Abstract
Objectives:
The aim of this study was to determine the prevalence of liver fibrosis in patients with human immunodeficiency virus (HIV) monoinfection versus those with HIV hepatitis-B virus (HBV) co-infection as assessed with shear wave elastography (SWE) in a tertiary sub-Saharan Africa hospital.
Materials and Methods:
A total of 105 consecutive patients, 70 with HIV monoinfection and 35 with HIV-HBV co-infection, had liver elastography obtained using SWE to assess for the presence of liver fibrosis the cutoff of which was 5.6 kPa. Assessment of aspartate aminotransferase-to-platelet ratio index (APRI) score (a noninvasive serum biomarker of liver fibrosis) in these patients was also done.
Results:
The prevalence of liver fibrosis was significantly higher (P < 0.0001) in patients with HIV-HBV co-infection, 25.7%, compared to those with HIV monoinfection, 7.1%. APRI score was greater in patients with HIV-HBV co-infection than those with HIV monoinfection. HIV co-infection with HBV accelerates progression to liver fibrosis. Association of a low cluster of differentiation 4 (CD-4) count with advanced fibrosis supports earlier starting of antiretroviral therapy to prevent rapid progression of liver disease in HIV-positive patients.
Conclusion:
In view of the high prevalence of liver fibrosis in patients with HIV-HBV co-infection, regular monitoring of the disease progression is recommended.
Keywords: Human immunodeficiency virus, Hepatitis-B, liver fibrosis, shear wave elastography

INTRODUCTION
Liver disease in the last decade has become one of the leading causes of morbidity and mortality in human immunodeficiency virus (HIV) infected individuals with chronic liver disease emerging as the leading cause of nonacquired immunodeficiency disease syndrome-related deaths in this population.[1] Chronic hepatitis-B virus (HBV) infection in patients with HIV occurs at a rate ten times higher than in the general population and is associated with increased rate of progression to chronic liver disease.[2]
Management of chronic liver disease is dependent on the grade of liver fibrosis to ascertain the urgency and choice of treatment and advice on further screening for cirrhosis and hepatocellular carcinoma. Though liver biopsy has traditionally been the gold standard for diagnosis and staging of liver fibrosis, the procedure has paramount shortfalls as a medical screening test. It lacks the safety profile, accuracy, and accessibility of a standard medical screening test. As a result, screening for liver fibrosis in patients with HIV is low. The low prevalence of liver disease staging in these patients probably contributes to their poor outcome.
In response to the lack of an ideal screening tool for liver fibrosis, there has been a quest to develop noninvasive methods for diagnosing fibrosis and cirrhosis in the last decade. In this respect, there has been significant development of special blood parameters and elastography. Serum biomarkers, such as aspartate aminotransferase (AST)-to-platelet ratio index (APRI) score and elastography are validated tools for assessment of liver fibrosis.[3,4] These noninvasive methods have several advantages over liver biopsy including lack of adverse effects, reduced sampling error, objectivity in the interpretation of results, and appropriateness for repeated examinations, all at a lower cost.[5]
Elastography has already been approved by most European countries as an evaluation tool for liver fibrosis in patients with viral hepatitis.[6]
In Africa, information on liver disease in HIV beyond liver enzyme measurements have not been studied.[7] Though the burden of HIV-HBV co-infection is known to be higher in sub-Saharan Africa as compared to the West, there is a paucity of data regarding its effect on chronic liver disease in this group of patients.[8]
The aim of this study was to determine the prevalence of liver fibrosis in patients with HIV monoinfection as compared to those with HIV-HBV co-infection using shear wave elastography (SWE) at a Tertiary Teaching Hospital in Kenya.
