Abstract
This ecological correlation study explores the marked differential in osteoporosis susceptibility between East and West Africans. African tsetse belt populations are lactase non-persistent (lactose intolerant) and possess none of the genetic polymorphisms carried by lactase persistent (lactose tolerant) ethnic populations. What appears paradoxical, however, is the fact that Niger-Kordofanian (NK) West African ethnicities are also at minimal risk of osteoporosis. Although East Africans share a genetic affinity with NK West Africans, they display susceptibility rates of the bone disorder closer to those found in Europe. Similar to Europeans, they also carry alleles conferring the lactase persistence genetic traits. Hip fracture rates of African populations are juxtaposed with a global model to determine whether it is the unique ecology of the tsetse-infested zone or other variables that may be at work. This project uses MINITAB 17 software for regression analyses. The research data are found on AJOL (African Journals Online), PUBMED and JSTOR (Scholarly Journal Archive). Data showing the risk of osteoporosis to be 80 times higher among East Africans with higher levels of lactase persistence than lactase non-persistence West Africans are compared with global statistics. Hip fracture rates in 40 countries exhibit a high Pearson's correlation of r=0.851, with P-value=0.000 in relation to dairy consumption. Lower correlations are seen for hip fracture incidence vis-à-vis lactase persistence, per capita income and animal protein consumption. Ethnic populations who lack lactase persistence single-nucleotide polymorphisms may be at low risk of developing osteoporosis.
Introduction
Osteoporosis is a degenerative bone disease, which is characterized by a low skeletal mass, micro-architectural deterioration of bone tissue and an increased risk of fracture. It afflicts an estimated 200 million people globally, placing a heavy burden on financial and health-care resources. Family and twin studies have identified a strong heritability component to this disorder. However, one of the most challenging areas of biogenetics research is the ongoing effort to decode the genetic signature of this complex bone disorder.1
Efforts to unmask the osteoporotic disease process by setting low-risk West Africans side by side with high-risk Northern Europeans are weighed down by a plethora of confounding variables. The differences that must be adjusted for involve not only genetic inheritance but also cultural factors, lifestyles, diet, habits of physical exertion, socio-economic status, life expectancy, climate, geography, epidemiological susceptibilities and qualitative differences in data collection. This study first examines data on hip fracture incidence among sub-Saharan African agriculturalists and pastoralists, that is, Niger-Kordofanians, Nilo-Saharans and Afro-Asiatics, whose genetic affiliations overlap with their linguistic groupings.2 Although sharing similar per capita incomes, and life expectancies, notable differences in osteoporosis rates exist.2 The appearance of a high correlation between pastoralism or dairy farming and osteoporosis in Africa is subsequently applied to a global data set of 40 countries. In addition to looking at the possible effects of dairy farming on hip fracture rates, it also applies regression analysis to such independent variables as lactase persistence single-nucleotide polymorphisms (SNP; derived from ethnic percentages of lactase persistence), per capita income and animal protein consumption.
Results
Hip fracture rates for females in the non-dairy, West African tsetse belt nations of Nigeria and Cameroon average 3.0 hip fractures per 100 000 for women aged 50 years and older.3,4 Among these Bantu-speaking (Niger-Kordofanian) agriculturalists, the rate of lactase non-persistence is 90+ percent. Kenya, on the other hand, is located outside the tsetse zone. Dairy farming/pastoralism is prevalent, and the rate of post-menopausal hip fractures averaged 243 per 100 000.5,6
In order to substantiate and expand the scope of these unusual findings, this study tests potentially meaningful independent variables, globally, using statistics from Europe, Asia, North America, Latin America and Africa (Tables 1, 2, 3: Data from 40 countries on hip fracture incidence, dairy consumption, lactase persistence SNPs, animal protein consumption and per capita income and references). An analysis using MINITAB 17 to compute correlations identifies dairy consumption as the independent variable with the highest Pearson correlation to hip fractures per 100 000 (r=0.851 with P-value=0.000 (Figure 1: Fitted Line Plot: Hip Fracture vs Dairy Consumption). The second highest independent variable, lactase persistence alleles, is r=0.735, P-value=0.000 (Figure 2 Fitted Line Plot: hip fracture incidence vs lactase persistence SNPs). The data on lactase persistence SNPs were derived from Population percentages that exhibited the LP trait as a function of their possessing the prescribed SNPs identified with this genotype. Per capita income and animal protein consumption are r=0.634, P-value=0.000 (Figure 3: Fitted Line Plot: Hip Fracture Incidence vs Animal Protein Consumption) and r=0.447, P-value=0.004 (Figure 4 Fitted Line Plot: Hip Fracture Incidence vs Per Capita Income). These calculations were made with a confidence interval of 95%.
Table 1. Data from 40 countries on hip fracture incidence, dairy consumption per annum, lactase persistence SNPs, animal protein consumption per annum and annual per capita income.
| C1-T | Country | Hip fracture per 100 000a | Dairy consumption (kg)b | Lactase persistence SNPsc | Animal protein (kg)d | Per capita incomee |
|---|---|---|---|---|---|---|
| North America | United States | 595 | 253.8 | 86.5 | 126.6 | 54 370 |
| Canada | 310.9 | 206.83 | 80 | 108.1 | 44 967 | |
| Europe | United Kingdom | 523.5 | 241.47 | 90 | 83.9 | 39 826 |
| Ireland | 488 | 247.17 | 95 | 106.3 | 51 284 | |
| Sweden | 802.8 | 355.86 | 95 | 77.1 | 46 219 | |
| Norway | 563 | 261.52 | 86 | 65.7 | 67 166 | |
| Denmark | 853 | 295.62 | 96 | 61.7 | 44 625 | |
| Finland | 440 | 361.19 | 88 | 67.4 | 40 661 | |
| Iceland | 385 | 223.68 | 95 | 84.8 | 44 029 | |
| Netherlands | 368.3 | 320.15 | 99 | 89.3 | 47 960 | |
| Belgium | 538.7 | 238.47 | 85 | 86.1 | 43 139 | |
| Switzerland | 413 | 315.78 | 90 | 72.9 | 58 149 | |
| Germany | 522 | 247.24 | 82.5 | 82.1 | 46 216 | |
| France | 443 | 260.48 | 65 | 101.1 | 40 538 | |
| Spain | 353 | 177.49 | 85 | 118.6 | 33 835 | |
| Portugal | 408 | 222.94 | 75 | 91.1 | 27 069 | |
| Italy | 498.4 | 256.1 | 81 | 90.4 | 35 131 | |
| Malta | 502.5 | 188.64 | 80 | 86.9 | 33 198 | |
| Hungary | 488 | 175.59 | 63 | 100.7 | 25 019 | |
| Russia | 249 | 172.46 | 75 | 51 | 24 449 | |
| Kazakhstan | 651.1 | 260 | 28.5 | 56.02 | 19 744 | |
| Oceania | Australia | 295 | 235.11 | 90 | 117.6 | 46 550 |
| New Zealand | 288 | 110.4 | 91 | 104 | 35 305 | |
| Latin America | Mexico | 169 | 115.18 | 45 | 62.2 | 17 950 |
| Argentina | 298 | 213.1 | 40 | 36.5 | 22 302 | |
| Brazil | 138 | 124.61 | 15 | 80.5 | 16 155 | |
| Venezuela | 150 | 87.29 | 20 | 56.6 | 17 759 | |
| Asia | China | 97 | 29.04 | 5 | 53.5 | 13 224 |
| India | 159 | 105.1 | 36.5 | 3.3 | 5808 | |
| South Korea | 262.8 | 71.5 | 10 | 48.9 | 35 379 | |
| Japan | 266 | 93 | 2.5 | 45.4 | 37 519 | |
| Hong Kong | 110 | 13.98 | 10 | 133.9 | 55 097 | |
| Thailand | 7.05 | 22.48 | 2 | 27.9 | 15 579 | |
| Turkey | 357 | 138.71 | 29 | 19.3 | 19 698 | |
| Jordan | 198 | 88.1 | 25 | 29.8 | 11 971 | |
| Africa | Morocco | 85.9 | 50 | 27 | 23.8 | 7813 |
| Cameroon | 3 | 14.4 | 5 | 13.5 | 3007 | |
| Kenya | 245 | 98.64 | 35 | 15.4 | 3009 | |
| Nigeria | 2 | 5.4 | 5 | 8.6 | 6054 | |
| South Africa | 20 | 57.92 | 16 | 39 | 13 094 |
aSee Table 2 for global references on hip fracture incidence per 100,000 per annum.
