Abstract
Purpose:
To compare the safety and efficacy of three methods for correcting pre-existing astigmatism during phacoemulsification.
Methods:
This prospective, comparative, non-randomized study was conducted from March 2010 to January 2011, and included patients with keratometric astigmatism ≥1.25 D undergoing cataract surgery. Astigmatism was corrected using the following approaches: limbal relaxing incisions (LRI) on the steep meridian, extension and suturing of the phaco incision created at the steep meridian (extended-on-axis incision, EOAI), and toric intraocular lens (tIOL) implantation. Keratometric and refractive astigmatism were evaluated 1, 8, and 24 weeks postoperatively.
Results:
Eighty-three eyes of 72 patients (35 male and 37 female) with mean age of 62.4 ± 14.3 (range, 41-86) years were enrolled. The astigmatism was corrected by using the LRI, EOAI and tIOL implantation methods in 17, 33 and 33 eyes, respectively. Postoperative uncorrected distance visual acuity (UDVA) was significantly improved in all three groups. The difference in postoperative UDVA was not statistically significant among the study groups throughout follow-up except at week 24, when UCVA was significantly better in the tIOL group as compared to the EOAI group (P = 0.024). There is no statistically significant difference of correction index and index of success between three groups at week 24 (P = 0.085 and P = 0.085 respectively).
Conclusion:
There was no significant difference in astigmatism reduction among the three methods of astigmatism correction during phacoemulsification. Each of these methods can be used at the discretion of the surgeon.
Keywords: Astigmatism Correction, Extended-on-axis Incision, Limbal Relaxing Incision, Phacoemulsification, Toric Intraocular Lens
INTRODUCTION
The effect of cataract incision on astigmatism has been known for more than a century.[1,2,3] In the past, replacement of the crystalline lens with intraocular lenses (IOLs) was used to correct spherical refractive error whereas corneal astigmatism remained uncorrected. Currently, refractive cataract surgery is performed to correct both spherical and astigmatic refractive errors.[4,5,6] Astigmatism of 1 to 3 diopters has been reported in 15% to 29% of eyes with cataracts.[4,7,8]
Optical methods including glasses or contact lenses can be used for astigmatism correction after cataract surgery. However, patients are usually reluctant to use glasses after surgery, and contact lenses have their own limitations including difficulty in keeping them sterile and the high long-term cost.[7] Features of a cataract incision including diameter of incision, location, and shape have variable effects on pre-existing corneal astigmatism. Other methods of intraoperative astigmatism correction include limbal relaxing incision, astigmatic keratotomy, and toric IOL (tIOL) implantation.[4]
Using corneal incisions for correction of postoperative astigmatism dates back to the early 1980s and was first introduced by Osher.[9] Several studies have been conducted to evaluate the effect of incision length, location, number and depth, as well as the diameter of the central optical zone in correcting astigmatism.[9,10,11,12,13] Limbal relaxing incisions (LRIs) are created on two sides of the steep corneal meridian in front of the limbus at an approximate depth of 600µ.[14,15,16] Primary studies conducted on a limited number of cases have shown that LRI can be used to reduce corneal astigmatism during cataract surgery.[17,18]
The effect of extended on axis incisions (EOAIs) in which the main surgical incision is extended depends on the length and location of the incisions; incisions created at the 90° meridian cause more corneal flattening than incisions created at 180°. The effect can also be enhanced by increasing the length of the incision. Disadvantages of this technique are difficulty in creating the incision at an exact meridian and the need for suturing in some cases.[4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]
Toric IOLs have been used in cataract surgery for more than a decade.[19,20,21,22] Several studies have demonstrated that tIOLs can accurately correct preexisting astigmatism.[23,24,25,26,27,28]
Herein, we compare the results of three methods of astigmatism correction including LRIs, EOAIs, and tIOL implantation in patients undergoing cataract surgery.
METHODS
This prospective, parallel, cohort, non-randomized study was performed at the Department of Ophthalmology, Shahid Beheshti University of Medical Sciences, Tehran, Iran and two private eye clinics between March 2010 and January 2011. The study was approved by the Ethics Committee of the Ophthalmic Research Center, affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran. Signed informed consent was obtained from all patients prior to surgery.