MATERIALS AND METHODS
This was a cross-sectional study at a tertiary hospital. The study obtained approval from the institution's research and ethics committee and written informed consent was obtained from all participants before enrollment in this study. Inclusion criteria were HIV-positive patients attending the HIV clinic at the tertiary hospital who consented to the study and had HBV and hepatitis-C virus (HCV) tests. Patients with HIV-HCV co-infection, massive ascites, those unable to hold their breath for adequate elastography readings, and those <18 years of age were excluded. The acquisition time for the machine used in this study is typically on the order of 100 ms which means none-overt breathing does not affect the readings. The technique also detects significant motion with no measurement recorded when there is a significant motion during the acquisition. One patient was excluded based on inability to acquire adequate readings.
Between August 2013 and January 2014, 105 patients were enrolled in the study. The HIV and HBV infections were determined using enzyme immunoassay. Chronic HBV infection was determined by a positive hepatitis-B surface antigen for more than 6 months. Based on these tests, two groups of patients were identified: Seventy patients with HIV monoinfection and thirty five patients with HIV-HBV co-infection [Figure 1].
Figure 1.
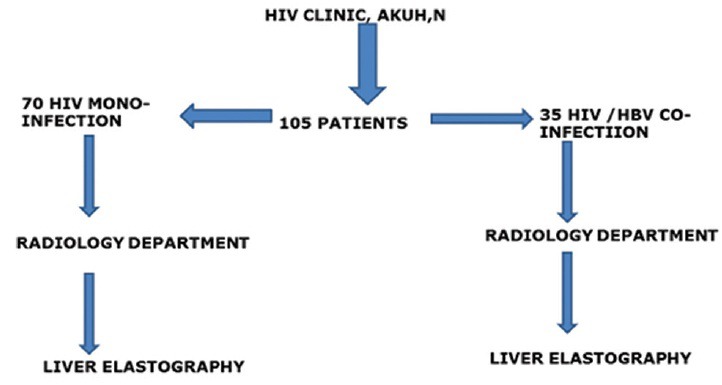
Flow chart showing process of recruitment of patients.
Each group had real-time SWE of the liver performed at the radiology department of the hospital. Only one machine, Phillips iU22 xMATRIX, was used for the liver elastography using a 5 MHz curvilinear probe. The liver elastography was carried out by one of two consultant radiologists in the radiology department with ultrasonography experience of 19 years and 7 years, respectively, a senior resident in the radiology department with 3 years’ experience or by a sonographer who has over two decades of experience. They underwent 3 weeks training on performance of elastography which was carried out by a specialist. Real-time SWE has been shown to have high intra-observer and inter observer reproducibility between an expert operator and a novice operator.[9,10]
The persons performing the scans were blind to the liver function tests of the patients. All liver stiffness measurements (LSMs) were acquired with the patient lying supine. In each patient and at the same session, 10 LSMs were acquired from the right lobe of the liver through an intercostal space with the probe in the region of the midclavicular line. The SWE box was placed at least 2.0 cm from the liver capsule. The area of the SWE box was 1 cm2 and was the same for all patients. Prior to starting the measurements, patients were trained on breath-hold to reduce erroneous readings resulting from inspiration. Each LSM acquisition took approximately 5 s. The acquisition was from an area free of large vessels [Figure 2a and b]. This technique reduces the effects of reverberation artifacts arising from the liver capsule and pulsations from large vessels on the measurements which could lead to erroneously high elastography readings. The median value of ten consecutive measurements in kilopascals (kPa) was used for statistical analyses.
Figure 2.
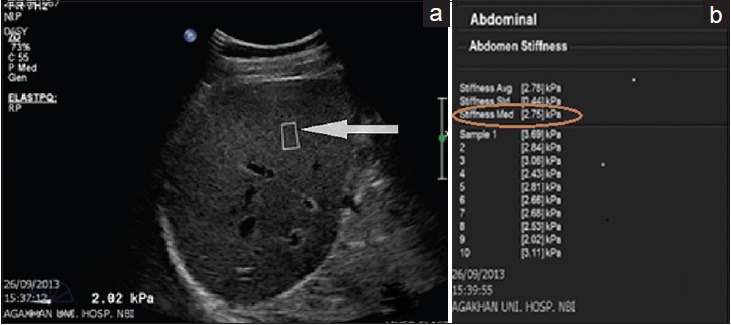
43-year-old male with human immunodeficiency virus monoinfection (a) Grayscale liver ultrasound image shows acquisition of liver stiffness measurement using shear wave elastography. The sample box (arrow) is away from the liver capsule and large blood vessels. (b) A table shows summary of ten liver stiffness measurement readings of the same patient with a median elastography score (encircled) of 2.75 kPa.