bFAO Statistics Division.47
cSee Table 3 for references. Lactase persistence (LP) single-nucleotide polymorphisms (SNP) are inferred from calculations of percentage of LP in national or ethnic populations.
dFAO.48
eInternational Monetary Fund.49
Table 2. References used to compute hip fracture incidences per 100 000.
| Country | Citation |
|---|---|
| Argentina | 50 |
| Australia | 51 |
| Belgium | 52 |
| Brazil | 53 |
| Cameroon | 4 |
| Canada | 54 |
| China | 55 |
| Denmark | 56 |
| Finland | 57 |
| France | 58 |
| Germany | 59 |
| Hong Kong | 60 |
| Hungary | 61 |
| Iceland | 62 |
| India | 60 |
| Ireland | 63 |
| Italy | 63 |
| Japan | 64 |
| Jordan | 65 |
| Kazakhstan | 66 |
| Kenya | 6 |
| Malta | 63 |
| Mexico | 55 |
| Morocco | 67 |
| Netherlands | 63 |
| New Zealand | 68 |
| Nigeria | 3 |
| Norway | 69 |
| Portugal | 70 |
| Russia | 71 |
| South Africa | 72 |
| South Korea | 73 |
| Spain | 74 |
| Sweden | 75 |
| Switzerland | 64 |
| Thailand | 76 |
| Turkey | 77 |
| United Kingdom | 78 |
| United States | 79 |
| Venezuela | 80 |
Table 3. References used to compute lactase persistence (LP) single-nucleotide polymorphisms (as a function of LP ethnic percentages)a (Multiple sources were averaged).
| Country | Citation |
|---|---|
| Argentina | 81 |
| Australia | 82 |
| Belgium | 83 |
| Brazil | 84 |
| Cameroon | 85 |
| Canada | 86 |
| China | 87 |
| 22 | |
| Denmark | 88 |
| Finland | 89 |
| France | 90 |
| Germany | 90 |
| Hong Kong | Duplicated from China data |
| Hungary | 91 |
| Iceland | Duplicated from Sweden data |
| India | 92 |
| Ireland | 85 |
| Italy | 93 |
| Japan | 94 |
| Jordan | 95 |
| Kazakhstan | 87 |
| Kenya | 96 |
| Malta | Duplicated from Spain data |
| Mexico | 90 |
| Morocco | 97 |
| Netherlands | 83 |
| New Zealand | 98 |
| Nigeria | 82 |
| Norway | 99 |
| Portugal | 100 |
| Russia | 95 |
| South Africa | 101 |
| 102 | |
| South Korea | Duplicated from China data |
| Spain | 103 |
| Sweden | 104 |
| Switzerland | 82 |
| Thailand | 82 |
| Turkey | 85 |
| United Kingdom | 90 |
| United States | 105 |
| Venezuela | 81 |
aThese data on lactase persistence (LP) single-nucleotide polymorphisms (SNPs) were derived from population percentages that exhibited the LP trait as a function of their possessing the prescribed SNPs identified with this genotype.
Figure 1.
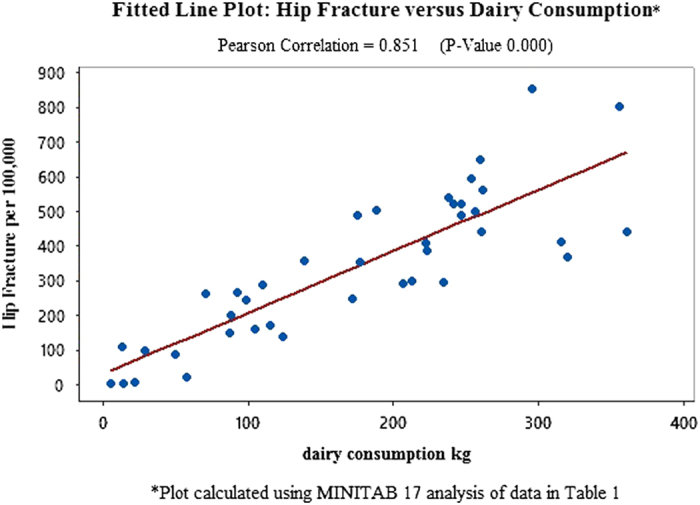
A Fitted Line Plot showing the correlation between Hip Fracture rates per 100 000 and Dairy Consumption, using data from 40 countries in Africa, Europe, Latin America, North America, Asia and Oceania.
Figure 2.
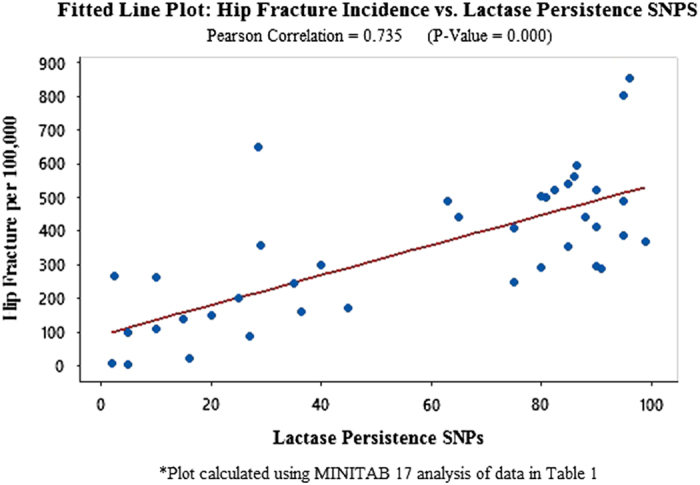
A Fitted Line Plot showing the correlation between Hip Fracture rates per 100 000 and the percentage of populations in 40 countries in Africa, Europe, Latin America, North America, Asia and Oceania, who display the lactase persistence (LP) genotype, which signals the presence of LP single-nucleotide polymorphisms.