Patients with senile cataract and corneal astigmatism exceeding 1.25D were enrolled in the study. Exclusion criteria were previous corneal or anterior segment surgery, previous corneal trauma, irregular astigmatism, corneal opacity, active blepharitis and meibomianitis, the presence of diabetes mellitus or collagen vascular diseases.
All patients underwent a complete ophthalmic examination that included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), refraction, slit lamp biomicroscopy, applanation tonometry, indirect ophthalmoscopy, manual keratometry and corneal topography (Placido disk videokeratography, TMS-5, Tomey, Nagoya, Japan.) Ultrasonic biometry (Standard-S France, Paris, France) was used to calculate IOL power using the SRK-T formula in all study groups. In the tIOL Group, data measured by ultrasonic biometry were entered into the AcrySof® Toric IOL calculator to determine an appropriate IOL cylinder power.
In Group 1, phacoemulsification with concomitant LRI was performed. In Group 2, the length of the phaco incision was increased to correct preexisting corneal astigmatism. The length of the incisions was selected based on an experimental nomogram.[19] In Group 3, a tIOL was implanted. Patients were evaluated at postoperative weeks 1, 8, and 24. More follow-up examinations were performed when indicated.
Surgical Technique
The procedures were performed under topical or general anesthesia, according to the patients’ condition, by three anterior segment surgeons (Group 1 by HMR, Group 2 by MAJ, and Group 3 by SJH) using the standard divide and conquer phacoemulsification technique. Preoperatively, the 6 and 12 o’clock positions of cornea were marked while the patient was sitting upright and looking at a distance target. The steepest meridian determined by topography was marked using a Mendez ring in the operating room, under the operating microscope.
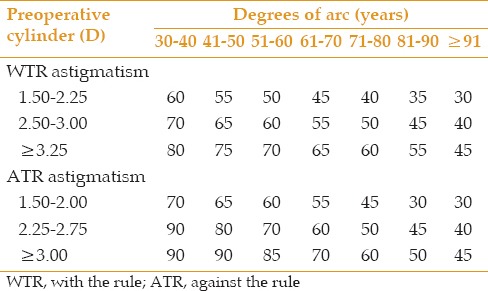
LRIs were created 1 mm anterior to the limbus at a depth of 600μ before phacoemulsification using a disposable preset knife (straight, full handle, preset [knife 72-6003] 600µm, Sharpoint™ Surgical Specialties Corp., Reading, USA), based on the modified Gills nomogram [Table 1].[29] After LRIs were created, phacoemulsification was performed using the divide and conquer technique through a 2.8 mm temporal clear cornea incision, and the IOL was inserted in the bag using the Monarch II injector and a C cartridge (Alcon Laboratories Inc., Fort Worth, TX, USA). After irrigation and aspiration, the anterior chamber was formed, and the effect of LRIs was evaluated using a handheld keratoscope. The length of the incisions was extended if the desired effect was not achieved.
Table 1.
Modified Gill's nomogram
In Group 2, the main incision was created on the steep meridian which was marked as described in group 1. At the conclusion of surgery, the length of the main incision was increased, based on an experimental nomogram [Table 2], to correct pre-existing corneal astigmatism.[19] If needed, two or three interrupted 10-0 nylon sutures were placed to close the wound. Selective suture removal was initiated two weeks after surgery based on keratometry.
Table 2.
Experimental extended on-axis incision nomogram

In Groups 1 and 2, a single piece acrylic foldable IOLs (AcrySof SA60AT, Alcon Laboratories Fort Worth, Texas, USA) was implanted through a self-sealing 2.8-mm clear corneal incision. In Group 3, AcrySof Toric IOL (SN60T3-5, Alcon Laboratories Inc., Fort Worth, Texas, USA) was inserted into the capsular bag, while the marked position on IOL was parallel to the marked steep meridian.
At the conclusion of surgery, subconjunctival betamethasone (4 mg) and ceftazidime (100mg) were injected, and the eyes were patched. The patients were followed on day 1 and also at weeks 1, 4, 8, and 24 after surgery. Postoperative medications consisted of betamethasone 0.1% eye drops (Sina Darou, Tehran, Iran) four times a day for one week and Chloramphenicol 0.5% eye drops (Sina Darou, Tehran, Iran) four times a day for one week. Betamethasone eye drops were tapered over 3 to 4 weeks, based on ocular inflammation.
