Abstract
Glaucoma is a degenerative optic neuropathy characterized by retinal ganglion cell (RGC) loss and visual field defects. It is known that in some glaucoma patients, death of RGCs continues despite intraocular pressure (IOP) reduction. Neuroprotection in the field of glaucoma is defined as any treatment, independent of IOP reduction, which prevents RGC death. Glutamate antagonists, ginkgo biloba extract, neurotrophic factors, antioxidants, calcium channel blockers, brimonidine, glaucoma medications with blood regulatory effect and nitric oxide synthase inhibitors are among compounds with possible neuroprotective activity in preclinical studies. A few agents (such as brimonidine or memantine) with neuroprotective effects in experimental studies have advanced to clinical trials; however the results of clinical trials for these agents have not been conclusive. Nevertheless, lack of compelling clinical evidence has not prevented the off-label use of some of these compounds in glaucoma practice. Stem cell transplantation has been reported to halt experimental neurodegenerative disease processes in the absence of cell replacement. It has been hypothesized that transplantation of some types of stem cells activates multiple neuroprotective pathways via secretion of various factors. The advantage of this approach is a prolonged and targeted effect. Important concerns in this field include the secretion of unwanted harmful mediators, graft survival issues and tumorigenesis. Neuroprotection in glaucoma, pharmacologically or by stem cell transplantation, is an interesting subject waiting for broad and multidisciplinary collaborative studies to better clarify its role in clinical practice.
Keywords: Brimonidine, Ginkgo Biloba Extract, Glaucoma, Memantine, Neuroprotection, Stem Cell Transplantation
INTRODUCTION
Glaucoma is a kind of degenerative optic neuropathy characterized by retinal ganglion cell (RGC) loss and visual field defects.[1] Although high intraocular pressure (IOP) is considered as the most important risk factor for the development of glaucoma, it is neither necessary nor sufficient. RGC loss continues in spite of IOP reduction in some glaucoma patients.[2] The risk of unilateral blindness in patients with treated open-angle glaucoma is estimated to be around 27%, which is higher than previously expected.[3] Thus, IOP reduction may not be sufficient for some glaucoma patients.
The pathophysiology of glaucoma is not completely understood. Clinically, there is progressive loss of the retinal nerve fiber layer (RNFL)[4] leading to axonal degeneration and characteristic optic nerve head cupping.[5] The most susceptible cell to glaucomatous damage is the RGC, which is located in the inner retina,[6,7] the axons of which constitute the RNFL and merge to form the optic nerve.
One of the areas of great interest in glaucoma is how RGC death occurs.[8] The molecular basis of RGC death stems from investigations on animal models of glaucoma. Deprivation of neurotrophic factors,[9] elevated concentrations of excitatory aminoacids such as glutamate,[10] and oxidative stress[11] may contribute to RGC apoptosis [Figure 1].
Figure 1.
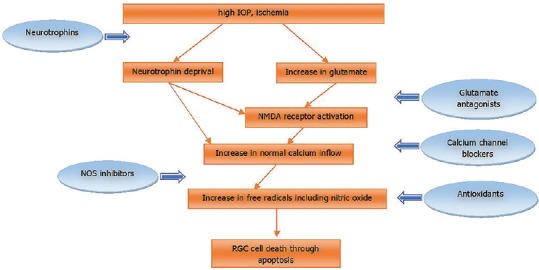
Simplified pathway of RGC death and assumed mechanisms of neuroprotective agents. IOP, intraocular pressure; NMDA, n-methyl-D-aspartate; NOS, nitric oxide synthase; RGC, retinal ganglion cell.
IOP reduction per se can prevent or delay RCG death in glaucomas and therefore is indirectly neuroprotective. However, neuroprotection in glaucoma is defined as any intervention, independent of IOP reduction, that can prevent RGC death. Several natural and synthetic compounds, have been reported to possess neuroprotective properties. Neuroprotection can affect glaucoma by direct protection of RGCs or neutralization of the deleterious effects of toxic factors. The present article reviews current evidence on neuroprotective compounds in the treatment of glaucoma.
GLUTAMATE ANTAGONISTS
Glutamate-induced exitotoxicity has been implicated as a common pathogenic mechanism in a broad variety of neurological diseases, including Alzheimer's disease and glaucoma.[12,13,14] The detrimental effect of glutamate on RGCs has been documented by exposing the retina to high glutamate levels both in vitro[15] and in vivo.[16] This effect of glutamate on RGCs occurs through interaction with glutamate receptors. Excitatory receptors are abundant in RGCs.[17] However, under normal conditions, homeostatic mechanisms prevent overexpression of the receptors.[18] Glutamate-induced excitotoxicity develops when extracelullar glutamate levels are increased.[19] Accumulation of excessive glutamate results in overstimulation of N-methyl-D-aspartate (NMDA) receptors, which in turn causes intracellular calcium influx leading to the activation of a complex cascade which attacks cell components and produces free radicals,[20] followed by programmed cell death or apoptosis.[21,22,23]
It has been shown in experimental models that after acute IOP elevation, there is an increase in intraocular glutamate levels.[24] In addition, analysis of the composition of vitreous fluid from dogs with glaucoma, experimental monkey glaucoma models and glaucomatous human eyes have revealed high levels of glutamate.[25,26] However, Carter-Dawson et al found normal levels of glutamate in the vitreous of monkeys with experimental glaucoma.[27] Based on these observations, inhibition of glutamate activity by modulation of NMDA-type receptors has been advocated as an important strategy for neuroprotection.[28]
MK801 (dizocilpine maleate), an uncompetitive NMDA antagonist, may be the most potent glutamate inhibitor[29] and neuroprotective agent in experimental glaucoma.[29,30] Nevertheless, because of the high affinity of the compound for the NMDA receptor, its long half-life and interference with normal physiologic functions of glutamate, MK801 is neurotoxic[31] and has never been evaluated in higher-level clinical trials.[32,33]
Memantine is a selective, non-competitive blocker of the NMDA receptor with moderate affinity.[28] In a study to assess the effect of glutamate and its antagonist (memantine) on RGCs, three groups of rats were studied; in the first group, the animals received serial intravitreal injections of glutamate to induce chronic elevations in glutamate levels. The second group of rats was treated with intraperitoneal memantine and glutamate, while the third group received vehicle injection with or without concurrent memantine. After 3 months, RGC survival was evaluated: Intravitreal injections of glutamate had raised its intravitreal levels up to 3 to 5 times its normal endogenous concentration. Glutamate elevation caused death of 42% of RGCs after 3 months. When memantine was administered alongside low-dose glutamate, it exhibited a partial protective effect against glutamate toxicity. However, memantine treatment alone, without concurrent injection of glutamate had no effect on ganglion cell survival.[22]
Although subsequent preclinical and experimental research with memantine appeared promising,[23,34,35] the phase III randomized, double-masked, placebo-controlled clinical trial conducted to test the efficacy of memantine as a neuroprotective agent in glaucoma, found no significant effect in preserving visual function.[36] These results came as a great disappointment for memantine, which had initially raised high hopes. It is possible that memantine may have actually benefited patients but to a level which was difficult to detect clinically, as observed in the study conducted by Hare et al In an experimental monkey model of glaucoma induced by laser destruction of the anterior chamber angle, Hare et al showed that memantine enhanced RGC survival only in animals with moderately high IOP. Furthermore, although memantine treatment had reduced the rate of RGC loss based on electroretinographic (ERG) measurements early during the study, this beneficial effect could not be observed if the injury was allowed to progress too long.[35] These observations suggest limited efficacy of memantine for reducing RGC death in glaucoma patients.