Figure 3.
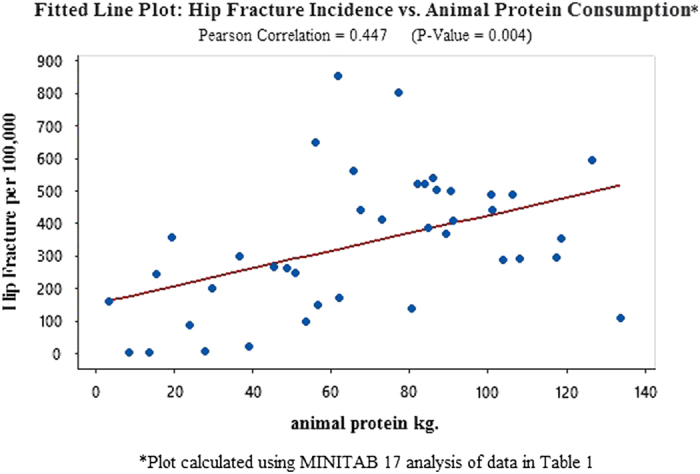
A Fitted Line Plot showing the correlation between Hip Fracture rates per 100 000 and Animal Protein Consumption, using data from 40 countries in Africa, Europe, Latin America, North America, Asia and Oceania.
Figure 4.
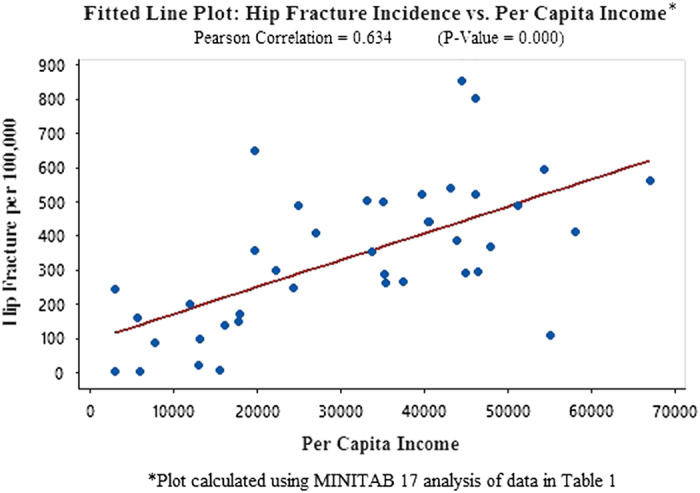
A Fitted Line Plot showing the correlation between Hip Fracture rates per 100 000 and per capita income, using data from 40 countries in Africa, Europe, Latin America, North America, Asia and Oceania.
Discussion
Genetic researchers using genome-wide association studies, and newer comprehensive genotyping platforms, have to date identified 150 candidate genes and SNP found to be associated with osteoporosis.7,8 However, that number is expected to rise, and some researchers now suggest that the final count could number in the thousands.9 Currently, the most popular candidates include genes encoding the vitamin D receptor, the alpha and beta estrogen receptors, apolipoprotein E, collagen type I, alpha 1 and methylene tetrahydrofolate reductase, among others.10 In short, biogenetic technology has widely increased our knowledge base of potential candidate genes for osteoporosis. However, the statistical power of such studies remains limited in their ability to assess gene–environment interaction.11 By shifting focus from the West, where this degenerative bone disorder is most prevalent, to Africa, where it is virtually unknown in some regions, but common in others, this study presents new insights into the disorder's etiology and its signature marker genes.
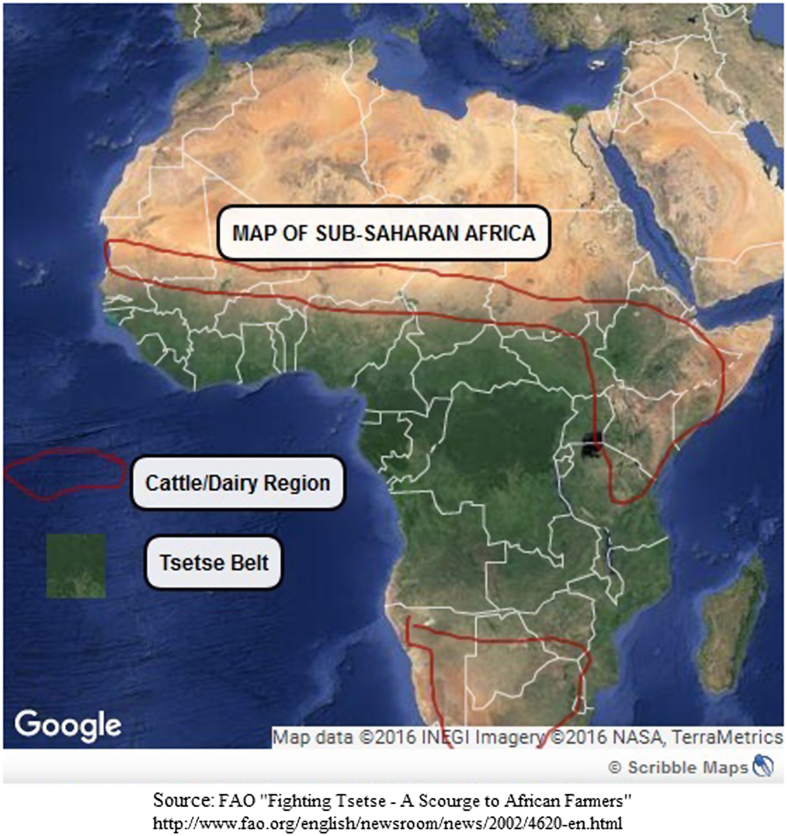
A unique phenomenon found in sub-Saharan Africa provides a natural laboratory for examining osteoporosis. An environmental line of demarcation runs through the continent, appearing to divide low osteoporotic risk West Africa from higher risk East Africa. It tracks the boundaries of the vast swathe of West and Central Africa infested by the tsetse fly glossina, which transmits parasites of the Genus Trypanosoma (Figure 5 Map of Sub-Saharan African Tsetse Zone & Cattle Rearing Areas).12 This tsetse-infected area covers nearly one-third of the African continent or roughly 10 million km2, including some of the most fertile and best watered regions of West Africa.13 Pastoralism is not possible in this zone. Although these parasites cause relatively mild infections in wild animals, in domestic livestock they cause a severe, often fatal disease, referred to as nagana.12 The exception is the trypanotolerant cattle breeds maintained by Fulbe pastoralists on the margins of the zone.14 Humans, however, show some level of resistance to all African trypanosome species with the exception of Trypanosoma brucei gambiense and T. b. rhodesiense.15
Figure 5.
A map highlighting the cattle/dairy farming regions and the Tsetse Fly Belt in Sub-Saharan Africa.
