disease aggressiveness (mean value: 23.8 ng/ml) compared with those without these features (mean value: 34.8 ng/ml). There was no significant difference in 25-(OH) vitamin D levels between those subjects with or without one or more characteristics of acute disease aggressiveness (Figure 1). When subjects with features of chronic disease aggressiveness were compared with those without these features, there was a significant difference in sporadic cases (mean difference: 14.04 ng/ml; 95%CI: [2.42–25.65]; p = 0.02) and a trend for significance in familial cases (mean difference: 12.32 ng/ml; 95%CI: [-1.62–26.25]; p = 0.08) (Table 2). We also observed the same results with 25-(OH) vitamin D3 (not shown).
Figure 1. . Total 25-(OH) vitamin D levels and chronic and acute disease aggressiveness of cerebral cavernous malformation.
(A) Total 25-(OH) vitamin D values were significantly lower between patients with and without chronic disease aggressiveness in all CCM (n = 22 and n = 21, respectively; p = 0.01), sporadic (n = 7 and n = 13, respectively; p = 0.02) and familial (n = 15 and n = 8, respectively; p = 0.08) patients. (B) Total 25-(OH) vitamin D levels were not different between patients with and without acute disease CCM aggressiveness (n = 15 and n = 28, respectively).
CCM: Cerebral cavernous malformation.
Table 2. . A summary of p-values for significant correlations with acute and chronic disease aggressiveness in all cerebral cavernous malformation subjects, and in familial and sporadic cohorts.
| Chronic disease aggressiveness | Acute disease aggressiveness | |||||||
|---|---|---|---|---|---|---|---|---|
| |
Unadjusted |
Season adjusted |
Age adjusted |
Sex adjusted |
Un adjusted |
Season adjusted |
Age adjusted |
Sex adjusted |
|
All CCM | ||||||||
| Total 25-(OH) vitamin D |
0.01 |
0.01 |
0.05 |
0.01 |
–† |
– |
– |
– |
| Non-HDL cholesterol |
0.01 |
0.01 |
– |
0.01 |
– |
– |
– |
– |
| HDL cholesterol |
0.03 |
0.04 |
– |
0.02 |
– |
– |
– |
– |
| ROCK activity |
0.02 |
0.02 |
– |
0.04 |
– |
– |
– |
– |
| CRP |
0.04 |
0.05 |
0.09 |
0.04 |
– |
– |
– |
– |
|
SPORADIC | ||||||||
| Total 25-(OH) vitamin D |
0.02 |
0.02 |
– |
0.08 |
– |
– |
– |
– |
| Non-HDL cholesterol |
– |
– |
– |
– |
– |
– |
– |
– |
| HDL cholesterol |
– |
– |
– |
– |
– |
– |
– |
– |
| ROCK activity |
– |
– |
– |
– |
– |
– |
– |
– |
| CRP |
– |
– |
– |
– |
– |
– |
– |
– |
|
FAMILIAL | ||||||||
| Total 25-(OH) vitamin D |
0.08 |
0.06 |
– |
– |
– |
– |
– |
– |
| Non-HDL cholesterol |
0.02 |
0.02 |
0.09 |
0.06 |
– |
– |
– |
– |
| HDL cholesterol |
– |
– |
– |
0.07 |
– |
– |
– |
– |
| ROCK activity |
– |
– |
– |
– |
– |
– |
– |
– |
| CRP | – | 0.06 | – | 0.07 | – | – | – | – |
† Dash (-) denotes no statistically significant correlation.
CCM: Cerebral cavernous malformation.
As reported by others, the 25-(OH) vitamin D level was significantly higher during summer (p = 0.02) [20]. After adjustment for season, the 25-(OH) vitamin D level remained statistically lower or showed a trend in patients with chronic disease aggressiveness, among all CCM subjects, and in sporadic and familial cohorts (p = 0.01, p = 0.02 & p = 0.06, respectively). These results were unchanged when the one subject on statin medication was excluded from the plasma biomarker analyses.