Bis(7)-tacrine is a newer NMDA receptor antagonist which possesses remarkable neuroprotective activity through concurrent inhibition of acetylcholinesterase[37,38] and nitric oxide synthase,[39] in addition to NMDA receptor blockade. Bis(7)-tacrine demonstrated more potent neuroprotective effect as compared to memantine in a study on cultured RGCs.[23] This agent still awaits further experimental and clinical studies for evaluation as an effective neuroprotective agent in glaucoma. Amantadine,[40] psychotropic tetrahydrobannabinol, and non-psychotropic cannabinol[41,42] are other potential neuroprotective agents that act via attenuation of NMDA activity.
GINKGO BILOBA EXTRACT
Ginkgo is an ancient species of tree similar to plants which were living 270 million years ago. This tree is widely grown in China and was introduced early in traditional Eastern medicine to treat a variety of problems such as asthma, vertigo, fatigue and tinnitus or circulatory disorders. In modern medical science, the extract from the leaves of ginkgo biloba, named as ginkgo biloba extract 761 (EGb761), has been shown to be beneficial for cognitive impairment and dementia.[43]
Because of biological and mechanistic similarities between Alzheimer's dementia and glaucoma,[44] investigators have studied ginkgo for glaucoma. Several studies have illustrated the role of mitochondrial dysfunction in the pathogenesis of glaucoma.[45] Only anti-oxidants capable of penetrating into the mitochondria can be of benefit as neuroprotective agents. Ginkgo contains certain substances, including poly-phenolic flavonoids which may theoretically prevent oxidative stress in the mitochondria and thereby protect RGCs.[46,47,48]
In a crossover randomized clinical trial, ginkgo biloba extract (GBE) improved pre-existing visual field defects of NTG patients. Twenty-seven NTG patients were included. Forty milligrams of GBE, three times a day, prescribed orally for 4 weeks, followed by 8 weeks washout period and then 4 weeks of placebo treatment were given. The other group of NTG patients were given the placebo first and GBE later on. Visual fields were examined at the end of each phase of the study and compared with the baseline perimetry. A significant improvement in visual field indices was recorded with GBE treatment in NTG patients.[49] More recently and in another short course placebo-controlled, crossover clinical trial, GBE could not improve contrast sensitivity or visual field damage in Chinese patients with NTG.[50]
The duration of follow-up in the above-mentioned studies was only 4 months; considering the chronic course of glaucoma, these studies were limited by short follow up period and small sample size. Despite the inconclusive results of clinical studies regarding the neuroprotective effect of GBE, because of its relatively safe profile,[51] some glaucomatologists have been prescribing GBE for their patients as adjuvant therapy for several years.[52] However, increasing risk of bleeding during surgery has been a cause of concern in patients using ginkgo.[53]
Efficacy and safety reports have recommended a daily dose of 120 mg of GBE.[51] Because of the beneficial effect of IOP reduction in most glaucoma cases and the economic burden associated with the use of GBE, its administration is recommended only in subjects with normal-tension glaucoma or in patients with high-pressure glaucoma whose condition progresses despite apparently adequate IOP reduction.[52]
NEUROTROPHIC FACTORS
Disruption of axonal transport has been demonstrated in experimental glaucoma models in monkeys and in human glaucoma.[5,54,55] These results suggest that interruption of the retrograde supply of a trophic factor to RGCs may play role in the RGC death observed in glaucomatous optic neuropathy.[56,57] In vivo and in vitro studies have revealed that neurons and glial cells within the mammalian retina possess receptors for different trophic factors, and that direct application of these factors may enhance the survival of injured ganglion cells.[58,59]
Among a variety of candidate growth and trophic factors for RGCs, brain-derived neurotrophic factor (BDNF), as a member of the nerve growth factor proteins, appears to be of particular importance to RGC function and survival.[60,61,62,63,64] BDNF has been shown to undergo both anterograde and retrograde axonal transport,[65] and has been effective in preventing lesion-induced axonal die-back in the rat optic nerve; however, it could not prevent the rapidly progressive degeneration of RGCs after axotomy. Weibel et al reported that BDNF has a selective influence on mechanisms responsible for survival of optic nerve axons.[66] Presence of the BDNF receptor, TrkB, in optic nerve axons and a change in its distribution with acute and chronic glaucoma in rats and monkeys was shown later by Pease et al.[57]
Therefore, disruption of BDNF supply to RGCs could be considered as a contributing factor in glaucomatous damage.[56] Several experimental investigations have demonstrated the protective effect of intravitreal injection of BDNF on RGCs in rat and primate models of optic nerve damage.[67,68,69] Di Polo et al observed a protective influence on RGCs by adenovirus-infected retinal Muller cells through production and release of BDNF.[70] Quigley et al suggested the optimal dose of BDNF to be 0.01 mg per milliliter of vitreous volume for intravitreal injections and found that higher intravitreal doses decrease the protective effect of BDNF on RGCs possibly due to down-regulation of Trk B, the BDNF receptor.[56]
In all preclinical studies mentioned above, the neuroprotective effect of BDNF on RGCs was assessed in the setting of optic nerve lesions such as transection and crushing.[59,71] However, experimental studies for demonstrating the protective effect of exogenous BDNF in models simulating glaucoma are scarce.
Another trophic factor undergoing preclinical investigation is the human ciliary neurotrophic factor (CNTF), which also showed a neurotrophic effect on RGCs. A single injection of CNTF protein into the vitreous significantly protected RGCs in a rat model of optic nerve axotomy[61,72] and against nitric oxide (NO) induced cell death.[73] CNTF promoted the survival of purified rat RGCs in culture[74] and it showed a promising effect on RGC protection after optic nerve axotomy when transferred by adenovirus vectors.[75]
Pease et al assessed virally-mediated over-expression of CNTF and BDNF in an experimental model of laser-induced glaucoma in rats. Loss of RGC axons was 15% lower in CNTF-treated retinas than in controls; however, neither the combined CNTF-BDNF group nor the BDNF over-expression group showed any significant improvement in RGC survival.[76]
Artemin,[77] basic fibroblast growth factor,[78] interleukin-6[79] and erythropoietin[80] are other trophic factors or cytokines for which a neuroprotective effect has been proposed.