The lack of osteoporosis risk found among the Niger-Kordofanian (Bantu-speaking) populations of West Africa may also help in reconstructing the etiology of this degenerative bone disorder. That is, the intersection of historical and genetic data may be able to shed light on the evolutionary epoch in which it began appearing among non-Niger-Kordofanian humans. According to the consensus ‘Recent African Origin' model, anatomically modern humans evolved in Africa around 200 Kya (thousand years ago). The Niger-Kordofanian Africans (Y-DNA Haplogroup E1b1a, also known as the E-V38 phylogenetic tree) have lived continuously on the continent from earliest times.16 If osteoporosis was not part of this ethnicity's bone morphology, when did it creep into the human genome? Several research studies have proposed that human susceptibility to osteoporosis and osteoporosis-related fractures is the result of evolutionary adaptation, in which clues might be found in weighing the selective advantages and disadvantages of changed environments or human ecology.17,18
This study's transdisciplinary approach takes up that challenge by identifying the African tsetse/non-tsetse geographic divide, which appears to have played a role in differentiating low- and high-risk osteoporotic populations. Although the post-menopausal hip fracture rate and lactase persistence trait among East African pastoralists are closer to those of Europeans, their phylogenetic classifications—Khoisan, Niger-Kordofanian, Nilo-Saharan and Afro-Asiatic—are African.19 The osteoporotic susceptibility of East Africans also appears to correlate with recently identified alleles, encoded by the mini-chromosome maintenance protein 6 (MCM6), which influences the nearby lactase (LCT) gene. This genetic variant produces the lactase-phlorizin hydrolase enzyme in the gut wall, which regulates the absorption of lactose, the main sugar component in milk.
Western researchers had once assumed that lactase persistence represented a global genotype because of the ubiquitous nature of dairy culture among the European populations with which they were most familiar. However, the contrary has turned out to be the case, with 65% of the world's population exhibiting the lactase non-persistence trait.20 As for what populations have these alleles and why, the answers have come through a series of studies examining genetic variation between dairy and non-dairy societies. Recent studies have shown that farming cultures have evolved the genetic variants required to allow adults to consume milk.21 In the case of Northern Europeans, the T allele of a SNP 13.9 kb upstream of the lactase gene 13910-T/T allele (also known as 13910-T/T or rs4988235-T) confers the lactase persistence trait and is found in 90–95% of this population group. Individuals carrying the 13910 C/T and 13910 C/C (rather than 13910-T/T) SNPs are likely to be lactase non-persistent. Another set of genetic variants found among certain Europeans, the Kazakhstanis and populations inhabiting Northern India is the 22018A (also known as rs182549) SNP, which confers the lactase persistence trait and 22018-G, associated with the lactase non-persistent genotype.22
Further research by the team of Sarah Tishkoff et al has shown that the genetic variants found among Europeans differ from those found in African dairying populations. East African ethnicities possess any of three of these LCT-associated SNPs (14010-G/C, 13915-T/G and 13907-C/G) in their genomes. They endow this group with the lactase persistence trait.23 The dominant lactase persistence polymorphism identified in Africa (c-14010) was found among Afro-Asiatic, Nilo-Saharan and Niger-Kordofanian populations at rates of 42.1%, 38.3 and 25% frequency. As expected, the East African branch of the Niger-Kordofanian group of farmers and agro-pastoralists had the smallest percentage of this dairy-derived SNP relative to the pastoralist populations. However, the West African Yoruba of Nigeria, which also belongs to the Niger-Kordofanian linguistic group, showed ‘0' percent frequency of the lactase persistence polymorphism C-14010.24
Bypassed by MCM6 mutation
Inhabiting the tsetse zone, with its special entomological challenges, the Niger-Kordofanians were passed over by one of the most significant developments in recent evolutionary genetics—the dairy revolution.21 This transition from cereal-grain agriculture to dairy pastoralism/farming swept through Europe, as well as parts of the Middle East and East Africa 11 000 years ago. The genomic consequences were significant and swift. Within two millennium, several mutations had emerged and spread rapidly, allowing adults in dairy regions to hydrolyze the lactose in milk without first having to ferment it. The introduction of milk products to the human food supply increased calcium intake in dairy societies by 190%. Although the norm in Western countries rose to 700–800 mg, dietary calcium intake for populations in the tsetse zone remained in the 200–400 mg. a day range.25 In comparison, the pastoralist Masai of East Africa have developed average daily intake of dairy calcium as high as 6000–7000, mg, based on a bovine milk diet.
Genetic studies also showed that, among Europeans, even the five to fifteen percent of such populations who exhibited the lactase non-persistence genotype nonetheless carried a variant of the lactase persistence allele. On the other hand, no lactase persistence variants were found in the West African Niger-Kordofanian population groups.
Osteoporosis in east and west Africa
Many West African-trained physicians in the tsetse belt have never seen, let alone treated a case of post-menopausal osteoporosis. However, their East African counterparts declare themselves to be facing an epidemic of such traumatic hip fractures, particularly among the agro-pastoralist population of Kenya.26 In fact, a 2008 study in the British Journal of Sports Medicine underscored the fact that osteoporosis has a presence in East Africa. It described the case of an elite Kenyan marathon runner, who presented at a London hospital with an osteoporotic fracture of the tibia, sustained during an international cross-country race.27 Although this man's case was singular, it did support the findings in two studies conducted by Kenyan doctors. One was a report prepared by Dr G. Omondi Oyoo, a Rheumatologist and Senior lecturer at the University of Nairobi (Kenya) entitled: ‘Stemming the tide of an osteoporosis epidemic.'26 The second was a 2004 study, ‘Is There Osteoporosis in Kenya?' in which Odawa et al.6 reported a diagnosis of osteoporosis among 24.3% of postmenopausal women and osteopenia in 32%. In 2010, Dr LN Gakuu28 of the Department of Orthopaedic Surgery, in the University of Nairobi College of Health Sciences, announced that osteoporosis had reached a crisis point and that all patients over 75 years of age with fragility fractures should be empirically treated for the bone disorder.29 Among pre-menopausal women, the rates were 0.9% and 20.5%, respectively.6 The Kenyan rate of osteoporosis for women 50 years of age and over averaged 243 per 100 000.6
The West African experience with osteoporosis appears to be uniquely different. A 2014 Nature study reviewing hip fracture incidence worldwide included a chart of age-standardized osteoporosis rates. The Nigerian values were 2 hip fractures per 100 000 females, whereas that of Norway was 532.3,30 A 2-year project conducted by Zebaze et al.31 in the West African nation of Cameroon, which was published in 2003, reported a low-energy trauma fracture rate for females over 35 at 4.1 per 100 000. The unusually low susceptibility rate for the West African nations did not raise eyebrows in the medical community because researchers had theorized as early as 1966 that Africans did not suffer from postmenopausal osteoporosis because of a short life expectancy, a more active lifestyle than industrialized westerners and the lack of medical facilities to treat and record osteoporotic disease.28,32 However, none of these assumptions proved valid when osteoporosis rates were compared within regions of Africa, sharing similar life expectancies and socio-economic conditions.