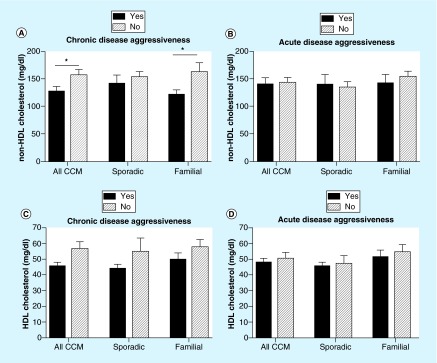
We further examined HDL and non-HDL cholesterol values since their levels are associated with pro- and antivascular inflammatory processes, and these biomarkers are minimally affected by fasting state [21]. Based on the same experimental design indicated for 25-(OH) vitamin D, we first examined the correlation of each parameter of disease aggressiveness with non-HDL cholesterol levels. There were no differences in non-HDL cholesterol levels between patients with or without individual parameters of disease aggressiveness, with the exception of age at first symptom less than or at 18 years old among all CCM subjects, and among those in the familial cohort (Supplementary Table 1). CCM patients with one or more features of chronic disease aggressiveness (mean value: 128.2 mg/dl) had a lower non-HDL cholesterol level (mean difference = 29.82 mg/dl; 95%CI: [6.50–53.14]; p = 0.01) (Figure 2) than those in the nonaggressive group (mean value: 158 mg/dl). This was true in the familial cohort (mean difference = 41.37 mg/dl; 95%CI: [7.03–75.70]; p = 0.02), but the trend did not reach significance in sporadic cases. There was no significant difference in HDL cholesterol in association with any parameter of chronic or acute disease aggressiveness (Table 2).
Figure 2. . Non-HDL and HDL cholesterol and chronic and acute disease aggressiveness of cerebral cavernous malformation.
(A) Non-HDL cholesterol value was statistically lower in CCM patients with chronic disease aggressiveness (22 patients with and 21 patients without chronic disease aggressiveness; p = 0.01). This result holds true in the familial patient cohort (n = 15 and n = 8, respectively; p = 0.02) but did not reach significance in the sporadic cohort. (B) There was no difference in non-HDL cholesterol levels between patients with and without acute disease aggressiveness, in CCM (n = 15 and n = 28, respectively), sporadic (n = 7 and n = 13, respectively) and familial (n = 8 and n = 15, respectively) cohort. There was no statistical difference in patients’ HDL cholesterol values with respect to chronic (C) or acute (D) disease aggressiveness.
CCM: Cerebral cavernous malformation.
In CCM and multifocal/familial cohort, both older (p = 0.001 and p < 0.001, respectively) and male (p = 0.06 and p = 0.06, respectively) subjects had higher non-HDL cholesterol values compared with younger and female subjects. In CCM and familial cohort after adjustment for sex (p = 0.01 and p = 0.06, respectively) and age at clinical evaluation (p = 0.11 and p = 0.09, respectively), subjects with one or more features of chronic disease aggressiveness still showed a statistically significant or trend toward lower non-HDL cholesterol values. These results remained unchanged when the one subject on statin medication was excluded from the plasma biomarker analyses.
Predictive thresholds & combination of two biomarkers as a predictor for cerebral cavernous malformation disease aggressiveness
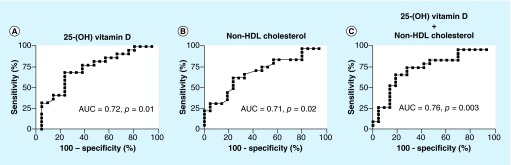
ROC curves [18,19] were generated and the area under the curve (AUC) was calculated to evaluate each individual biomarker and their combination’s ability to predict CCM chronic disease aggressiveness. ROC curves for 25-(OH) vitamin D and non-HDL cholesterol showed ‘fair’ accuracy: AUC = 0.72 (p = 0.01) and 0.71 (p = 0.02), respectively. The predictive threshold values for chronic disease aggressiveness were calculated for each biomarker based on the best sensitivity and specificity. The values were 25.68 ng/ml for 25-(OH) vitamin D and 138.5 mg/dl for non-HDL cholesterol. The sensitivity of single biomarker detection was 76% for 25-(OH) vitamin D and 76% for non-HDL cholesterol. A specificity of 68% was achieved for 25-(OH) vitamin D, and 64% for non-HDL cholesterol.