The measurements obtained were automatically stored in the department's picture archiving and communication systems and also recorded in a data sheet. The data were then classified into two with elastography scores <5.6 kPa classified as having no significant fibrosis and scores ≥5.6 kPa representing the presence of fibrosis. Elastographic reference ranges have been developed for distinguishing presence or absence of fibrosis and the presence of cirrhosis using histology as the reference standard.[11,12,13] Based on these studies, the following cutoffs have been recommended for elastographic scores in liver fibrosis using SWE: On average, 5.6 kPa represents the upper limit for normal, 7.1 kPa the cutoff for significant fibrosis, while 12 kPa is the cutoff for the presence of cirrhosis.
Calculation of the APRI score was done using the most current AST and platelet count available for the patient. These were within 3 months of the elastography measurement. For patients with more than one set of laboratory test results available, the set of results closest to the time of the elastography were used. These data were obtained from a computerized medical register. The score was then calculated using Wai's formula:[3]
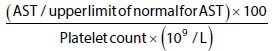
Based on their APRI score, patients were placed into one of three classes as follows - Class 1: <0.5, Class 2: 0.51-1.5, and Class 3: >1.5.
Statistical analysis
Study data were captured in a spreadsheet (Excel, Microsoft Corporation). The analysis was done using STATA version 11.2 StataCorp, Texas, USA.
The continuous metric data obtained from APRI and SWE converted to categorical ordinal data using standard cut-offs as explained in the methods above.
Differences in baseline characteristics between patients with and without liver fibrosis by SWE were assessed using Chi-square or Fisher's exact tests, as indicated, for categorical data and Wilcoxon rank-sum tests for continuous data.
Prevalence of liver fibrosis as assessed by SWE was calculated by dividing the number of patients with liver fibrosis based on SWE cutoff score with the total number of patients who had undergone the test.
Association between risk factors such as a low cluster of differentiation 4 (CD4), cell count (below 200 cells/mm3), detectable HIV viral load (HIV viral load > 40 copies/mL), and obesity, with liver fibrosis, were assessed by stratification and summary odds ratios presented using Mantel-Haenszel method. Those found to have a significant effect were subjected to multivariable logistic regression to control for the confounding. Pearson's correlation coefficient was used to assess for correlation between SWE and APRI score.
Differences and associations were considered statistically significant if the P value was <0.05.
RESULTS
Baseline characteristics
A total of 105 participants were consecutively enrolled; 70 with HIV monoinfection and 35 with HIV-HBV co-infection. All patients were on highly active antiretroviral therapy. The baseline characteristics of the two groups are provided in Table 1. 59% were male, and this was not statistically different between the two groups. The median age was also similar in the two groups. The body mass index (BMI) was statistically lower in patients with HIV-HBV co-infection (P < 0.02).
Table 1.