Outcome Measures
Pre- and postoperative keratometry measurements in group 1 and 2, and refractive astigmatism in group 3 were compared to evaluate the effect of each intervention in the study groups. The change in astigmatism was evaluated using subtraction and vector analysis methods. The Alpins Goggins method[30] was used to measure surgically induced astigmatism (SIA). Additionally, correction index (CI) was calculated to evaluate the achieved power effect versus the targeted power. CI >1.0 D indicates overcorrection, while CI <1.0 D indicates undercorrection.[16]
Statistical analysis was performed using SPSS software (version 17.0, SPSS Co, Chicago, IL). Data were presented as mean, standard deviation, range, frequency, and percentage values as appropriate. The study groups were compared using analysis of variance, and Chi-square or Fisher exact test and analysis of covariance (ANCOVA) were used to adjust for baseline values. The Bonferroni method was used for multiple comparisons. P values < 0.05 were considered as statistically significant.
RESULTS
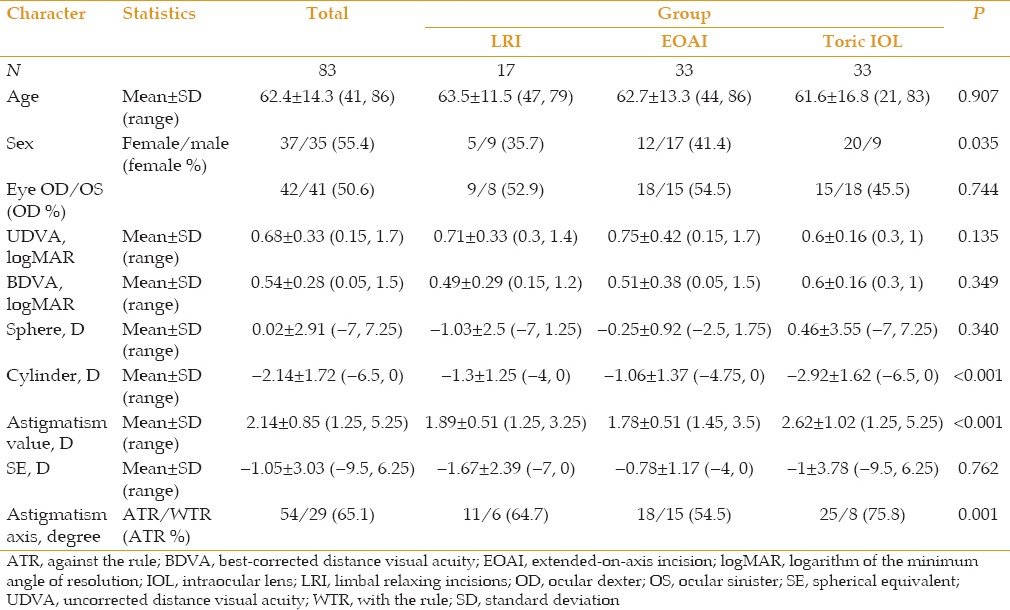
Overall, 83 eyes of 72 patients underwent phacoemulsification and intraocular lens implantation. Mean patient age was 62.4 ± 14.3 (range, 41-86) years. Thirty-five patients (48.6%) were male, and 37 patients (51.4%) were female. The LRI group consisted of 17 eyes, the EOAI group included 33 eyes, and the tIOL group contained 33 eyes. Patients’ demographic data are presented in Table 3. Preoperative keratometric astigmatism was significantly higher in the tIOL group as compared to the other two groups (P = 0.003).
Table 3.
Demographic data according to treatment groups
UDVA was significantly improved from 0.71 ± 0.27 logMAR preoperatively to 0.44 ± 0.24 at week 1, 0.33 ± 0.21 at week 4, and 0.33 ± 0.21 logMAR at week 8 in the LRI group. Corresponding figures in the EOAI group were 0.98 ± 0.38 logMAR at baseline, and 0.34 ± 0.24, 0.23 ± 0.24 and 0.22 ± 0.25 logMAR, respectively. Improvement in UDVA in this group was statistically significant at all time points. In the tIOL group, UDVA was 0.73 ± 0.32 logMAR preoperatively which was significantly improved to 0.27 ± 0.15, 0.25 ± 0.17, and 0.25 ± 0.17 logMAR at weeks 1,4 and 8, respectively. There was no inter-group difference among the study groups in terms of postoperative UDVA up to week 24 when UDVA was significantly better in the tIOL group than in the EOAI group (P = 0.024; Table 4 and Figure 1).