The challenge facing the application and efficacy of these trophic factors is how to accomplish effective and sustainable delivery to the retina. The blood-retina barrier impedes such large proteins from reaching the retina with systemic administration. Intravitreal injection is an alternative route to deliver purified recombinant trophic factors to the retina, but this may not be feasible for life-long administration in chronic conditions such as glaucoma. The integration of neurotrophic factors in drug delivery devices for intraocular implantation is one possible approach for long-term provision of such agents.
Although viral vector-delivery of trophic factors in animal models of retinal degeneration have demonstrated protective effects, certain issues such as precise control of dosage make the clinical application of this approach questionable.[81]
CALCIUM CHANNEL BLOCKERS
The neurotoxic effect of NMDA is mediated by calcium influx into neural cells, followed by apoptosis and cell death.[82] Thus, calcium-channel blockers (CCBs) seem to be a rational alternative for neuroprotection in glaucoma. CCBs theoretically rescue RGCs by prevention of cell death mediated by calcium influx and by improving local blood flow in ischemic tissues by inducing vasodilation.[83]
Different calcium channel blockers such as iganidipine, nimodipine and lomerizine have been shown to significantly increase purified rat RGC viability under hypoxia.[84] In another laboratory study, unlike nilvadipine, diltiazem could not prevent glutamate-induced RGC apoptosis.[85] The effect of topical 2% flunarizine on the rabbit retina under ischemic conditions induced by high IOP was evaluated by ERG; topical flunarizine reduced IOP and attenuated injury to the retina, including RGCs.[86]
Other members of this family, brovincamine and nilvadipine, have high blood–brain barrier permeability and are expected to induce favorable effects in the optic nerve or retina with minimal influence on systemic blood pressure.[87] They were shown to improve visual field defects and ocular circulation in NTG patients and diminished the rate of deterioration in visual field sensitivity of NTG patients in randomized clinical trials.[88,89,90]
There seem to be drawbacks to the use of CCBs in glaucoma. Inadequate perfusion pressure at the ONH, may play a role in the pathogenesis of glaucoma.[91,92,93,94] There is concern that although nilvadipine or other CCBs may increase blood flow, these agents may impair the autoregulation of blood circulation at the ONH during acute IOP elevation.[95] One should keep in mind that oral CCBs prescribed for systemic hypertension may be harmful to the optic nerve in glaucoma patients; lower systemic blood pressure seems to reduce ONH blood flow, which is a risk factor in the pathogenesis of glaucoma.[96]
ANTIOXIDANTS
A number of investigations have supported the role of oxidative stress in the pathogenesis of glaucoma.[97] These mainly demonstrated lower levels of antioxidants[98,99] and elevated oxidative stress markers in the aqueous humor of eyes with glaucoma,[99] antibodies against glutathione-S-transferase,[100] decreased plasma levels of glutathione[101] and increased lipid peroxidation products in the plasma of glaucoma patients.[102] Furthermore, tissue analysis studies comparing cultured human trabecular meshwork (TM) from eyes with POAG to that of non-glaucomatous eyes have revealed higher concentrations of reactive oxygen species, decreased cell membrane potentials and reduced ATP production in the TM of eyes with POAG.[103] Insufficiency of reactive oxygen species (ROS)-neutralizing mechanisms has been proposed as the cause of accumulation of oxidative free radicals in the TM.[104,105,106] Oxidative free radicals have been implicated in human TM degeneration and subsequent IOP increase and glaucoma.[107] In another study, the correlation between DNA oxidative damage in the TM, increased IOP and visual field defects was reported.[108]
Theoretically, inhibition of ROS and up-regulation of cell defense systems may enhance RGC survival.[109,110,111,112] Cell defense mechanisms against oxidative stress include the superoxide dismutase, glutathione (GSH) and thioredoxin (TRX) systems.[110] The TRX system mitigates oxidative damage by scavenging intracellular ROS. The reaction leads to TRX oxidation, which is returned to its reduced form by TRX reductase in the presence of NADPH.
In a rat glaucoma model induced by laser damage to the TM, it was shown that overexpression of thioredoxins 1 and 2 could decrease RGC death following IOP elevation.[110]
In an experimental study, an association was found between a vitamin E-deficient diet and increased RGC death in a rat glaucoma model. The vitamin-E deficient group demonstrated greater lipid peroxidation as compared to rats with the usual diet. This study suggested that accelerated RGC death in the vitamin E-deficient group could be related to increased lipid peroxidation.[113]
Coenzyme Q10 (CoQ10), cofactor of the electron transport chain, is assumed to protect neuronal cells against oxidative stress by stabilizing the mitochondrial membrane potential, supporting ATP synthesis and inhibiting the generation of ROS.[114,115,116] A study by Nakajima, demonstrated that CoQ10 protected retinal neurons against hydrogen peroxide–induced oxidative stress in vitro and NMDA-induced glutamate excitotoxicity in vivo.[117] Moreover, CoQ10 prevented retinal damage caused by transient ischemic injury due to acutely elevated IOP.[118,119] The level of CoQ10 in the human retina has been shown to decrease by about 40% with age. The senile decrease in CoQ10 suggested the possibility that it may contribute to age-related RGC loss.[120] In a mouse model of glaucoma, diet supplemented with coenzyme Q10 inhibited glutamate excitotoxicity, and oxidative stress-mediated RGC and axonal degeneration by 29%.[121] To evaluate the effect of antioxidants in a clinical trial, Coqun eye drops (coenzyme Q10 combined with vitamin E) were administrated to 22 glaucoma patients twice daily in addition to beta-blockers. Retinal and cortical evoked responses of treated patients were compared to that of glaucoma patients treated with beta-blockers alone after 6 and 12 months. This topical preparation demonstrated a beneficial effect on inner retinal function as measured by pattern ERG with consequent improvement of visual cortical responses assessed by visually evoked potentials (VEPs).[122]
Natural substances such as polyphenolic flavoids in green tea, coffee, wine and dark chocolate; anthocyanosides in bilberry; vitamins including thiamin (vitamin B1) and even melatonin have been suggested to possess antioxidant activity.[123] Further studies are required to investigate the effect of antioxidants in glaucoma. Another open question is whether antioxidants are beneficial for all glaucoma patients or only those with reduced antioxidant reserve.