Baseline characteristics
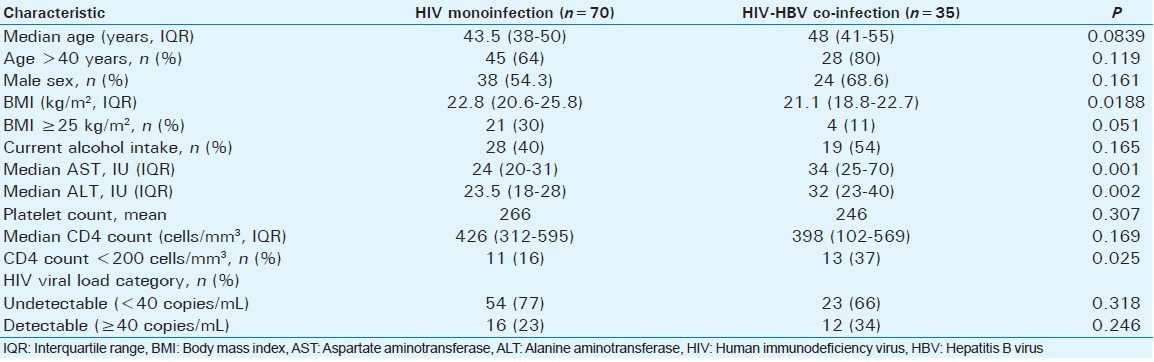
The median AST and alanine aminotransferase (ALT) were significantly higher in the HIV-HBV co-infected group (P = 0.001 and P = 0.002, respectively).
At the time of enrollment, the participants had a median CD4 count of 418 cells per μL (IQR 256–569). This was comparable for the two groups. The majority of patients (72%) had undetectable HIV viral load which was not significantly different in the two groups (P = 0.318). HBV viral load was undetectable in 68% of the patients with HIV-HBV co-infection.
The median elastography score was significantly higher in the HIV-HBV co-infected group (P < 0.001). Figure 3a and b show SWE measurement for one of the patients with HIV-HBV co-infection who had liver fibrosis as per the median score of 6.35 kPa.
Figure 3.
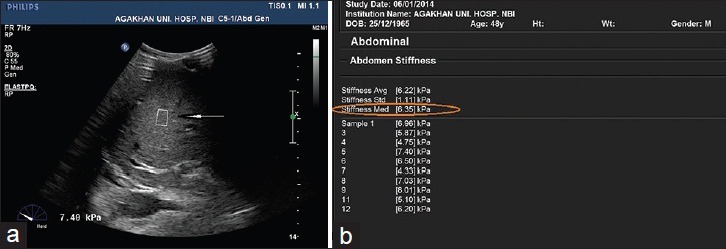
A 39-year-old male with the human immunodeficiency virus hepatitis-B virus co-infection (a) Grayscale ultrasound image of the liver shows a shear wave elastography acquisition box (arrow) with a high elastography score of 7.4 kPa. (b) A table showing ten liver stiffness measurements readings for the same patient with a high median elastography score of 6.35 kPa (encircled).
Prevalence of liver fibrosis
Overall, the prevalence of liver fibrosis based on an SWE score >5.6 kPa was 13.3%. The prevalence was significantly higher (P < 0.0001) in HIV-HBV co-infected patients (25.7%) than in the HIV-monoinfected patients (7.1%) as shown in the bar graph in Figure 4.
Figure 4.
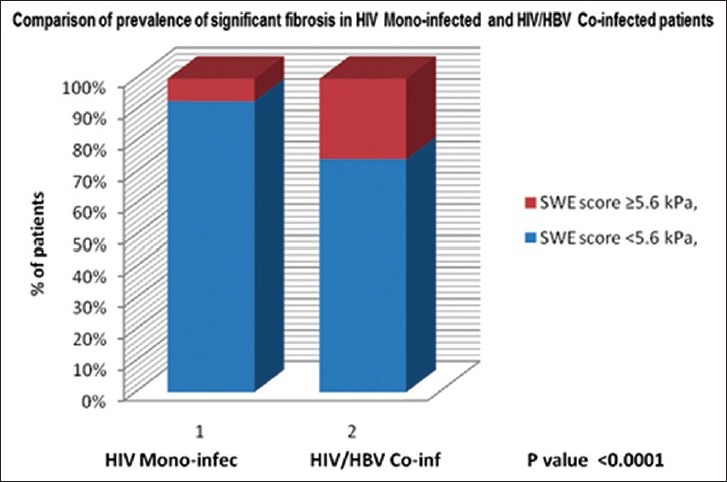
Bar graph comparing prevalence of liver fibrosis in the human immunodeficiency virus- monoinfected and human immunodeficiency virus hepatitis-B virus co-infected patients show a higher prevalence in the co-infected group of approximately 26%.