Animal protein and osteoporosis
The identification of candidate genes involved in the immediate pathogenesis of osteoporosis lies beyond the scope of this study, which, instead, examines broad ecological and evolutionary patterns of osteoporotic susceptibility. However, in recent years, a growing number of medical researchers have endorsed what is commonly referred to as the ‘acid-ash' theory. It stipulates that low circulating 25-hydroxyvitamin D, caused by excess acidity produced during the metabolism of animal protein, raises the risk of osteoporosis.33 However, the correlation analyses presented in this paper suggest that animal protein may not be as pivotal a factor in the disease's etiology as dairy calcium (Figures 1 and 3). It is generally true that the consumption of animal protein is greatest in the West, where susceptibility to osteoporosis is highest.34 Dairy farming and beef consumption are naturally correlated, as the availability of cows for dairy farming enhances the availability of beef in the food supply. The one exception does represent 17% of the global human population—India. Although that country's inhabitants consume 105.10 kilograms of dairy per capita each year, the consumption of animal protein for this predominantly vegetarian nation is only 3.3 kilograms. Osteoporosis is widely prevalent in India and is a common cause of morbidity and mortality in both men and women.31,35
Data reliability
In comparing hip fracture rates among the African ethnicities, this study has eliminated some of the confounding factors that might otherwise arise in comparing osteoporotic risk among culturally diverse European and African populations. It then compares these findings with a regression analysis of hip fracture rates and several relevant independent variables on a global basis (Figure 6). However, attesting to the reliability of what appear to be such marked differences in post-menopausal hip fracture rates between East Africa (Kenya-243) and West Africa (Cameroon-3) when the data are so scanty requires a different approach.
Figure 6.
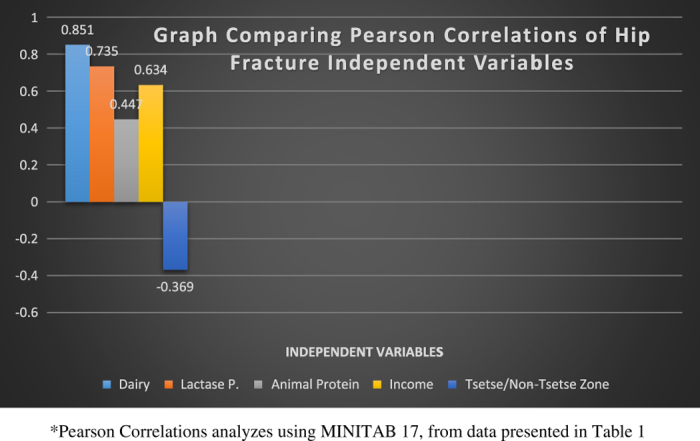
A graph depicting the degree of correlation between Hip Fracture Rates per 100 000 and 5 independent variables: dairy consumption, lactase persistence, animal protein consumption, per capita income and habitation in tsetse or non-tsetse zones.
For nearly three decades, medical researchers had grappled with the ‘paradox' of African-Americans being deemed calcium deficient by national nutritional standards, while suffering the lowest rate of osteoporosis and highest bone mineral density (BMD) levels of any American ethnic group.36,37 In terms of genetic ancestry, American blacks are an admixed ethnic population of ∼80% West African/Niger-Kordofanian and ∼20% European ancestral quanta. Their low dairy consumption rate is attributable to the fact that 70% of this population is also lactase non-persistent.38
However, a series of clinical studies begun in the 1990s showed that Black children and adults excreted less urinary calcium than whites on essentially the same diets and consequently retained more calcium in their skeletons.39 Greater calcium retention generated faster rates of bone growth during adolescence. Also, parathyroid hormone concentrations did not result in increased bone loss as seems to be the cause in European ethnicities that have been studied, because of skeletal resistance to that hormone.39 In short, the more efficient process of calcium homeostasis found in the physiology of this low to non-dairy consuming ethnic population more than made up for the reduced dietary calcium intake.39 African-Americans' verifiably low rate of osteoporosis did in fact support the sketchy data pointing to their Niger-Kordofanian genetic ancestors' low susceptibility to the disease.
Hip fracture vs BMD
Hip or femoral fracture rates are used in this study because this fragility fracture pattern is commonly applied in diagnosing osteoporosis. It is often due to a fall or minor trauma in someone with weakened osteoporotic bone. Also, as a point of clarification, this study relies on hip fracture rather than BMD data, whose lumbar and spinal measures are used in the US and Europe to diagnose osteoporosis in women with low bone density. Although low rates of BMD have correlated with high susceptibility to osteoporosis among European populations, a series of studies have shown this not to be the case among all ethnicities. Blacks in South Africa as well as the West African nation of Gambia have exhibited BMD measurements lower than those of age-matched Whites, but these groups retained low osteoporosis rates.40 Also, BMD data were not available in the areas covered by this study. Only one dual-energy X-ray absorptiometry scanner, used to diagnose BMD, exists in the entire East Africa region of 131.1 million inhabitants.41 Although the development of the Fracture Risk Assessment Tool algorithm by the World Health Organization has improved osteoporosis detection in other parts of the world, Kenya is one of the few African nations that has adopted the less technology-dependent Fracture Risk Assessment Tool.42
Materials and methods
This study uses ecological correlation modeling to assess associations between post-menopausal female hip fracture rates and factors identified by comparing sub-Saharan populations and a global sampling with differing osteoporotic risks. The pinpointed independent variables include per capita dairy consumption, lactase persistence alleles, animal protein consumption, per capita GDP and location in or outside the African tsetse belt. Pearson correlations and Fitted Line/Scatter Plots produced using MINITAB 17 software (Figure 6). In the absence of fracture registries in Africa, this research uses data and observations found in AJOL (African Journals Online—an index of peer-reviewed African scholarly journals based in South Africa).43 For the global distribution of age-adjusted hip fracture risk, per capita dairy, animal protein consumption and lactase persistence alleles, it uses an interdisciplinary review of epidemiological and medical literature found in a search of PUBMED and JSTOR (Scholarly Journal Archive), which is a digitized library of academic articles in history, geography and a wide variety of other disciplines (Tables 1 and 2). The search period dated from 1 January 1970 to 30 April 2015. The terms, some of which had been searched singly then merged through the use of AND, were taken from peer-reviewed articles and included the following keywords: osteoporosis, hip fracture, fragility fracture and Africa, ethnic, blacks in US, tsetse fly, tsetse belt, trypanosomiasis, LCT, MCM6 polymorphisms, C/T−13 910, C-14010, G-13907, G-13915 genotype, lactase persistence/lactose tolerance, lactase non-persistence/lactose intolerance, hypolactasia, l tsetse fly dairy, milk production, milk consumption, calcium homeostasis, Africa, African-Americans, India, global and NHANES.
Conclusion
Most genetic research involved in identifying osteoporotic-candidate genes has not targeted the MCM6 gene or its LCT-associated SNPs as critical factors. Although this regression study does show an association between dairy pastoralism/farming, osteoporotic risk and the possession of lactase persistence alleles, the correlations do not in and of themselves establish a causal relationship between the two variables. However, when the differential osteoporosis rates of East and West Africans are juxtaposed with studies showing global correlations and a more efficient calcium homeostasis among low dairy consuming African-American descendants of Niger-Kordofanians, the evolutionary link between hip fracture rates and dairy consumption becomes compelling.