The linear combination of 25-(OH) vitamin D and non-HDL cholesterol was generated using the canonical linear discriminant analysis [22,23], with the equation shown below:
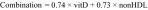 |
The best predictive threshold for the combination was 118.8 with a sensitivity of 81% and a specificity of 68% for detecting chronic disease aggressiveness. By combining the two parameters, the accuracy was increased to AUC = 0.76 (p = 0.003) (Figure 3). The two biomarkers taken together were better predictors of CCM chronic disease aggressiveness than each biomarker considered separately.
Figure 3. . The combination of 25-(OH) vitamin D and non-HDL cholesterol values is a better predictor of chronic disease aggressiveness of cerebral cavernous malformation.
(A) Using 25-(OH) vitamin D values alone, AUC was 0.72 (95% CI: 0.57–0.88), compared with (B) AUC of 0.71 for non-HDL cholesterol levels (95% CI: 0.56–0.87). (C) Combination of the two biomarkers gave the higher AUC of 0.76 (95% CI: 0.62–0.91), suggesting a better prognostic value of chronic disease aggressiveness.
AUC: Area under the curve.
ROCK activity in leukocytes & plasma CRP activity are not correlated with cerebral cavernous malformation disease aggressiveness
Several studies have previously reported that in vitro knockdown of CCM genes using siRNA causes ROCK-mediated vascular hyperpermeability, a phenomenon central to disease pathogenesis [3,24,25]. Thus, we examined in a smaller cohort whether ROCK activity in the peripheral leukocytes was also a biomarker of CCM disease aggressiveness (see Supplementary Methods section). Our analyses found no significant difference in ROCK activity in subjects with and without disease aggressiveness (Supplementary Figure 1). These results remain unchanged when the two subjects on statin medication were excluded from the ROCK activity analyses. In addition, we did not observe any differences in plasma CRP levels in relation to either chronic or acute CCM disease aggressiveness (Supplementary Figure 2).
Discussion
CCMs are a significant public health problem, affecting 0.5–1% of the population [26]. Lifetime risk of clinically overt hemorrhage is >30%. Clinical disease aggressiveness is portended by early age of onset [3,27], high lesion burden [3] and multiple clinical bleeds resulting in disability [4]. Acute disease aggressiveness is characterized by recent clinical hemorrhage, which predicts a higher likelihood of future bleeding [5–7]. Lesion genesis and asymptomatic hemorrhagic proliferation (lesion growth) have been considered as features of disease aggressiveness in preclinical studies [10,11,28] with potential clinical implications. This prospective case–control study is the first to examine one or more features of CCM disease aggressiveness with peripheral blood biomarkers. It focused on a limited panel of common inflammatory biomarkers derived from CCM patients’ peripheral blood and investigated their association with CCM disease severity. We found that CCM patients with one or more features of the more aggressive chronic clinical course had lower 25-(OH) vitamin D and non-HDL cholesterol values.
The ROC curve generated threshold of 25.68 ng/ml for vitamin D found in the chronic aggressive group, represents a suboptimal value according to published consensus statement [29] and systematic reviews [20,30] designating 30 ng/ml as the value below which vitamin D is insufficient. This shows that CCM subjects with chronic disease aggressiveness not only have lower vitamin D levels compared with those with nonaggressive disease, but are actually deficient compared with the general population.
Our results are consistent with previous reports showing an influence of vitamin D level deficiency on chronic inflammatory, vascular and brain diseases [17]. Vitamin D is known to play a pivotal role in immunomodulation by regulating proinflammatory cells and the production of inflammatory cytokines, both of which are crucial steps in the pathogenesis of cardiovascular and inflammatory diseases [15]. Other studies have demonstrated a correlation between 25-(OH) vitamin D level deficiency and early disease onset in multiple sclerosis [31]. Investigators have rightly cautioned that a biomarker of disease aggressiveness does not necessarily portend a benefit of therapeutic modification, and any effects of vitamin D supplementation in multiple sclerosis remain controversial [32]. Cholecalciferol (vitamin D3) supplement has been shown to decrease lesion burden in a murine model of Ccm2 disease [16]. It is reasonable to postulate whether vitamin D supplementation will be helpful in modulating human CCM disease over time. Given the association with chronic rather than recent disease aggressiveness, we speculate that such supplementation would likely need to be long term, and started early during disease development. Unfortunately, the cross-sectional design of this study did not allow a rigorous analysis of historical vitamin D intake or over the counter supplementation in our subjects.