Risk factors for liver fibrosis
In multivariate analysis, HBV co-infection, low CD4 count (<200 cells/mm3), and detectable viral load was found to have a significant association with the presence of advanced liver fibrosis.
HBV co-infection was associated with 4.5 times increased risk of having significant fibrosis (CI: 1.3–15.4, P = 0.0086). Although AST, ALT, and BMI were statistically different in the two groups, they were not found to have a significant association with liver fibrosis. There was no statistically significant difference in current alcohol intake between the two groups and this was not found to have a significant association with the presence of liver fibrosis.
Aspartate aminotransferase-to-platelet ratio index score
APRI score was significantly higher in patients with HIV-HBV co-infection compared to those with HIV monoinfection (P = 0.0068) as shown in the bar graph in Figure 5. Exactly, 33.4% of the co-infected patients had an APRI score of >0.5 compared to 8.6% in the monoinfected group.
Figure 5.
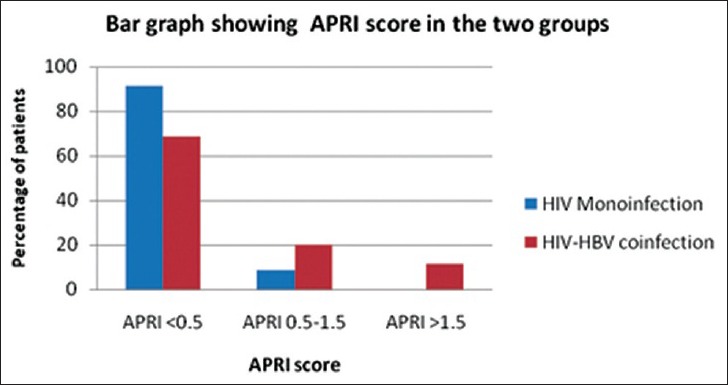
Bar graph shows distribution of aspartate aminotransferase-to-platelet ratio index score in the human immunodeficiency -virus monoinfected and human immunodeficiency virus hepatitis-B virus co-infected groups and indicates that only the co-infected group had patients with an aspartate aminotransferase-to-platelet ratio index score above 1.5.
The Pearson's correlation coefficient between APRI score and median elastography score was 0.33 (P = 0.0026) representing moderate correlation. A scatter plot between the two [Figure 6] showed strong positive correlation for patients with an APRI score <1. After fitting a linear regression line, for every unit increase in APRI score, there is a 0.64 (CI = 0.23–1.06) increase in the median elastography score.
Figure 6.
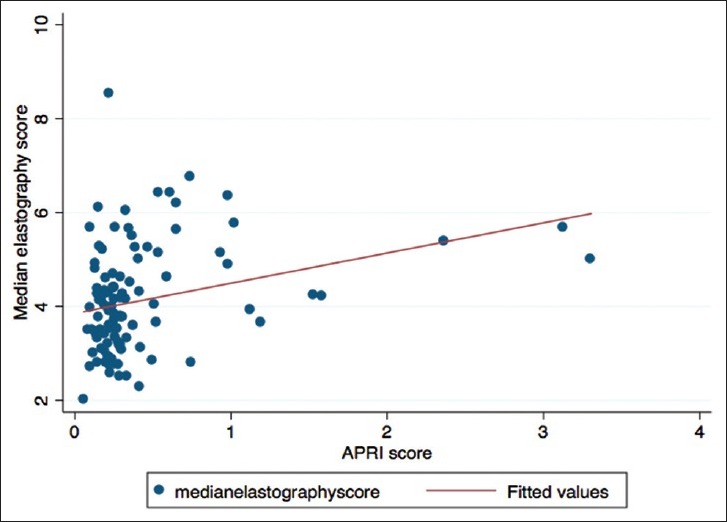
A scatter plot between median elastography scores and aspartate aminotransferase-to-platelet ratio index scores with a fitted linear regression line. There was a better correlation between the two for patients with aspartate aminotransferase-to-platelet ratio index score less than 0.5 as opposed to those with higher scores.