ALPHA 2 ADRENERGIC AGONISTS INCLUDING BRIMONIDINE
The presence of alpha-adrenergic receptors in human, bovine and porcine retinas, particularly in RGCs and the inner nuclear layer of the rat retina has been demonstrated by immunohistochemical studies.[124,125] In a histological study, brimonidine (a selective alpha-2 receptor adrenergic agonist) increased retinal metabolism and promoted neuronal growth in cultured retinal cells.[126]
It has been suggested that brimonidine may prevent RGC death by direct interaction with alpha-2 adrenergic receptors, leading to reduced accumulation of extracellular glutamate and blockade of NMDA receptors; this protective effect is thought to be independent of IOP reducing mechanisms attributed to this agent.[127,128,129] Elimination of the protective effect of brimonidine by co-administration of an alpha 2-antagonist confirms that the mentioned effect is secondary to alpha-2 receptor activation.[127,130]
In a pre-clinical study, continuous subcutaneous treatment with brimonidine significantly improved RGC survival exposed to elevated IOP for 8 weeks. Brimonidine treatment also preserved morphology, density and the total number of axons in the optic nerve subjected to high IOP.[131]
It is increasingly recognized that ocular blood flow alteration is involved in the pathogenesis of glaucomatous optic neuropathy.[132,133] In contrast to alpha-1 receptor activation which leads to vasoconstriction of ocular and systemic blood vessels, there is no evidence that alpha-2 agonists alter optic nerve, retinal, choroidal or retrobulbar blood flow.[134,135]
A randomized clinical trial from Singapore compared the effect of brimonidine and timolol on the incidence of glaucomatous visual field defects and the rate of visual field deterioration after acute IOP rise. Evaluation of the visual field tests during the 16-week follow-up period did not show any protective effect from brimonidine.[136] In another study, however, measurement of RNFL thickness by scanning laser polarimetry (GDx) demonstrated less RNFL loss with brimonidine in comparison to timolol 0.5% in ocular hypertensive patients over 12 months of treatment.[137]
The industry-supported “Low-Pressure Glaucoma Treatment Study (LoGTS)” evaluated the neuroprotective effect of brimonidine versus timolol in 190 NTG patients over four-year follow-up.[138] This study suggested that brimonidine may halt visual field deterioration more than timolol, but the authors did not consider the higher rate of incomplete follow up in the brimonidine group (55% and 29% missing data in the brimonidine and timolol groups, respectively).[139]
NITRIC OXIDE SYNTHASE INHIBITORS
Evidence in the literature points to a possible role for NO in RGC degeneration.[140,141,142] Increased levels of NO to twice normal values was shown in rat retinas with induced glaucoma.[143] Aslan et al suggested that excessive NO could result in apoptosis and necrosis of RGCs.[144] There are three forms of nitric oxide synthase (NOS): NOS-1 (neuronal NOS) and NOS-3 (constitutive NOS) act as vasodilators or neurotransmitters in normal retinal tissue, however NOS-2 (inducible NOS) contributes to RGC neurotoxicity.[145] An increased expression of NOS has been shown in optic nerve head (ONH) of glaucoma patients.[146,147] In an experimental study, in vitro elevation of hydrostatic pressure upregulated NOS-2 expression in cultured rat RGCs and astrocytes of the human lamina Cribrosa.[148,149] The ability of aminoguanidine as a NOS-2 inhibitor in protecting RGCs in the rat cautery model of retinopathy led to the suggestion that NOS-2 inhibition may be protective in glaucoma.[141,150] The possibility that NOS-2 inhibition could be neuroprotective in glaucoma was strengthened by reports showing that another NOS-2 inhibitor (N-nitro-L-arginine) delayed RGC degeneration.[151] The non-psychotropic component of marijuana, cannabidiol (CBD), and the synthetic cannabinoids, tetrahydrocannabinol and HU-211 have been demonstrated to possess protective actions in part due to an effect on reducing formation of lipid peroxides, nitrite/nitrate and nitrotyrosine.[42,152,153]
These data suggest that activation of NOS, especially NOS-2, may play a significant role in glaucomatous optic neuropathy and that nitric oxide synthase inhibitors could halt neurodegeneration.
On the other hand, the role of NOS-2 in optic neuropathy has been argued by subsequent studies. Pang et al did not find any evidence for NOS-2 in glaucomatous neurodegeneration in their study. They induced elevated IOP in rats by injection of hypertonic saline into episcleral veins. No significant increase in NOS-2 expression was found in the optic nerve head. Furthermore, aminoguanidine treatment had no effect on glaucomatous damage in rats.[154] In another study conducted by Libby et al with the same IOP elevation method, a similar result was achieved.[155] Kasmala et al found that oral administration of another inhibitor of NOS2, SC-5, did not prevent optic neuropathy induced by saline injection ocular hypertension.[156]
Technical differences in the simulation of glaucoma and different mouse races in experimental studies may explain the discrepancy between different investigations.[157] In short, preclinical evidence regarding the effect of NOS in neurodegeneration is inconclusive and NOS inhibitors have not yet been tested in any clinical study.
ANTI-GLAUCOMA MEDICATIONS WITH BLOOD REGULATION EFFECT
Vascular dysregulation has been implicated in the pathogenesis of glaucoma,[158] therefore a neuroprotective effect has been suggested for agents which can improve regulation of ocular blood perfusion.[159] Some anti-glaucoma medications have additional ocular blood perfusion effects. For instance, carbonic anhydrase inhibitors increase ocular perfusion.[160] Improvement of ocular blood has also been reported with latanoprost.[161,162]
Betaxolol is a putative selective B1-adrenoceptor blocker. Experimental studies have suggested a neuroprotective effect from betaxolol in animal models of retinal ischemia.[163,164] However, the reports did not provide evidence on how betaxolol modulates neurodegeneration and which types of retinal cell are affected by betaxolol.[165,166] Some studies have suggested that betaxolol reduces the NMDA-stimulated influx of calcium into isolated cells of rat retinas by direct interaction with voltage-dependent calcium channels or sodium channels.[167]
Anti-glaucoma medications have a large preservative effect on RGCs by IOP reduction, therefore clinical studies to evaluate their action as a “neuroprotective” agent independent of their protective action due to IOP reduction are difficult to conduct and interpret.
STEM CELL TRANSPLANTATION FOR RGC NEUROPROTECTION
Stem cell transplantation has gained significant interest because of its potential to treat neuro-degenerative diseases such as glaucoma. There are two mechanisms by which stem cell therapy might be applicable to glaucoma. The most important therapeutic power of stem cells lies in their ability to generate new cells of many types and to induce RGC regeneration.[168] Nevertheless, RGC replacement would require that the cells become integrated into the complex circuitry and be capable of synapsing at precise brain locations. Thus, protection of RGCs from degeneration might be a more accessible goal in glaucoma therapy in the short term.[169]
It has been hypothesized that implantation of some types of stem cells activates multiple neuroprotective pathways simultaneously via secretion of various factors.[170] Transplanted stem cells may be utilized as an intraocular delivery device for diffusible bioactive factors. Supply of various neurotrophic factors is the most widely acceptable mechanism by which transplanted cells can modulate excitotoxicity. This method may provide the advantage of long-lasting and localized effect. Delivery of a single prolonged effective treatment could also prevent the common problem of patient noncompliance with pharmacologic therapy. Even though cells which fulfill all the required criteria for stem cell transplantation have not yet been identified, multiple cells have been suggested in different studies, which can secrete different neurotrophic factors, such as embryonic or adult tissue-derived stem cells.[171]
There are important concerns in this field since implanted cells may secrete other agents with unknown activity, in addition to the desired neurotrophic factor. Some of these factors may even be harmful. Therefore, it is necessary to determine all factors produced by the transplanted stem cells before they come into clinical practice.[172]
Another limitation in this field is graft survival. Prolonged survival is necessary to achieve continuous benefit from stem cell transplantation; however, longer survival times are associated with an increased risk of tumorigenesis.[173] Consequently, careful selection of stem cells, thorough long-term observation, and safety evaluation will be necessary to ensure that the potential benefit of neuroprotection outweighs the risk of inducing tumors. On the other hand, stem cell transplantation has been shown to induce reactive gliosis in the host retina which caused retinal folding, up-regulation of intermediate filaments, and recruitment of macrophages. Inhibition of stem cell-induced reactive gliosis would be fundamental for successful transplantation-based strategies.[174]
Even once these questions are resolved, numerous issues should be addressed during translation of successful laboratory models to the clinic. Difference in animal models used for glaucoma, the rapid time course of optic nerve damage in the laboratory setting and different mechanisms for simulating RCG injuries are some of the issues which should be taken into account in order to achieve the desired results in the clinical setting.[171]
SUMMARY
Over the past 30 years, numerous pharmacologic agents have been advocated as neuroprotective agents in glaucoma, however few of them such as brimonidine or memantine have advanced to clinical trials. In a systematic review by the Cochrane group in 2013 for neuroprotection in glaucoma, from dozens of clinical trials, only one study (LoGTS) fulfilled the criteria for review which itself faced criticism. Through an updated search for the current review, as of July 2015, no more completed clinical trials corroborating neuroprotection in glaucoma have been published after the Cochrane review in October 2012. This has occurred despite encouraging evidence from laboratory and preclinical studies.