We also showed that CCM patients with more aggressive chronic clinical course exhibit a lower non-HDL cholesterol level. The ROC curve generated threshold of 138.5 mg/ml for non-HDL cholesterol is in the range of common variations of this parameter in clinical practice [33–35]. Notably, the mean value of 158 mg/dl in the nonaggressive group is considered to be ‘borderline high.’ This is in-line with a recent study by Choquet et al. reporting that obese CCM patients with higher BMI harbored a lower CCM lesion burden than lean subjects [14]. BMI is positively correlated with non-HDL cholesterol values [36]. Choquet et al. failed to find a correlation between hyperlipidemia and CCM severity [14], but the discrepancy might be due to our use of continuous variables in lipid levels, while Choquet et al. considered a clinical history of hyperlipidemia as a categorical variable. Similarly, a lack of correlation of CRP levels with CCM disease aggressiveness in our subjects may reflect the categorical reporting of clinical laboratory CRP results. A greater sensitivity may be demonstrated in correlation with calculated CRP levels by ELISA. Neither 25-(OH) vitamin D nor non-HDL cholesterol levels were associated with parameters of acute disease aggressiveness. This may be explained by the small number of cases in this cohort (15 of 43) with one or more features of acute disease aggressiveness. It is also possible that proinflammatory genotypes [9] predispose certain patients more than others to disease aggressiveness, which would be expected to continue throughout a patient’s lifetime.
The significant correlations were apparently driven mostly by early age of symptom onset, an acknowledged index of aggressive CCM disease [3,5–7,27]. We recognize that the weaker correlations with other features may reflect small numbers of subjects with individual parameters of disease aggressiveness. Analysis in larger cohorts is currently underway, and may uncover other associations, including those with more recent disease behavior. This study, while prospective, was a cross-sectional case control as opposed to a longitudinal series. We are currently looking at prospective disease behavior in association with high versus low biomarkers for positive and negative predictive value. Despite these limitations, this is one of the largest prospectively enrolled cohorts of CCM subjects ever published in the literature, and the associations observed herein are novel, and will generate cogent hypotheses for future testing.
Our group has recently shown that brain permeability measured by dynamic contrast enhanced MRI may also represent a biomarker of CCM aggressiveness, presumably reflecting CCM-associated vascular leak mediated by ROCK activity [1]. Yet we found herein no correlation of disease aggressiveness with peripheral ROCK activity. This may represent a confounding effect of ROCK1 subtype on circulating leukocytes, rather than the more brain-specific ROCK2 subtype, as the latter likely modulates brain permeability and may be more reflective of CCM disease activity.
Although statins may have therapeutic benefits in the treatment of CCM by lowering ROCK-mediated hyperpermeability [1], this pleiotropic effect may conflict with a consequent lowering of non-HDL cholesterol levels. Indeed, this ‘lipid paradox’ (i.e., a reduction of serum lipids levels and an increased risk of symptomatic events) has been observed in numerous autoimmune inflammatory diseases [37]. However, we cannot conclude herein that statins are harmful in CCM merely because they may lower non-HDL cholesterol. The beneficial versus harmful effects of statins in CCM, and any relationship to lower levels of circulating lipids, remain speculative [38]. These questions are best addressed in hypothesis-driven laboratory studies and clinical trials specifically focused on CCM.
Finally, we found that the combination of 25-(OH) vitamin D and non-HDL cholesterol is a better predictor of chronic disease aggressiveness than each individual biomarker alone.
Conclusion
This study is a first step toward identifying biological signatures associated with human CCM disease severity. However, a larger cohort of CCM patients and age-matched controls needs to be investigated during an intra- and inter-subject longitudinal study to confirm these findings by controlling for multivariate codependence. Further studies using transgenic murine models of CCM disease could also examine causality between these biomarker levels and inflammation or disease progression.
Future perspective
To further enhance the sensitivity, specificity and positive predictive value of disease aggressiveness, we are currently correlating a larger panel of inflammatory biomarkers, including cytokines, chemokines and VEGF, with CCM patients’ features, using an integrative computational approach, and multivariate control of demographic and disease features. This computational approach provides us with an opportunity to discover a potential biomarker signature portending CCM disease aggressiveness. This would enhance our mechanistic understanding of CCM disease instability and its potential rescue. The biomarkers may predict clinical outcomes of CCM patients and serve as a new therapeutic target in CCM disease.