DISCUSSION
This study reveals a significantly higher prevalence of liver fibrosis in patients with HIV-HBV co-infection compared to those with HIV monoinfection as assessed by SWE. HBV co-infection was associated with 4.5 times increase in the prevalence of liver fibrosis. This suggests a significant impact of HBV infection on the progress of liver disease with its potential associated morbidity and mortality in patients with HIV as has been observed in the data collection on adverse events of anti-HIV drugs study.[14] HIV co-infection with HBV accelerates progression to significant liver fibrosis which is in keeping with the higher prevalence of liver fibrosis observed in the HIV-HBV co-infected group in our study.[15,16]
Prior to this study, evaluation of liver disease in patients with HIV at our institution was limited to liver enzyme studies. These tests are however poor markers of liver fibrosis, especially in HIV-infected persons as a significant liver fibrosis may be present in individuals with normal transaminases.[17] As a result, there may have been underestimation of the significance of HIV and HBV on liver disease. Though liver biopsy is traditionally the gold standard for the evaluation of liver fibrosis, it is not a practical diagnostic tool in this cohort of patients. Liver elastography offers a noninvasive means of assessment of liver fibrosis, especially in such patients. The examination is quick, painless, and highly acceptable to patients with results obtained almost instantly. It has been widely validated as a noninvasive means for evaluation of liver fibrosis.[6]
To our knowledge, only two other studies in Africa, in Uganda and Nigeria, have assessed liver fibrosis in HIV patients using elastography.[18,19] Both used transient elastography as opposed to real-time SWE used in this study. The prevalence of significant liver fibrosis in patients with HIV monoinfection was higher in the Ugandan study (17%) compared to the Nigerian study (4.7%). Our study found a prevalence of 7.1% which is slightly higher than that in the Nigerian study. A study using APRI score found a prevalence of 8.3% in HIV - monoinfected patients.[20]
The 25.7% prevalence of liver fibrosis in patients with HIV-HBV co-infection was comparable to the 22.5% found in the Nigerian study. The average age of subjects was slightly higher in our study. The median age in our study was 45 years compared to 34 years for the Nigerian study. The progression of liver fibrosis is strongly dependent on age.[21] This is probably related to the higher vulnerability to environmental factors, especially oxidative stress and the reduction in immune capacity of an individual as they age. Although the Nigerian study subjects were ART naive while those in the current study were not, studies have shown that ART does not cause significant histological liver disease and may be protective.[22] The results of our study are similar to those of the Nigerian study despite the different elastography techniques used SWE has been shown to be more accurate and reproducible in the assessment of liver fibrosis than TE.[9] Monitoring the effect of therapy is one the major challenges in the management of chronic liver diseases. In a study involving patients with chronic hepatitis-B infection, there was a significant decrease in liver elastography measurements compared to the baseline 1 year posttreatment with entecavir.[23] Liver elastography, being noninvasive, would thus be useful for monitoring of liver fibrosis in patients on management for chronic liver disease.
This study was not powered to assess the effect of potential confounders such as age, alcohol use, CD4 count, and viral load on the presence of liver fibrosis. However, low CD4 count and detectable HIV viral load were associated with the presence of liver fibrosis in our study. Other studies have shown both to be associated with a significant increase in the risk of having advanced liver fibrosis.[19,24] These findings are as a result of the worse clinical progression of liver fibrosis observed in patients with deeper immunosuppression. This would support starting of ART earlier so as to prevent rapid progression of liver disease in HIV-positive patients.