Table 4.
Uncorrected visual acuity before and after the operation at different time points according to treatment groups
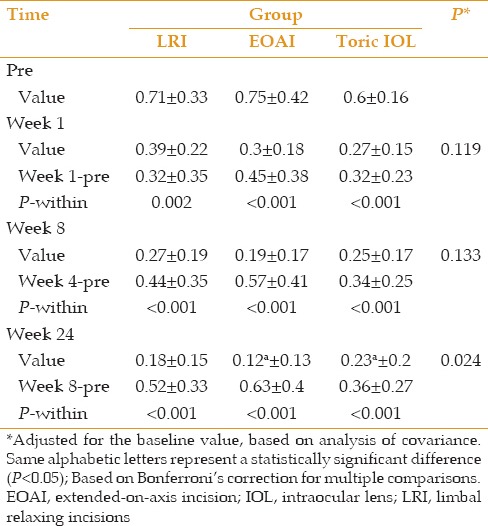
Figure 1.
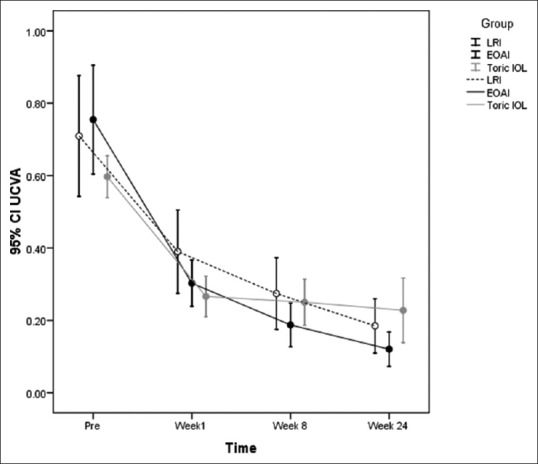
Pre- and post-operative UDVA at different time points compared among the treatment groups. UDVA, uncorrected distance visual acuity.
CDVA was increased from 0.63 ± 0.29 logMAR in the LRI group preoperatively to 0.23 ± 0.18, 0.14 ± 0.11 and 0.13 ± 0.1 logMAR at postoperative weeks 1, 4, and 8, respectively. Corresponding figures were 0.63 ± 0.33, 0.25 ± 0.24, 0.16 ± 0.24, and 0.15 ± 0.24 logMAR, respectively, in the EOAI group, and 0.61 ± 0.13, 0.27 ± 0.15, 0.16 ± 0.13, and 0.16 ± 0.13 logMAR, respectively, in the tIOL group.
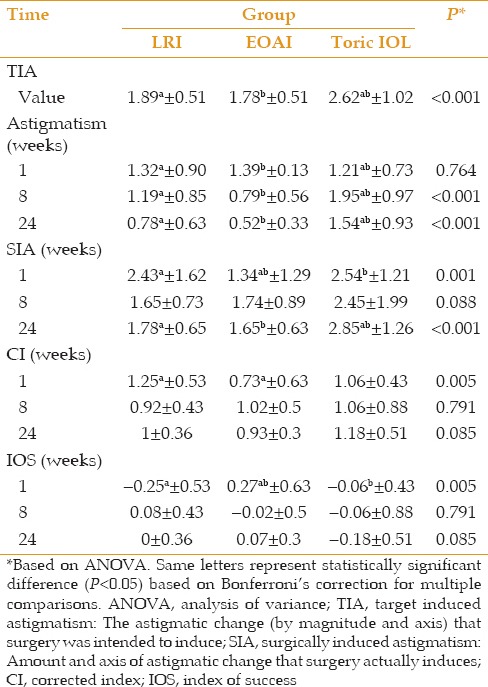
SIA measured at week 24 was higher in the tIOL group than in the other two groups (P < 0.001). This difference was explained by the fact that the tIOL group had a higher amount of preoperative astigmatism. However, CI measured at week 24 was comparable among the three groups (P = 0.85; Table 5).
Table 5.