Executive summary.
Background
An association of disease aggressiveness with vitamin D has been suggested in preclinical studies, and other evidence supports a role of inflammation in the pathobiology of cerebral cavernous malformation (CCM).
Patients & methods
Plasma samples from 43 CCM subjects were analyzed for levels of vitamin D and cholesterol. These were correlated with the presence or absence of chronic and acute CCM clinical disease aggressiveness features.
Results
Patients with one or more features of chronically aggressive CCM disease had significantly lower vitamin D and non-HDL cholesterol levels. Combination of the two biomarkers at identified thresholds had 81% sensitivity with 68% specificity at detecting chronic aggressiveness.
Conclusion
This is the first evidence of correlation of peripheral blood biomarkers with the severity of human CCM disease. Validation of these biomarkers and their potential modification may influence the clinical care of CCM disease.
Supplementary Material
Footnotes
Financial & competing interests disclosure
This work was supported by the NIH/NINDS research grant R21 NS087328 to IA Awad, the NIH National Center for Advancing Translational Sciences grants UL1 RR024999 and UL1 TR000430 to the University of Chicago, the Scientist Development Grant of the American Heart Association (AHA-11SDG4890009) to C Shi and the Safadi Translational Research Fellowship at the University of Chicago to R Girard. The current address for C Shi is Department of Neurosurgery at The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China. The sponsors had no input in the study design or data analysis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Mikati AG, Khanna O, Zhang L, et al. Vascular permeability in cerebral cavernous malformations. J. Cereb. Blood Flow Metab. 2015;35(10):1632–1639. doi: 10.1038/jcbfm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Champfleur NM, Langlois C, Ankenbrandt WJ, et al. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery. 2011;68(3):647–648. doi: 10.1227/NEU.0b013e31820773cf. [DOI] [PubMed] [Google Scholar]
- 3.Shenkar R, Shi C, Rebeiz T, et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet. Med. 2015;17(3):188–196. doi: 10.1038/gim.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shahi Salman R, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. 2012;11(3):217–224. doi: 10.1016/S1474-4422(12)70004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Holou WN, O’lynnger TM, Pandey AS, et al. Natural history and imaging prevalence of cavernous malformations in children and young adults. J. Neurosurg. Pediatr. 2012;9(2):198–205. doi: 10.3171/2011.11.PEDS11390. [DOI] [PubMed] [Google Scholar]
- 6.Barker FG, 2nd, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49(1):24–15. doi: 10.1097/00006123-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Hao SY, Tang J, et al. Clinical course of untreated pediatric brainstem cavernous malformations: hemorrhage risk and functional recovery. J. Neurosurg. Pediatr. 2014;13(5):471–483. doi: 10.3171/2014.2.PEDS13487. [DOI] [PubMed] [Google Scholar]
- 8.Flemming KD, Link MJ, Christianson TJ, Brown RD., Jr Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012;78(9):632–636. doi: 10.1212/WNL.0b013e318248de9b. [DOI] [PubMed] [Google Scholar]
- 9.Choquet H, Pawlikowska L, Nelson J, et al. Polymorphisms in inflammatory and immune response genes associated with cerebral cavernous malformation type 1 severity. Cerebrovasc. Dis. 2014;38(6):433–440. doi: 10.1159/000369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui AA, Jooma R. Neoplastic growth of cerebral cavernous malformation presenting with impending cerebral herniation: a case report and review of the literature on de novo growth of cavernomas. Surg. Neurol. 2001;56(1):42–45. doi: 10.1016/s0090-3019(01)00505-5. [DOI] [PubMed] [Google Scholar]