However, a caveat must also be acknowledged here. This project has called attention to the importance of differentiating the lactase non-persistence genotype found among non-dairy consuming ethnic groups from that found in Northern European individuals, who carry a variant of the lactase persistence polymorphism. Some level of dairy consumption may be needed to support bone health in Europeans, East Africans, Middle Easterners and others who carry this LCT-associated allele.44 However, the same prescription might prove less than beneficial in lactase non-persistence ethnic populations, whose bone health or that of their descendants could be compromised by calcium overload. Thus, an additional issue in need of further study is the long-term consequences of feeding dairy products to those lactase non-persistence populations who exhibit high levels of bone health and low osteoporotic risk on account of biological differences in calcium homeostasis.
Lactase persistence and lactase non-persistence traits may be used to estimate osteoporosis risk in aggregated ethnic populations. However, these phenotypes do not determine the disease risk of self-identified members of ethnic groups, who have not been genetically tested for the presence of the requisite gene variants.
This research also identifies a simple, phenotypic criterion for determining osteoporotic susceptibility in ethnic populations—lactase non-persistence. Its predictive value will aid in determining which developing nations should allocate future public health resources to osteoporosis. The current assumption that this bone disorder is a function of attaining higher standards of living and increased animal protein consumption is not borne out by the data. These findings also suggest that ethnic minorities in the West who are lactase lactase non-persistent may benefit from lower dietary calcium levels than lactase persistence majority populations.
Acknowledgments
I offer my gratitude to those colleagues who have given generous encouragement to this research project. They are Dr Joseph Oppong, Professor of Medical Geography at the University of North Texas, Marjorie Elizabeth Starkman, MD, Assistant Professor of Medicine emerita, Division of Endocrinology and Metabolism, Albany Medical College and Dr Oluwadiya Kehinde, Consultant Orthopaedic Surgeon and Traumatologist, Faculty of Clinical Sciences, College of Medicine, Ekiti State University, and Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria.
Footnotes
The author declares no conflict of interest.
References
- Alonso N, Ralston SH. Unveiling the mysteries of the genetics of osteoporosis. J Endocrinol Invest 2014; 37: 925–934. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Rev Genomics Hum Genet 2008; 9: 403–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebajo AO, Cooper C, Evans JG. Fractures of the hip and distal forearm in West Africa and the United Kingdom. Age and Ageing 1991; 20: 435–438. [DOI] [PubMed] [Google Scholar]
- Zebaze RMD, Seeman E. Epidemiology of hip and wrist fractures in Cameroon, Africa. Osteoporos Int 2003; 14: 301–305. [DOI] [PubMed] [Google Scholar]
- Oyoo GO, Kariuki JG. Osteoporosis-from hormal replacement therap7 to bisphosphonates and beyond: a review. East African Med J 2008; 84: 535. [DOI] [PubMed] [Google Scholar]
- Odawa F, Ojwang S, Muia N. The prevalence of post menopausal osteoporosis in black Kenyan women. J Obstet Gynaecol 2004; 17: (Supp 1): 45–46. [Google Scholar]
- Richards JB, Zheng H-F, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 2012; 13: 576–588. [DOI] [PubMed] [Google Scholar]
- Zmuda YT, Sheu SP, Moffett J. The search for human osteoporosis genes J.M. Musculoskelet Neuronal Interact 2006; 6: 3–15. [PubMed] [Google Scholar]
- Clark GR, Duncan EL. The genetics of osteoporosis. Br Med Bull 2015; 113: 73–81. [DOI] [PubMed] [Google Scholar]
- Richards JB. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 2009; 151: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Ferrell RE. Recent progress in understanding the genetic susceptibility to osteoporosis. Genet Epidemiol 1999; 16: 356–367. [DOI] [PubMed] [Google Scholar]
- Steverding D. The history of African trypanosomiasis. Parasit Vectors 2008; 1: 7.http://www.parasitesandvectors.com/content/1/1/3 . (Accessed on December 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan KJR, Putt SNH, Na'isa SNH, Toure SM. ‘Tsetse Transmitted Trypanosomiasis. Joint IFS/ILCA workshop on small ruminant research in the tropics. http://www.fao.org/wairdocs/ilri/x5539e/x5539e08.htm . (Accessed on January 2016).
- Mattioli R, Feldmann U, Hendrickx G, Wint W, Jannin J, Slingenbergh J. 2004; Tsetse and trypanosomiasis intervention policies supporting sustainable animal-agricultural development. J Food Agric Environ 2: 310–314. [Google Scholar]
- Lambrecht FL. Trypanosomes and hominid evolution. Bioscience 1985; 35: 640–646. [Google Scholar]
- Campbell MC, Tishkoff SA. The evolution of human genetic and phenotypic variation in Africa. Curr Biol 2010; 20: R166–R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik D. Osteoporosis: an evolutionary perspective. Hum Genet 2008; 124: 349–356. [DOI] [PubMed] [Google Scholar]
- Cotter MM, Loomis DA, Simpson SW, Latimer B, Hernandez CJ. Human evolution and osteoporosis-related spinal fractures. PLoS ONE 2011; 6: e26658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HY, Underhill PA, Cavalli-Sforza LL, Ibrahim ME. Y-chromosome variation among sudanese: restricted gene flow, concordance with language, geography, and history. Am J Phys Anthropol 2008; 137: 316–323. [DOI] [PubMed] [Google Scholar]
- Swallow DM. Genetics of lactase persistence and lactose intolerance. Ann Rev Genet 2003; 37: 197–219. [DOI] [PubMed] [Google Scholar]
- Curry A. The milk revolution: when a single genetic mutation first let ancient Europeans drink milk, it set the stage for a continental upheaval. Nature. 2013; 1 500: 20–22. [Google Scholar]
- Xu L, Sun H, Zhang X, Wang J, Sun D, Chen F et al. The -2 2018A allele matches the lactase persistence phenotype in northern Chinese populations. Scand J Gastroenterol 2010; 45: 168–174. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet 2006; 39: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranciaro A, Campbell C, Hirbo J, Ko WY, Froment A, Anagnostou P et al. Genetic origins of lactase persistence and the spread of pastoralism in Africa. Am J Hum Genet 2014; 94: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A. Calcium intake and risk of fracture: systematic review. Br Med J 2015; 351: h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoo G. Stemming the tide of osteoporosis epidemic in Kenya (Conference Paper), University of Nairobi, 2010. http://www.kapkenya.org/repository/CPDs/Conferences/Annual.2014/Osteoporosis%20for%20KOGs%20nyeri.pdf . (Accessed on December 2015).