Comparison of vector analysis between the three surgical methods
No serious complications were noted in the study groups. There was one case of wound leakage in the EOAI group which ceased after a day of patching and use of oral acetazolamide. Misalignment of tIOLs was less than 10° except for one eye with misalignment of 15°. This patient was reluctant to undergo IOL repositioning.
The stability of SIA, CI, and IOS (Index of Success) was investigated using linear mixed model analysis and multiple comparisons corrected by Bonferroni method. In all three groups, IOS changes after week 1 were not statistically significant comparing to the weeks 8 and 24 (LRI group: P=0.067, P=0.388, respectively), (EOAI group: P=0.063, P=0.367, respectively) and (tIOL group: P>0.99 for both times). Also, another linear mixed model analysis revealed that CIA changes from baseline to week 1 was statistically significant (P<0.001 in all three groups). In the LRI group this reduction continues from weak 1 to weak 8, but then it was stable compared to week 24 (P=0.017 for changes from week 1 to week 8, P=0.068 for changes from week 1 to week 24 and P=0.544 for changes from week 8 to week 24). The same pattern happened in the EOAI group, reduction of the CIA continues from weak 1 to weak 8 but then it was stable compared to week 24 (P=0.009 for changes from week 1 to week 8, P=0.061 for changes from week 1 to week 24, P=0.442 for changes from week 8 to week 24). On the other hand, there was no statistically significant change from week 1 to week 8 (P=0.978) or week 24 (P=0.432) nor a statistically significant change from week 8 to week 24 (P=0.436). The third model study the changes in SIA and the results were as following. The SIA changes after week 1 compared to week 8 and 24 revealed that it was not statistically significant in the LRI group (P=0.280, P>0.99, respectively) nor in EOAI (P=0.280, P>0.99, respectively) and neither in the tIOL group (P>0.99 for both times).
DISCUSSION
The results of this study showed that UDVA ≥20/40 at week 24 was achieved in 97% of eyes in the EOAI group, 82.4% of eyes in the LRI group, and 76.2% of eyes in the tIOL group. The change in SIA from week 1 to 24 in the LRI and EOAI groups did not reach a significant level. In contrast, the difference in CI measured between weeks 1 and 24 in both LRI and EOAI groups was statistically significant. The results of the current study demonstrate that SIA and CI in the toric IOL group were stable throughout the follow-up period, and that participants of this group experienced faster visual recovery.
Several studies have evaluated the effect of LRIs to correct low to moderate corneal astigmatism during phacoemulsification.[17,18,19] Carvalho et al[31] who found LRIs as a safe approach to correct astigmatism during phacoemulsification reported a postoperative UDVA ≥20/40 in 75% of cases which is better than our results.
On axis incisions are basic approaches for correcting corneal astigmatism, with simplicity being their main advantages over other incisional techniques.[18,19,20] The simplest approach for correcting corneal astigmatism is to extend the surgical wound created on the steep meridian. The effect of EOAIs can be enhanced by creating a limbal relaxing incision just opposite the main wound. This approach, however, necessitates wound suturing.[6,19] The main drawbacks of this approach are the need for wound suturing and extended follow-up examination resulting in slower visual recovery which can last a few weeks until complete suture removal. Additionally, if one end of an incision is closer to visual axis, an asymmetric correction will take place resulting in the shift toward this end of the wound.
In spite of being expensive, tIOLs yield more predictive results than other approaches and do not require additional corneal incisions, hence hastening visual recovery. One major complication is IOL rotation that can result in residual astigmatism. This complication, however, can be reduced by the new generations of toric IOLs.
In the current study, there was no significant change in SIA one week after toric IOL implantation. Mingo-Botín et al[32] compared astigmatism reduction by tIOLs versus corneal relaxing incisions and reported that refractive astigmatism was decreased in both groups. However, tIOLs more effectively and predictably reduced astigmatism. At the last follow-up examination, 15% of patients in the toric group and 45% in the relaxing incision group needed spectacles for distance vision. In a similar study conducted by Gangwani et al,[33] mean residual astigmatism was 0.45 ± 0.49 D in the tIOL group and 0.72 ± 0.61 D in the peripheral corneal relaxing incision (PCRI) group. They concluded that tIOLs were more predictable than PCRIfor reducing astigmatism.