Although the co-infected group had a significantly lower BMI, this was not found to have a significant effect on the stage of liver fibrosis following multivariate analysis. Furthermore, weight loss has been postulated to result in a reduction in abnormal liver enzyme and improvement in liver fibrosis.[25]
The co-infected group had significantly higher APRI scores compared to the monoinfected patients as a result of higher AST levels in this group. This is in keeping with a higher prevalence of advanced liver fibrosis observed in the co-infected patients using SWE. These findings are similar to those observed by Post et al., in which HIV patients with viral co-infection (HCV or HBV) had higher APRI scores compared to the monoinfected patients.[26] Although HIV is associated with thrombocytopenia, high APRI score in this referenced study was attributed to elevated AST and not low platelets. AST was normal in the monoinfected patients in our study, which would explain the lower APRI scores in this group. Although most patients with HIV may have normal transaminases despite advanced liver fibrosis, a high APRI score (which is in essence as a result of high AST) is associated with increased mortality in these patients.[26] A comparison between the APRI score and the median liver elastography scores showed a moderate correlation between the two with a stronger correlation for patients with a low APRI score. APRI score is a more readily available screening tool than SWE as it only requires AST and platelet count and would, therefore, be very useful in resource -poor settings. The poor correlation in patients with higher APRI score is likely due to the small number of patients with significant fibrosis as assessed by SWE and APRI scores and the fact that this study was not powered to assess for this correlation.
Limitations
Our study had some limitations. First, the elastographic and APRI score findings in the current study were not compared to liver biopsy due to technical and ethical challenges. The SWE cut-offs used in the study were based on studies done mainly in Europe with most of the subjects having HCV. Though there is no reason to believe that the cut-offs would be different in the African population, a study to validate the cut-offs in African population by comparing SWE with histology is recommended. Second, the study did not take into consideration the effect of other potential causes of liver fibrosis which may be unique to sub-Saharan Africa, for example, tropical schistosomiasis and exposure to aflatoxins. It is however important to state that the study population was not from an area where these two are endemic in Kenya. Although the cut-off of 5.6 kPa is based on the highest sensitivity and specificity for detecting fibrosis, there is the inherent risk of misclassifying some patients with high normal as having fibrosis. The liver elastography scans were performed by only one radiologist per patient. Although internal quality evaluation of the technique revealed a high intra- and inter-observer reproducibility between the two radiologists of over 90%, lack of two reviewers per patient in this study is a limitation. SWE also has inherent limitations with falsely elevated scores seen in patients with acute hepatitis, cholestasis, and congestive cardiac disease in the absence of fibrosis which were not controlled for in our study. Compared to magnetic resonance MR elastography, SWE has shown moderate correlation and similar diagnostic performance in the diagnosis of hepatic fibrosis of METAVIR stage F2 or greater.[27]
CONCLUSION
Given the higher prevalence of liver fibrosis in patients with HIV-HBV co-infection compared to those with HIV monoinfection, the authors recommend routine monitoring of this group of patients to check for progression to cirrhosis. Significant reduction in LSM has been shown to correlate well with response to therapy and since SWE is noninvasive, it would form an excellent monitoring tool.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge Jyoti Shah, senior sonographer, and Dr. Joyce Sande, consultant radiologist, both of Department of Radiology and Imaging, Aga Khan University Hospital, Nairobi who were of great help in the performance of the liver elastography scans. This work would not have been possible without the services of Dr. Edward Chege, who helped with data analysis, formatting, and other technical aspects.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/22/183582
REFERENCES