Several conceptual and methodological issues hinder the translation of experimental results to clinical glaucoma practice. First of all, glaucoma is a chronic heterogenous group of disorders, and no animal model can fully mimic the course of human disease. Furthermore, considerable disease variability exists in human clinical trials; these include the presence of comorbidities, polypharmacy in elderly glaucoma patients, and minimal control over a myriad of physiologic factors. Another basic difference between animal and human clinical trials in the neuroprotection field is the time of the intervention. In most experimental studies, the neuroprotective agent is given at the time or even prior to injury, unlike human studies, in which the patient is eligible for enrollment after the disease is well establishment. Another basic difference between experimental studies and human clinical trials is outcome measure. Most animal studies employ histopathologic endpoints to assess the treatment efficacies. Clinical trials, however, judge efficacy by using functional outcomes, which most often take months to show any change. Extrapolation of the appropriate dose of a neuroprotective agent for use in humans from animal or laboratory studies is another issue. Many of these agents are toxic or ineffective, at concentrations higher or lower than optimum. Human ocular bioavailability with a given dose also is often difficult to predict.
Broad and multidisciplinary collaborative effort is required to design a set of guidelines for experimental and clinical studies on neuroprotection in ophthalmic disease. A consensus on how to design and execute translational research in neuroprotection in ophthalmic disease would optimize the use of resources and facilitate the development of effective neuroprotective agents.[36] To that day, the main therapeutic option for glaucoma treatment will remain to decrease intraocular pressure and the selection of anti-glaucoma medications should be based on their ability to reduce IOP.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta N, Weinreb RN. New definitions of glaucoma. Curr Opin Ophthalmol. 1997;8:38–41. doi: 10.1097/00055735-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Brubaker RF. Delayed functional loss in glaucoma. LII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1996;121:473–483. doi: 10.1016/s0002-9394(14)75421-2. [DOI] [PubMed] [Google Scholar]
- 3.Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–2104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- 4.Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 6.Nickells RW. Retinal ganglion cell death in glaucoma: The how, the why, and the maybe. J Glaucoma. 1996;5:345–356. [PubMed] [Google Scholar]
- 7.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 8.Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: An update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43(Suppl 1):S151–S161. doi: 10.1016/s0039-6257(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 9.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 10.Epstein FH, Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 11.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 12.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S102–S128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 13.Salt TE, Cordeiro MF. Glutamate excitotoxicity in glaucoma: Throwing the baby out with the bathwater? Eye (Lond) 2006;20:730–731. doi: 10.1038/sj.eye.6701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by beta-amyloid (1-40) Brain Res. 2002;958:210–221. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 15.Levy DI, Lipton SA. Comparison of delayed administration of competitive and uncompetitive antagonists in preventing NMDA receptor-mediated neuronal death. Neurology. 1990;40:852–855. doi: 10.1212/wnl.40.5.852. [DOI] [PubMed] [Google Scholar]
- 16.Sisk DR, Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch Clin Exp Ophthalmol. 1985;223:250–258. doi: 10.1007/BF02153655. [DOI] [PubMed] [Google Scholar]
- 17.Sisk DR, Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch Clin Exp Ophthalmol. 1985;223:250–258. doi: 10.1007/BF02153655. [DOI] [PubMed] [Google Scholar]
- 18.Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion cell death in glaucoma: What do we really know? Br J Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Campos J, Villena A, Díaz F, Vidal L, Moreno M, Pérez de Vargas I. Morphological and functional changes in experimental ocular hypertension and role of neuroprotective drugs. Histol Histopathol. 2007;22:1399–1411. doi: 10.14670/HH-22.1399. [DOI] [PubMed] [Google Scholar]
- 20.Naskar R, Dreyer EB. New horizons in neuroprotection. Surv Ophthalmol. 2001;45(Suppl 3):S250–S255. doi: 10.1016/s0039-6257(01)00198-9. [DOI] [PubMed] [Google Scholar]
- 21.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 22.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996;37:1618–1624. [PubMed] [Google Scholar]
- 23.Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis (7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci. 2010;11:31. doi: 10.1186/1471-2202-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapin MA, Doshi R, Scatton B, DeSantis LM, Chandler ML. Neuroprotective effects of eliprodil in retinal excitotoxicity and ischemia. Invest Ophthalmol Vis Sci. 1999;40:1177–1182. [PubMed] [Google Scholar]
- 25.Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE. Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res. 1997;58:864–867. [PubMed] [Google Scholar]
- 26.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 27.Carter-Dawson L, Crawford ML, Harwerth RS, Smith EL, 3rd, Feldman R, Shen FF, et al. Vitreal glutamate concentration in monkeys with experimental glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2633–2637. [PubMed] [Google Scholar]
- 28.Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol. 2003;48(Suppl 1):S38–S46. doi: 10.1016/s0039-6257(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhary P, Ahmed F, Sharma SC. MK801-a neuroprotectant in rat hypertensive eyes. Brain Res. 1998;792:154–158. doi: 10.1016/s0006-8993(98)00212-1. [DOI] [PubMed] [Google Scholar]
- 30.Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipton SA. Prospects for clinically tolerated NMDA antagonists: Open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993;16:527–532. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- 32.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 33.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: Mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 34.Lagrèze WA, Knörle R, Bach M, Feuerstein TJ. Memantine is neuroprotective in a rat model of pressure-induced retinal ischemia. Invest Ophthalmol Vis Sci. 1998;39:1063–1066. [PubMed] [Google Scholar]