The results of the present study should be interpreted in the context of its limitations. First, the study was not randomized which explains why preoperative astigmatism was significantly higher in the tIOL group. Some patients, especially, in the LRI group were lost to follow-up. Therefore, we had no access to the all patients’ data for all follow-up examinations. Third, three surgeons performed the operation. Although the surgeons were experienced, this could result in bias in the results.
In summary, considering CI at final follow-up examination, the three methods of astigmatism correction can effectively and interchangeably be applied during phacoemulsification depending on patient compliance for follow-up examinations, his or her ability to pay for the cost of tIOLs, surgeon's preference, and the need for rapid visual recovery.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Schiotz HA. [Ein fall von hochgradigemhornhaut astigmatismus nachstarr extraction. Besserung auf operativemwege] Arch Augenheilkd. 1885;15:178–181. Article in German. [Google Scholar]
- 2.Faber E. [Operative behandeling van astigmatisme] Ned Tijdschr Geneeskd. 1895;2:495–496. Article in German. [Google Scholar]
- 3.Lans LJ. [Experimentelle untersuchugen uber entstehung von astigmatisus durch nich-perforivende corneawunden] Albrecht Von Graefes Arch Ophthalmol. 1898;45:117–152. Article in German. [Google Scholar]
- 4.Steinert RF. 2nd ed. Philadelphia, PA: WB Saunders; 2004. Cataract surgery: Technique, complication and management; pp. 253–266. [Google Scholar]
- 5.Zare MH, Tehrani MH, Gohari M, Jabbarvand M, Hashemian MN, Mohammadpour M, et al. Management of corneal astigmatism by limbal relaxing incisions during cataract surgery. Iran J Ophthalmol. 2010;22:15–20. [Google Scholar]
- 6.Brint SF. Refractive cataract surgery. Int Ophthalmol Clin. 1994;34:1–11. doi: 10.1097/00004397-199403440-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bobrow JC, Blecher MH, Glasser DB, Mitchell KB, Rosenberg LF, Isbey EK, et al. Surgery for cataract. Lens and cataract. 2008-2009. Section 11. Singapore: American Academy of Ophthalmology (AAO); 2008. p. 160. [Google Scholar]
- 8.Hoffer KJ. Biometry of 7,500 cataractous eyes. Am J Ophthalmol. 1980;90:360–368. doi: 10.1016/s0002-9394(14)74917-7. [DOI] [PubMed] [Google Scholar]
- 9.Osher RH. Paired transverse relaxing keratotomy: A combined technique for reducing astigmatism. J Cataract Refract Surg. 1989;15:32–37. doi: 10.1016/s0886-3350(89)80137-3. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd JR. Induced astigmatism in small incision cataract surgery. J Cataract Refract Surg. 1989;15:85–88. doi: 10.1016/s0886-3350(89)80145-2. [DOI] [PubMed] [Google Scholar]
- 11.Davison JA. Transverse astigmatic keratotomy combined with phacoemulsification and intraocular lens implantation. J Cataract Refract Surg. 1989;15:38–44. doi: 10.1016/s0886-3350(89)80138-5. [DOI] [PubMed] [Google Scholar]
- 12.Hall GW, Campion M, Sorenson CM, Monthofer S. Reduction of corneal astigmatism at cataract surgery. J Cataract Refract Surg. 1991;17:407–414. doi: 10.1016/s0886-3350(13)80847-4. [DOI] [PubMed] [Google Scholar]
- 13.Gills JP. Relaxing incisions reduce postop astigmatism. Ophthalmol Times. 1991;15:11. [Google Scholar]
- 14.Betes WH. A suggestion of an operation to correct astigmatism. Arch Ophthalmol. 1894;23:9–13. [PubMed] [Google Scholar]
- 15.Frranks JB, Binder PS. Keratotomy procedure for the correction of astigmatism. J Refract Surg. 1985;1:11–17. [Google Scholar]
- 16.Rapuano CJ, Belin MW, Boxer Wachler BS, Donnenfeld ED, Feder RS, Rosenfeld SI, et al. Collagen shrinkage procedures. Refractive Surgery. 2008-2009. Section 13. Singapore: American Academy of Ophthalmology (AAO); 2008. pp. 150–163. [Google Scholar]