- Pollock N, Hamilton B. Osteoporotic fracture in an elite male Kenyan athlete. Br J Sports Med 2007; 42: 10001001. [DOI] [PubMed] [Google Scholar]
- Adebajo AO. Dietary calcium, physical activity, and risk of hip fracture. BMJ 1989; 299: 1165–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakuu LN. The challenge of fracture management in osteoporotic bones. East African Orthopaed J 2010; 4: 5. [Google Scholar]
- Cauley JA, Chalhoub D, Kassem AM, Fuleihan Gel-H. Geographic and ethnic disparities in osteoporotic fractures. Natl Rev Endocrinol 2014; 10: 338–351. [DOI] [PubMed] [Google Scholar]
- Gupta A. Osteoporosis in India. Natl Med J India 1996; 9: 268–274. [PubMed] [Google Scholar]
- Nordin BEC. International patterns of osteoporosis. Clin Orthop Relat Res 1966; 45: 17–30. [PubMed] [Google Scholar]
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005; 90: 3215–3224. [DOI] [PubMed] [Google Scholar]
- Abelow BJ, Holford TR, Insogna KL. Cross-cultural association between dietary animal protein and hip fracture: a hypothesis. Calcif Tissue Int 1992; 50: 14–18. [DOI] [PubMed] [Google Scholar]
- Tandon RK, Joshi YK, Singh DS, Narendranathan M, Balakrishnan V, Lal K. Lactose intolerance in North and South Indians. Am J Clin Nutr 1981; 34: 943–946. [DOI] [PubMed] [Google Scholar]
- Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 2007; 92: 2087–2099. [DOI] [PubMed] [Google Scholar]
- Leslie WD. Ethnic differences in bone mass—clinical implications. J Clin Endocrinol Metab 2012; 97: 4331. [DOI] [PubMed] [Google Scholar]
- Tian C, Hinds DA, Shigeta R, Kittles R, Ballinger DG, Seldin MF. A genomewide single-nucleotide–polymorphism panel with high ancestry information for African American admixture mapping. Am J Hum Genet 2006; 79: 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond J, Jarjou LMA, Zhou B, Prentice A, Schoenmakers I. Ethnic differences in calcium, phosphate and bone metabolism. Proc Nutr Soc 2014; 73: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A. Diet, nutrition and the prevention of osteoporosis. MRC Human Nutrition Research. Public Health Nutr 2004; 7: 227. [DOI] [PubMed] [Google Scholar]
- ‘Diagnostic Services,'. The Aga Khan University Hospital, Nairobi (Kenya) hospitals.aku.edu/Nairobi/ourservices/Pages/AKUHN_Services_Diagnostic.Aspx. (Accessed on December 2015).
- Kanis JA, Johansson H, Oden A, Cooper C, McCloskey EV. Epidemiology and quality of life working group of IOF Worldwide uptake of FRAX position paper. Arch Osteoporos 2014; 9: 166. [DOI] [PubMed] [Google Scholar]
- African Journals Online. http://www.ajol.info/index.php (accessed on December 2015).
- Lemay DG. Milk intake and risk of mortality and fractures in women and men: cohort studies. Br Med J 2014; 349: g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. GDP per capita, PPP (current international $). World Development Indicators database (updated on 14 April 2015). http://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD (accessed 14 April 2015).
- Central Intelligence Agency. GDP—per capita (PPP), The World Factbook. https://www.cia.gov/library/publications/the-world-factbook/rankorder/2004rank.html (accessed on 7 March 2014).
- FAO Statistics Division. Milk Consumption—Excluding Butter (Total) (kg per capita per year), 2011. http://faostat.fao.org/site/610/DesktopDefault.aspx?PageID=610#ancor. (accessed 19 May 2011).
- FAO. Current Worldwide Annual Meat Consumption per capita, Livestock and Fish Primary Equivalent, 2013. http://faostat.fao.org/site/610/DesktopDefault.aspx?PageID=610#ancor. (accessed 31 March 2013).
- International Monetary Fund. World Economic Outlook Database, October 2015. https://www.imf.org/external/pubs/ft/weo/2015/02/weodata/index.aspx (accessed 6 October 2015).
- International Osteoporosis Foundation. Latin America Regional Audit, 2012. http://www.iofbonehealth.org/data-publications/regional-audits/latin-america-regional-audit (accessed April 2016).
- International Osteoporosis Foundation. Asia-Pacific Regional Audit, 2013. http://www.iofbonehealth.org/data-publications/regional-audits/asia-pacific-regional-audit. (accessed April 2016).
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J et al. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro MM, Ciconeli RM, Jacques Nde O. The burden of osteoporosis in Brazil: regional data from fractures in adult men and women – The Brazilian Osteoporosis Study (BRAZOS). Rev Bras Reumatol 2010; 50: 113–127. [PubMed] [Google Scholar]
- Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C et al. Public Health. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health 2012; 12: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanwal DK, Cooper C, Dennison EM. Geographic variation in osteoporotic hip fracture incidence: the growing importance of Asian influences in coming decades. J Osteoporos 2010; 2010: 757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J et al. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J et al. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J et al. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanwal DK, Dennison EM, Harvey NC. Epidemiology of hip fracture: worldwide geographic variation. Indian J Orthop 2011; 45: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggeirsdottir K, Aspelund T, Jonsson BY, Mogensen B, Gudmundsson EF, Gudnason V et al. Epidemiology of fractures in Iceland and secular trends in major osteoporotic fractures 1989-2008. Osteoporos Int 2014; 25: 211–219. [DOI] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara NK, Marti B, Gutzwiller F. Hip fracture mortality and morbidity in Switzerland and Japan: a cross-cultural comparison. Soz Praventivmed 1993; 38: 8–14. [DOI] [PubMed] [Google Scholar]
- Azar ES, Abulmajeed S, Masri BK, Kanis JA. The prevalence of osteoporotic hip fractures in Jordan. Osteoporos Int 2011; 22(Suppl 5): S715. [Google Scholar]
- International Osteoporosis Foundation. Eastern European and Central Asia Audit, 2010. http://www.iofbonehealth.org/eastern-european-central-asian-audit (accessed April 2016).