- 35.Hare WA, WoldeMussie E, Lai RK, Ton H, Ruiz G, Chun T, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Invest Ophthalmol Vis Sci. 2004;45:2625–2639. doi: 10.1167/iovs.03-0566. [DOI] [PubMed] [Google Scholar]
- 36.Danesh-Meyer HV, Levin LA. Neuroprotection: Extrapolating from neurologic diseases to the eye. Am J Ophthalmol. 2009;148:186–191.e2. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Pang YP, Quiram P, Jelacic T, Hong F, Brimijoin S. Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer's disease. J Biol Chem. 1996;271:23646–23649. doi: 10.1074/jbc.271.39.23646. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Carlier PR, Ho WL, Wu DC, Lee NT, Li CP, et al. Effects of bis(7)-tacrine, a novel anti-Alzheimer's agent, on rat brain AChE. Neuroreport. 1999;10:789–793. doi: 10.1097/00001756-199903170-00023. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Lee NT, Fu H, Kan KK, Pang Y, Li M, et al. Neuroprotection via inhibition of nitric oxide synthase by bis(7)-tacrine. Neuroreport. 2006;17:471–474. doi: 10.1097/01.wnr.0000209014.09094.72. [DOI] [PubMed] [Google Scholar]
- 40.Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77:309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- 41.El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, et al. Neuroprotective effect of (-) Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am J Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-) Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Re v. 2009:CD003120. doi: 10.1002/14651858.CD003120.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Ghiso JA, Doudevski I, Ritch R, Rostagno AA. Alzheimer's disease and glaucoma: Mechanistic similarities and differences. J Glaucoma. 2013;22(Suppl 5):S36–S38. doi: 10.1097/IJG.0b013e3182934af6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 46.Ritch R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Med Hypotheses. 2000;54:221–235. doi: 10.1054/mehy.1999.0025. [DOI] [PubMed] [Google Scholar]
- 47.Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 48.Eckert A, Keil U, Scherping I, Hauptmann S, Müller WE. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann N Y Acad Sci. 2005;1056:474–485. doi: 10.1196/annals.1352.023. [DOI] [PubMed] [Google Scholar]
- 49.Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110:359–362. doi: 10.1016/S0161-6420(02)01745-1. [DOI] [PubMed] [Google Scholar]
- 50.Guo X, Kong X, Huang R, Jin L, Ding X, He M, et al. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: A randomized, crossover clinical trial. Invest Ophthalmol Vis Sci. 2014;55:110–116. doi: 10.1167/iovs.13-13168. [DOI] [PubMed] [Google Scholar]
- 51.Le Bars PL, Kastelan J. Efficacy and safety of a Ginkgo biloba extract. Public Health Nutr. 2000;3:495–499. doi: 10.1017/s1368980000000574. [DOI] [PubMed] [Google Scholar]
- 52.Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: An adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. 2012;18:390–402. [PMC free article] [PubMed] [Google Scholar]
- 53.Chan AL, Leung HW, Wu JW, Chien TW. Risk of hemorrhage associated with co-prescriptions for Ginkgo biloba and antiplatelet or anticoagulant drugs. J Altern Complement Med. 2011;17:513–517. doi: 10.1089/acm.2010.0295. [DOI] [PubMed] [Google Scholar]
- 54.Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol Vis Sci. 1974;13:771–783. [PubMed] [Google Scholar]
- 55.Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- 56.Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 57.Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:764–774. [PubMed] [Google Scholar]
- 58.Reh TA, McCabe K, Kelley MW, Bermingham-McDonogh O, editors . Chichester: John Wiley & Sons, Ltd; 1996. Growth factors in the treatment of degenerative retinal disorders. [DOI] [PubMed] [Google Scholar]
- 59.Aguayo AJ, Clarke DB, Jelsma TN, Kittlerova P, Friedman HC, Bray GM. Effects of neurotrophins on the survival and regrowth of injured retinal neurons. Ciba Found Symp. 1996;196:135–144. doi: 10.1002/9780470514863.ch10. [DOI] [PubMed] [Google Scholar]
- 60.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thanos S, Bähr M, Barde YA, Vanselow J. Survival and axonal elongation of adult rat retinal ganglion cells. Eur J Neurosci. 1989;1:19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 62.Bonnet D, Garcia M, Vecino E, Lorentz JG, Sahel J, Hicks D. Brain-derived neurotrophic factor signalling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res. 2004;1007:142–151. doi: 10.1016/j.brainres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 63.Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol. 2004;60:319–327. doi: 10.1002/neu.20028. [DOI] [PubMed] [Google Scholar]
- 64.Arango-González B, Cellerino A, Kohler K. Exogenous brain-derived neurotrophic factor (BDNF) reverts phenotypic changes in the retinas of transgenic mice lacking the BDNF gene. Invest Ophthalmol Vis Sci. 2009;50:1416–1422. doi: 10.1167/iovs.08-2244. [DOI] [PubMed] [Google Scholar]
- 65.Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, et al. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weibel D, Kreutzberg GW, Schwab ME. Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve. Brain Res. 1995;679:249–254. doi: 10.1016/0006-8993(95)00238-l. [DOI] [PubMed] [Google Scholar]
- 67.Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001;42:966–974. [PubMed] [Google Scholar]
- 68.Peinado-Ramón P, Salvador M, Villegas-Pérez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 69.Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci. 1996;16:3887–3894. doi: 10.1523/JNEUROSCI.16-12-03887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Pérez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: A quantitative in vivo study. Exp Eye Res. 2009;89:32–41. doi: 10.1016/j.exer.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 73.Takahata K, Katsuki H, Kume T, Nakata D, Ito K, Muraoka S, et al. Retinal neuronal death induced by intraocular administration of a nitric oxide donor and its rescue by neurotrophic factors in rats. Invest Ophthalmol Vis Sci. 2003;44:1760–1766. doi: 10.1167/iovs.02-0471. [DOI] [PubMed] [Google Scholar]
- 74.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 75.Weise J, Isenmann S, Klöcker N, Kügler S, Hirsch S, Gravel C, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- 76.Pease ME, Zack DJ, Berlinicke C, Bloom K, Cone F, Wang Y, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:2194–2200. doi: 10.1167/iovs.08-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omodaka K, Kurimoto T, Nakamura O, Sato K, Yasuda M, Tanaka Y, et al. Artemin augments survival and axon regeneration in axotomized retinal ganglion cells. J Neurosci Res. 2014;92:1637–1646. doi: 10.1002/jnr.23449. [DOI] [PubMed] [Google Scholar]
- 78.Unoki K, LaVail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994;35:907–915. [PubMed] [Google Scholar]