- 17.Budak K, Friedman NJ, Koch DD. Limbal relaxing incisions with cataract surgery. J Cataract Refract Surg. 1998;24:503–508. doi: 10.1016/s0886-3350(98)80292-7. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Jensen K, Fischer P, Siepe U. Limbal relaxing incisions to correct astigmatism in clear corneal cataract surgery. J Refract Surg. 1999;15:586–589. doi: 10.3928/1081-597X-19990901-12. [DOI] [PubMed] [Google Scholar]
- 19.Beardsley TL, Bobrow JC, Parrish CM. Management of astigmatism in lens-based surgery. Focal Points. 2008;2:1–13. [Google Scholar]
- 20.Ruhswurm I, Scholz U, Zehetmayer M, Hanselmayer G, Vass C, Skorpik C. Astigmatism correction with a foldable toric intraocular lens in cataract patients. J Cataract Refract Surg. 2000;26:1022–1027. doi: 10.1016/s0886-3350(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 21.Amm M, Halberstadt M. Implantation of toric intraocular lenses for correction of high post-keratoplasty astigmatism. Ophthalmologe. 2002;99:464–469. doi: 10.1007/s003470100542. [DOI] [PubMed] [Google Scholar]
- 22.Till JS, Yoder PR, Jr, Wilcox TK, Spielman JL. Toric intraocular lens implantation: 100 consecutive cases. J Cataract Refract Surg. 2002;28:295–301. doi: 10.1016/s0886-3350(01)01035-5. [DOI] [PubMed] [Google Scholar]
- 23.De Silva DJ, Ramkissoon YD, Bloom PA. Evaluation of a toric intraocular lens with a Z-haptic. J Cataract Refract Surg. 2006;32:1492–1498. doi: 10.1016/j.jcrs.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Dick HB, Krummenauer F, Tröber L. Compensation of corneal astigmatism with toric intraocular lens: Results of a multicentre study. Klin Monbl Augenheilkd. 2006;223:593–608. doi: 10.1055/s-2006-926652. [DOI] [PubMed] [Google Scholar]
- 25.Gimbel HV, Ziémba SL. Management of myopic astigmatism with phakic intraocular lens implantation. J Cataract Refract Surg. 2002;28:883–886. doi: 10.1016/s0886-3350(01)01098-7. [DOI] [PubMed] [Google Scholar]
- 26.Kottler UB, Tehrani M, Dick HB. Impact of the line of sight on toric phakic intraocular lenses for hyperopia. J Cataract Refract Surg. 2004;30:1799–1801. doi: 10.1016/j.jcrs.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 27.Werblin TP. Multicomponent intraocular lens. J Refract Surg. 1996;12:187–189. doi: 10.3928/1081-597X-19960101-33. [DOI] [PubMed] [Google Scholar]
- 28.Jan AK, Bansal R, Nawani N, John T. Acrysof toric IOL implantation in phaco surgery in keratoconus patient with cataract. Tech Ophthalmol. 2009;7:131–133. [Google Scholar]
- 29.Gills JP, Cherchio MN. Tarpon Springs, Florida: St. Luke's Cataract and Laser Institute; 1999. Nomogram for limbal relaxing incision with cataract surgery. [Google Scholar]
- 30.Alpins NA, Goggin M. Practical astigmatism analysis for refractive outcomes in cataract and refractive surgery. Surv Ophthalmol. 2004;49:109–122. doi: 10.1016/j.survophthal.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho MJ, Suzuki SH, Freitas LL, Branco BC, Schor P, Lima AL. Limbal relaxing incisions to correct corneal astigmatism during phacoemulsification. J Refract Surg. 2007;23:499–504. doi: 10.3928/1081-597X-20070501-14. [DOI] [PubMed] [Google Scholar]
- 32.Mingo-Botín D, Muñoz-Negrete FJ, Won Kim HR, Morcillo-Laiz R, Rebolleda G, Oblanca N. Comparison of toric intraocular lenses and peripheral corneal relaxing incisions to treat astigmatism during cataract surgery. J Cataract Refract Surg. 2010;36:1700–1708. doi: 10.1016/j.jcrs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 33.Gangwani V, Hirnschall N, Findl O, Maurino V. Multifocal toric intraocular lenses versus multifocal intraocular lenses combined with peripheral corneal relaxing incisions to correct moderate astigmatism. J Cataract Refract Surg. 2014;40:1625–1632. doi: 10.1016/j.jcrs.2014.01.037. [DOI] [PubMed] [Google Scholar]