- 11.Nikoubashman O, Wiesmann M, Tournier-Lasserve E, et al. Natural history of cerebral dot-like cavernomas. Clin. Radiol. 2013;68(8):e453–e459. doi: 10.1016/j.crad.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Shi C, Shenkar R, Kinloch A, et al. Immune complex formation and in situ B-cell clonal expansion in human cerebral cavernous malformations. J. Neuroimmunol. 2014;272(1–2):67–75. doi: 10.1016/j.jneuroim.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Shi C, Shenkar R, Du H, et al. Immune response in human cerebral cavernous malformations. Stroke. 2009;40(5):1659–1665. doi: 10.1161/STROKEAHA.108.538769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choquet H, Nelson J, Pawlikowska L, et al. Association of cardiovascular risk factors with disease severity in cerebral cavernous malformation type 1 subjects with the common Hispanic mutation. Cerebrovasc. Dis. 2014;37(1):57–63. doi: 10.1159/000356839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson CC, Zhu W, Davis CT, et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation. 2015;131(3):289–299. doi: 10.1161/CIRCULATIONAHA.114.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anagnostis P, Athyros VG, Adamidou F, Florentin M, Karagiannis A. Vitamin D and cardiovascular disease: a novel agent for reducing cardiovascular risk? Curr. Vasc. Pharmacol. 2010;8(5):720–730. doi: 10.2174/157016110792006978. [DOI] [PubMed] [Google Scholar]
- 18.Campbell PG, Jabbour P, Yadla S, Awad IA. Emerging clinical imaging techniques for cerebral cavernous malformations: a systematic review. Neurosurg. Focus. 2010;29(3):E6. doi: 10.3171/2010.5.FOCUS10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Souza JM, Domingues RC, Cruz LC, Jr., Domingues FS, Iasbeck T, Gasparetto EL. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with t2-weighted fast spin-echo and gradient-echo sequences. AJNR Am. J. Neuroradiol. 2008;29(1):154–158. doi: 10.3174/ajnr.A0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111(1):23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 21.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118(20):2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 22.Rencher AC, Christensen WF. Methods Of Multivariate Analysis (3rd Edition). Wiley; NJ, USA: 2012. [Google Scholar]
- 23.Huberty CJ. Applied Discriminant Analysis. Wiley; NY, USA: 1994. [Google Scholar]
- 24.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J. Exp. Med. 2010;207(4):881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson BT, Dibble CF, Borikova AL, Johnson GL. Cerebral cavernous malformation is a vascular disease associated with activated RhoA signaling. Biol. Chem. 2013;394(1):35–42. doi: 10.1515/hsz-2012-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otten P, Pizzolato GP, Rilliet B, Berney J. [131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies] Neurochirurgie. 1989;35(2):82–83. [PubMed] [Google Scholar]
- 27.Riant F, Bergametti F, Fournier HD, et al. CCM3 mutations are associated with early-onset cerebral hemorrhage and multiple meningiomas. Mol. Syndromol. 2013;4(4):165–172. doi: 10.1159/000350042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flemming KD, Bovis GK, Meyer FB. Aggressive course of multiple de novo cavernous malformations. J. Neurosurg. 2011;115(6):1175–1178. doi: 10.3171/2011.8.JNS11751. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 30.Wahl DA, Cooper C, Ebeling PR, et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012;7:155–172. doi: 10.1007/s11657-012-0093-0. [DOI] [PubMed] [Google Scholar]
- 31.Simpson S, Jr., Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann. Neurol. 2010;68(2):193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 32.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet. Diabetes Endocrinol. 2014;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 33.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am. J. Cardiol. 2005;96(4A):E53–E59. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Virani SS. Non-HDL cholesterol as a metric of good quality of care: opportunities and challenges. Tex. Heart Inst. J. 2011;38(2):160–162. [PMC free article] [PubMed] [Google Scholar]
- 35.Arsenault BJ, Boekholdt SM, Kastelein JJ. Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 2011;8(4):197–206. doi: 10.1038/nrcardio.2010.223. [DOI] [PubMed] [Google Scholar]
- 36.Hoenig MR. Implications of the obesity epidemic for lipid-lowering therapy: non-HDL cholesterol should replace LDL cholesterol as the primary therapeutic target. Vasc. Health Risk Manag. 2008;4(1):143–156. doi: 10.2147/vhrm.2008.04.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson J, Peters MJ, Mcinnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat. Rev. Rheumatol. 2013;9(9):513–523. doi: 10.1038/nrrheum.2013.91. [DOI] [PubMed] [Google Scholar]
- 38.Eisa-Beygi S, Wen XY, Macdonald RL. A call for rigorous study of statins in resolution of cerebral cavernous malformation pathology. Stroke. 2014;45(6):1859–1861. doi: 10.1161/STROKEAHA.114.005132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.