- 79.Perígolo-Vicente R, Ritt K, Gonçalves-de-Albuquerque CF, Castro-Faria-Neto HC, Paes-de-Carvalho R, Giestal-de-Araujo E. IL-6, A1 and A2aR: A crosstalk that modulates BDNF and induces neuroprotection. Biochem Biophys Res Commun. 2014;449:477–482. doi: 10.1016/j.bbrc.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 80.Bartesaghi S, Marinovich M, Corsini E, Galli CL, Viviani B. Erythropoietin: A novel neuroprotective cytokine. Neurotoxicology. 2005;26:923–928. doi: 10.1016/j.neuro.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- 83.Crish SD, Calkins DJ. Neurodegeneration in glaucoma: Progression and calcium-dependent intracellular mechanisms. Neuroscience. 2011;176:1–11. doi: 10.1016/j.neuroscience.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamada H, Chen YN, Aihara M, Araie M. Neuroprotective effect of calcium channel blocker against retinal ganglion cell damage under hypoxia. Brain Res. 2006;1071:75–80. doi: 10.1016/j.brainres.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 85.Otori Y, Kusaka S, Kawasaki A, Morimura H, Miki A, Tano Y. Protective effect of nilvadipine against glutamate neurotoxicity in purified retinal ganglion cells. Brain Res. 2003;961:213–219. doi: 10.1016/s0006-8993(02)03951-3. [DOI] [PubMed] [Google Scholar]
- 86.Osborne NN, Wood JP, Cupido A, Melena J, Chidlow G. Topical flunarizine reduces IOP and protects the retina against ischemia-excitotoxicity. Invest Ophthalmol Vis Sci. 2002;43:1456–1464. [PubMed] [Google Scholar]
- 87.Mayama C. Calcium channels and their blockers in intraocular pressure and glaucoma. Eur J Pharmacol. 2014;739:96–105. doi: 10.1016/j.ejphar.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 88.Sawada A, Kitazawa Y, Yamamoto T, Okabe I, Ichien K. Prevention of visual field defect progression with brovincamine in eyes with normal-tension glaucoma. Ophthalmology. 1996;103:283–288. doi: 10.1016/s0161-6420(96)30703-3. [DOI] [PubMed] [Google Scholar]
- 89.Koseki N, Araie M, Yamagami J, Shirato S, Yamamoto S. Effects of oral brovincamine on visual field damage in patients with normal-tension glaucoma with low-normal intraocular pressure. J Glaucoma. 1999;8:117–123. [PubMed] [Google Scholar]
- 90.Koseki N, Araie M, Tomidokoro A, Nagahara M, Hasegawa T, Tamaki Y, et al. A placebo-controlled 3-year study of a calcium blocker on visual field and ocular circulation in glaucoma with low-normal pressure. Ophthalmology. 2008;115:2049–2057. doi: 10.1016/j.ophtha.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Anderson DR. Glaucoma, capillaries and pericytes 1.Blood flow regulation. Ophthalmologica. 1996;210:257–262. doi: 10.1159/000310722. [DOI] [PubMed] [Google Scholar]
- 92.Langham ME. Ocular blood flow and vision in healthy and glaucomatous eyes. Surv Ophthalmol. 1994;38(Suppl):S161–S168. doi: 10.1016/0039-6257(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 93.Prünte C, Orgül S, Flammer J. Abnormalities of microcirculation in glaucoma: Facts and hints. Curr Opin Ophthalmol. 1998;9:50–55. doi: 10.1097/00055735-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995;113:216–221. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 95.Takayama J, Tomidokoro A, Ishii K, Tamaki Y, Fukaya Y, Hosokawa T, et al. Time course of the change in optic nerve head circulation after an acute increase in intraocular pressure. Invest Ophthalmol Vis Sci. 2003;44:3977–3985. doi: 10.1167/iovs.03-0024. [DOI] [PubMed] [Google Scholar]
- 96.Iwase A, Tomidokoro A, Leung C, Zeitz O, Vingrys A, Schmetterer L, et al. Ocular Blood Flow in Glaucoma. Amsterdam: Kugler Publications; 2009. Clinical relevance of ocular blood flow (OBF) measurements including effects of general medications or specific glaucoma treatment; p. 59. [Google Scholar]
- 97.Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 99.Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39:823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- 100.Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001;42:1273–1276. [PubMed] [Google Scholar]
- 101.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–883. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- 102.Yildirim O, Ates NA, Ercan B, Muslu N, Unlü A, Tamer L, et al. Role of oxidative stress enzymes in open-angle glaucoma. Eye (Lond) 2005;19:580–583. doi: 10.1038/sj.eye.6701565. [DOI] [PubMed] [Google Scholar]
- 103.He Y, Leung KW, Zhang YH, Duan S, Zhong XF, Jiang RZ, et al. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: Protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- 104.Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010;133:2612–2625. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moreno MC, Campanelli J, Sande P, Sánez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Yuki K, Ozawa Y, Yoshida T, Kurihara T, Hirasawa M, Ozeki N, et al. Retinal ganglion cell loss in superoxide dismutase 1 deficiency. Invest Ophthalmol Vis Sci. 2011;52:4143–4150. doi: 10.1167/iovs.10-6294. [DOI] [PubMed] [Google Scholar]
- 107.Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: The trabecular meshwork. J Cell Physiol. 1999;180:182–189. doi: 10.1002/(SICI)1097-4652(199908)180:2<182::AID-JCP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 108.Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in human trabecular meshwork and its correlation with intraocular pressure and visual field in primary open angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 109.Geiger LK, Kortuem KR, Alexejun C, Levin LA. Reduced redox state allows prolonged survival of axotomized neonatal retinal ganglion cells. Neuroscience. 2002;109:635–642. doi: 10.1016/s0306-4522(01)00493-6. [DOI] [PubMed] [Google Scholar]
- 110.Caprioli J, Munemasa Y, Kwong JM, Piri N. Overexpression of thioredoxins 1 and 2 increases retinal ganglion cell survival after pharmacologically induced oxidative stress, optic nerve transection, and in experimental glaucoma. Trans Am Ophthalmol Soc. 2009;107:161–165. [PMC free article] [PubMed] [Google Scholar]
- 111.Swanson KI, Schlieve CR, Lieven CJ, Levin LA. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci. 2005;46:3737–3741. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]
- 112.Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ko ML, Peng PH, Hsu SY, Chen CF. Dietary deficiency of Vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr Eye Res. 2010;35:842–849. doi: 10.3109/02713683.2010.489728. [DOI] [PubMed] [Google Scholar]
- 114.Forsmark-Andrée P, Lee CP, Dallner G, Ernster L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic Biol Med. 1997;22:391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- 115.McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 116.Noack H, Kube U, Augustin W. Relations between tocopherol depletion and coenzyme Q during lipid peroxidation in rat liver mitochondria. Free Radic Res. 1994;20:375–386. doi: 10.3109/10715769409145637. [DOI] [PubMed] [Google Scholar]