- El-Maghraoui A, Ngbanda AR, Bensaoud N. Age-adjusted incidence rates of hip fractures between 2006 and 2009 in Rabat, Morocco. Osteoporos Int 2013; 24: 1267–1273. [DOI] [PubMed] [Google Scholar]
- Brown P, McNeill R, Rawan E, Willingale J. The burden of osteoporosis in New Zealand: 2007–2020 Osteoporosis New Zealand, Inc.2007; . [Google Scholar]
- Emaus N, Olsen LR, Ahmed LA, Balteskard L, Jacobsen BK, Magnus T et al. Hip fractures in a city in Northern Norway over 15 years: time trends, seasonal variation and mortality: the Harstad Injury Prevention Study. Osteoporos Int 2011; 22: 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnyak O, Ershova O, Belova K, Gladkova E, Sinitsina O, Ganert O. The development of a FRAX model for the Russian Federation. Archives of Osteoporosis Combined data 2008-2010 from Yaroslavl and Pervouralsk Olga Yershova, Olga Lesnyak, 2012. [DOI] [PubMed]
- Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide on behalf of the IOF Working Group on Epidemiology and Quality of Life Osteoporosis International. 21 February 2012. [DOI] [PMC free article] [PubMed]
- Park C, Jang S, Lee A, Kim HY, Lee YB, Kim TY et al. Incidence and mortality after proximal humerus fractures over 50 years of age in South Korea: National Claim Data from 2008 to 2012. J Bone Metab 2015; 22: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyawongpaisal P, Siriwongpairat P, Loahachareonsombat W, Angsachon T, Kumpoo U, Sujaritputtangkul S et al. A multicenter study on hip fractures in Thailand. J Med Assoc Thai 1994; 77: 488–495. [PubMed] [Google Scholar]
- Tuzun S, Eskiyurt N, Akarirmak U, Saridogan M, Senocak M, Johansson H et al. Incidence of hip fracture and prevalence of osteoporosis in Turkey: the FRACTURK study. Osteoporos Int 2012; 23: 949–955. [DOI] [PubMed] [Google Scholar]
- Svedbom A, Hernlund E, Ivergård M. The EU review panel of the International Osteoporosis Foundation. Osteoporosis in the European Union & a compendium of country-specific reports. Arch Osteoporos 2013; 8: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013; 178: 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera-Espinoza G. Epidemiology of osteoporosis in Latin America 2008. Salud Publica Mex 2009; 51(Suppl 1): S52–S55. [DOI] [PubMed] [Google Scholar]
- Scrimshaw NS, Murray E. Lactose tolerance and milk consumption: myths and realities. Arch Latinoam Nutr 1988; 38: 543–567. [PubMed] [Google Scholar]
- Norman Kretchmer, Lactose and Lactase, Scientific American, October, 1972. [PubMed]
- Koek WN, van Meurs JB, van der Eerden BC, Rivadeneira F, Zillikens MC, Hofman A et al. The T-13910C polymorphism in the lactase phlorizin hydrolase gene is associated with differences in serum calcium levels and calcium intake. J Bone Miner Res 2010; 25: 1980–1987. [DOI] [PubMed] [Google Scholar]
- Mattar R, Monteiro MS, Villares CA, Santos AF, Silva JMK, Carrilho FJ. Frequency of LCT-13910C.T single nucleotide polymorphism associated with adult-type hypolactasia/lactase persistence among Brazilians of different ethnic groups. Nutr J 2009; 8: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcare CA, Weale ME, Jones AL, Connell B, Zeitlyn D, Tarekegn A et al. The allele of a singlenucleotide polymorphism 13.9 kb upstream of the lactase gene (LCT) (C13.9kbT) does not predict or cause the lactase-persistence phenotype in Africans. Am J Hum Genet 2004; 74: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr SI. Perceived lactose intolerance in adult Canadians: a national survey. Appl Physiol Nutr Metab 2013; 38: 830–835. [DOI] [PubMed] [Google Scholar]
- Wang YG, Yan YS, Xu JJ, Du RF, Flatz SD, Kühnau W et al. Prevalence of primary adult lactose malabsorption in three populations of northern China. Hum Genet. 1984; 67: 103–106. [DOI] [PubMed] [Google Scholar]
- Anne Charlotte Jäger, "Laktose-intolerans: Gentest for laktose-intolerans - hurtig og billig diagnostik", DSKB-NYT, no. 2006.
- Kokkonen J, Tikkanen S, Savilahti E. Residual intestinal disease after milk allergy in infancy. J Pediatr Gastroenterol Nutr. 2001; 32: 156–161. [DOI] [PubMed] [Google Scholar]
- De Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. Probiotics—compensation for lactase insufficiency. American Journal of Clinical Nutrition 2001; 73: (No. 2): 421S–429s. [DOI] [PubMed] [Google Scholar]
- Bogacsi-Szabo ND, Varkonyi E, Csanyi A, Czibula B, Bede A, Tari O et al. Prevalence of adult-type hypolactasia as diagnosed with genetic and lactose hydrogen breath tests in Hungarians. Eur J Clin Nutr 2009; 63: 909–912. [DOI] [PubMed] [Google Scholar]
- Rana SV, Bhasin DK, Naik N. Lactose malabsorption in apparently healthy adults in northern India, assessed using lactose hydrogen breath test. Indian J Gastroenterol 2004; 23: 78. [PubMed] [Google Scholar]
- Cavalli-Sforza LT, Strata A, Barone A, Cucurachi L. Primary adult lactose malabsorption in Italy: regional differences in prevalence and relationship to lactose intolerance and milk consumption. Am J Clin Nutr 1987; 45: 748–754. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Sasaki G, Goto S, Yanagiya S, Takashina K. Studies on the etiology of milk intolerance in Japanese adults. Gastroenterol Jpn 1975; 10: 29–34. [DOI] [PubMed] [Google Scholar]
- Enattah NS, Jensen TG, Nielsen M, Lewinski R, Kuokkanen M, Rasinpera H et al. Independent introduction of two lactase-persistence alleles into human populations reflects different history of adaptation to milk culture. Am J Hum Genet 2008; 82: 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Ranciaro A. Convergent adaptation of human lactase persistence in Africa and Europe. Nature Genetics 2006; 39: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enattah NS, Trudeau A, Pimenoff V, Maiuri L, Auricchio S, Greco L et al. Evidence of still-ongoing convergence evolution of the lactase persistence T-13910 alleles in humans. Am J Hum Genet 2007; 81: 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton J, George P. The prevalence of lactose intolerance (adult hypolactasia) in a randomly selected New Zealand population. N Z Med J 2010; 123: 117–118. [PubMed] [Google Scholar]
- Vuorisalo T, Arjamaa O, Vasemägi A, Taavitsainen JP, Tourunen A, Saloniemi I. High lactose tolerance in North Europeans: a result of migration, not in situ milk consumption. Perspect Biol Med 2012; 55: 163–174. [DOI] [PubMed] [Google Scholar]
- Coelho M, Luiselli D, Bertorelle G, Lopes AI, Seixas S, Destro-Bisol G et al. Microsatellite variation and evolution of human lactase persistence. Hum Genet. 2005; 117: 329–339. [DOI] [PubMed] [Google Scholar]
- Torniainen S, Parker MI, Holmberg V, Lahtela E, Dandara C, Jarvela I. Screening of variants for lactase persistence/non-persistence in populations from South Africa and Ghana. BMC Genet 2009; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal I, Gagjee PP, Essop AR, Noormohamed AM. Lactase deficiency in the South African black population. Am J Clin Nutr 1983; 38: 901–905. [DOI] [PubMed] [Google Scholar]
- Rasinperä H, Forsblom C, Enattah NS, Halonen P, Salo K, Victorzon M et al. The C/C-13910 genotype of adult-type hypolactasia is associated with an increased risk of colorectal cancer in the Finnish population. Gut 2005; 54: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorisalo T, Arjamaa O, Vasemägi A, Taavitsainen JP, Tourunen A, Saloniemi I. High lactose tolerance in North Europeans: a result of migration, not in situ milk consumption. Perspect Biol Med 2012; 55: 163–174. [DOI] [PubMed] [Google Scholar]
- Nicklas TA, Qu H, Hughes SO, Wagner S, Foushee H, Shewchuk R et al. Prevalence of self-reported lactose intolerance in a multi-ethnic sample of adults. Nutr Today 2009; 44: 222–227. [Google Scholar]