- 1.Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, et al. Data Collection on Adverse Events of Anti-HIV drugs (D: A: D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D: A: D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 Suppl):S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 4.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology. 2011;53:726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 5.Duarte-Rojo A, Altamirano JT, Feld JJ. Noninvasive markers of fibrosis: Key concepts for improving accuracy in daily clinical practice. Ann Hepatol. 2012;11:426–39. [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55:245–64. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Rockstroh JK, Peters L, Wedemeyer H. Is there a need for liver disease monitoring in HIV patients in Africa? Antivir Ther. 2011;16:287–9. doi: 10.3851/IMP1787. [DOI] [PubMed] [Google Scholar]
- 8.Uneke CJ, Ogbu O, Inyama PU, Anyanwu GI, Njoku MO, Idoko JH. Prevalence of hepatitis-B surface antigen among blood donors and human immunodeficiency virus-infected patients in Jos, Nigeria. Mem Inst Oswaldo Cruz. 2005;100:13–6. doi: 10.1590/s0074-02762005000100002. [DOI] [PubMed] [Google Scholar]
- 9.Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, et al. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012;81:3102–6. doi: 10.1016/j.ejrad.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Guzmán-Aroca F, Reus M, Berná-Serna JD, Serrano L, Serrano C, Gilabert A, et al. Reproducibility of shear wave velocity measurements by acoustic radiation force impulse imaging of the liver: A study in healthy volunteers. J Ultrasound Med. 2011;30:975–9. doi: 10.7863/jum.2011.30.7.975. [DOI] [PubMed] [Google Scholar]
- 11.Ferraioli G, Parekh P, Levitov AB, Filice C. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med. 2014;33:197–203. doi: 10.7863/ultra.33.2.197. [DOI] [PubMed] [Google Scholar]
- 12.Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: Comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910–8. doi: 10.1148/radiol.13130128. [DOI] [PubMed] [Google Scholar]
- 13.Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: A prospective study of 935 patients. J Hepatol. 2007;46:628–34. doi: 10.1016/j.jhep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: The D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 15.Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, et al. Liver disease as a major cause of death among HIV infected patients: Role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Carbonero L, de Ledinghen V, Moreno A, Maida I, Foucher J, Barreiro P, et al. Liver fibrosis in patients with chronic hepatitis C and persistently normal liver enzymes: Influence of HIV infection. J Viral Hepat. 2009;16:790–5. doi: 10.1111/j.1365-2893.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 18.Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Ndyanabo A, Kiggundu V, et al. High prevalence of liver fibrosis associated with HIV infection: A study in rural Rakai, Uganda. Antivir Ther. 2011;16:405–11. doi: 10.3851/IMP1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins C, Agbaji O, Ugoagwu P, Thio CL, Auwal MM, Ani C, et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis. 2013;57:e189–92. doi: 10.1093/cid/cit564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Torres M, Poynard T. Risk factors for liver fibrosis progression in patients with chronic hepatitis C. Ann Hepatol. 2003;2:5–11. [PubMed] [Google Scholar]
- 22.Mehta SH, Thomas DL, Torbenson M, Brinkley S, Mirel L, Chaisson RE, et al. The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection. Hepatology. 2005;41:123–31. doi: 10.1002/hep.20541. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto M, Mori M, Ogawa T, Fujii H, Kobayashi S, Iwai S, et al. Usefulness of transient elastography for assessment of liver fibrosis in chronic hepatitis B: Regression of liver stiffness during entecavir therapy. Hepatol Res. 2010;40:853–61. doi: 10.1111/j.1872-034X.2010.00687.x. [DOI] [PubMed] [Google Scholar]
- 24.Pineda JA, González J, Ortega E, Tural C, Macías J, Griffa L, et al. Prevalence and factors associated with significant liver fibrosis assessed by transient elastometry in HIV/hepatitis C virus-coinfected patients. J Viral Hepat. 2010;17:714–9. doi: 10.1111/j.1365-2893.2009.01229.x. [DOI] [PubMed] [Google Scholar]
- 25.Price JC, Seaberg EC, Badri S, Witt MD, D’Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis. 2012;205:1005–13. doi: 10.1093/infdis/jir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post FA, Sabin CA. UK Collaborative HIV Cohort Study Steering Committee. Aspartate aminotransferase-to-platelet ratio index is a powerful predictor of mortality among HIV-positive patients. J Infect Dis. 2013;207:367–8. doi: 10.1093/infdis/jis666. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, et al. Hepatic fibrosis: Prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772–82. doi: 10.1148/radiol.14132000. [DOI] [PubMed] [Google Scholar]