- 117.Nakajima Y, Inokuchi Y, Nishi M, Shimazawa M, Otsubo K, Hara H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008;1226:226–233. doi: 10.1016/j.brainres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 118.Nucci C, Tartaglione R, Cerulli A, Mancino R, Spanò A, Cavaliere F, et al. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int Rev Neurobiol. 2007;82:397–406. doi: 10.1016/S0074-7742(07)82022-8. [DOI] [PubMed] [Google Scholar]
- 119.Russo R, Cavaliere F, Rombolà L, Gliozzi M, Cerulli A, Nucci C, et al. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. Prog Brain Res. 2008;173:575–582. doi: 10.1016/S0079-6123(08)01139-4. [DOI] [PubMed] [Google Scholar]
- 120.Qu J, Kaufman Y, Washington I. Coenzyme Q10 in the human retina. Invest Ophthalmol Vis Sci. 2009;50:1814–1818. doi: 10.1167/iovs.08-2656. [DOI] [PubMed] [Google Scholar]
- 121.Lee D, Shim MS, Kim KY, Noh YH, Kim H, Kim SY, et al. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2014;55:993–1005. doi: 10.1167/iovs.13-12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parisi V, Centofanti M, Gandolfi S, Marangoni D, Rossetti L, Tanga L, et al. Effects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J Glaucoma. 2014;23:391–404. doi: 10.1097/IJG.0b013e318279b836. [DOI] [PubMed] [Google Scholar]
- 123.Mozaffarieh M, Grieshaber MC, Orgül S, Flammer J. The potential value of natural antioxidative treatment in glaucoma. Surv Ophthalmol. 2008;53:479–505. doi: 10.1016/j.survophthal.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Bylund DB, Chacko DM. Characterization of alpha2 adrenergic receptor subtypes in human ocular tissue homogenates. Invest Ophthalmol Vis Sci. 1999;40:2299–2306. [PubMed] [Google Scholar]
- 125.Wheeler LA, Gil DW, WoldeMussie E. Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv Ophthalmol. 2001;45(Suppl 3):S290–S294. doi: 10.1016/s0039-6257(01)00206-5. [DOI] [PubMed] [Google Scholar]
- 126.Prokosch V, Panagis L, Volk GF, Dermon C, Thanos S. Alpha2-adrenergic receptors and their core involvement in the process of axonal growth in retinal explants. Invest Ophthalmol Vis Sci. 2010;51:6688–6699. doi: 10.1167/iovs.09-4835. [DOI] [PubMed] [Google Scholar]
- 127.Donello JE, Padillo EU, Webster ML, Wheeler LA, Gil DW. Alpha (2)-Adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J Pharmacol Exp Ther. 2001;296:216–223. [PubMed] [Google Scholar]
- 128.Kalapesi FB, Coroneo MT, Hill MA. Human ganglion cells express the alpha-2 adrenergic receptor: Relevance to neuroprotection. Br J Ophthalmol. 2005;89:758–763. doi: 10.1136/bjo.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoles E, Wheeler LA, Schwartz M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest Ophthalmol Vis Sci. 1999;40:65–73. [PubMed] [Google Scholar]
- 130.Dong CJ, Guo Y, Agey P, Wheeler L, Hare WA. Alpha2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity. Invest Ophthalmol Vis Sci. 2008;49:4515–4522. doi: 10.1167/iovs.08-2078. [DOI] [PubMed] [Google Scholar]
- 131.Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol Neurodegener. 2011;6:4. doi: 10.1186/1750-1326-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harris A, Sergott RC, Spaeth GL, Katz JL, Shoemaker JA, Martin BJ. Color Doppler analysis of ocular vessel blood velocity in normal- tension glaucoma. Am J Ophthalmol. 1994;118:642–649. doi: 10.1016/s0002-9394(14)76579-1. [DOI] [PubMed] [Google Scholar]
- 133.Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clin Ophthalmol. 2008;2:849–861. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harris A, Jonescu-Cuypers CP. The impact of glaucoma medication on parameters of ocular perfusion. Curr Opin Ophthalmol. 2001;12:131–137. doi: 10.1097/00055735-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 135.Jonescu-Cuypers CP, Harris A, Ishii Y, Kagemann L, Gazozi HJ, Rotenstreich Y, et al. Effect of brimonidine tartrate on ocular hemodynamics in healthy volunteers. J Ocul Pharmacol Ther. 2001;17:199–205. doi: 10.1089/108076801750295236. [DOI] [PubMed] [Google Scholar]
- 136.Aung T, Oen FT, Wong HT, Chan YH, Khoo BK, Liu YP, et al. Randomised controlled trial comparing the effect of brimonidine and timolol on visual field loss after acute primary angle closure. Br J Ophthalmol. 2004;88:88–94. doi: 10.1136/bjo.88.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsai JC, Chang HW. Comparison of the effects of brimonidine 0.2% and timolol 0.5% on retinal nerve fiber layer thickness in ocular hypertensive patients: A prospective, unmasked study. J Ocul Pharmacol Ther. 2005;21:475–482. doi: 10.1089/jop.2005.21.475. [DOI] [PubMed] [Google Scholar]
- 138.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–681. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 139.Sena DF, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev. 2013;2:CD006539. doi: 10.1002/14651858.CD006539.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hangai M, Yoshimura N, Hiroi K, Mandai M, Honda Y. Inducible nitric oxide synthase in retinal ischemia-reperfusion injury. Exp Eye Res. 1996;63:501–509. doi: 10.1006/exer.1996.0140. [DOI] [PubMed] [Google Scholar]
- 141.Neufeld AH, Kawai SI, Das S, Vora S, Gachie E, Connor JR, et al. Loss of retinal ganglion cells following retinal ischemia: The role of inducible nitric oxide synthase. Exp Eye Res. 2002;75:521–528. doi: 10.1006/exer.2002.2042. [DOI] [PubMed] [Google Scholar]
- 142.Shareef S, Sawada A, Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1999;40:2884–2891. [PubMed] [Google Scholar]
- 143.Siu AW, Leung MC, To CH, Siu FK, Ji JZ, So KF. Total retinal nitric oxide production is increased in intraocular pressure-elevated rats. Exp Eye Res. 2002;75:401–406. [PubMed] [Google Scholar]
- 144.Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45:367–376. doi: 10.1016/j.freeradbiomed.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 145.Nucci C, Morrone L, Rombolà L, Nisticò R, Piccirilli S, Cerulli L. Multifaceted roles of nitric oxide in the lateral geniculate nucleus: From visual signal transduction to neuronal apoptosis. Toxicol Lett. 2003;139:163–173. doi: 10.1016/s0378-4274(02)00430-7. [DOI] [PubMed] [Google Scholar]
- 146.Liu B, Neufeld AH. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia. 2000;30:178–186. doi: 10.1002/(sici)1098-1136(200004)30:2<178::aid-glia7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 147.Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol. 1997;115:497–503. doi: 10.1001/archopht.1997.01100150499009. [DOI] [PubMed] [Google Scholar]
- 148.Hu Z, Du S. Pressure influence on mRNA and protein expression of inducible nitric oxide synthase in purified retinal ganglion cells of rats. Zhonghua Yan Ke Za Zhi. 2002;38:495–498. [PubMed] [Google Scholar]
- 149.Liu B, Neufeld AH. Nitric oxide synthase-2 in human optic nerve head astrocytes induced by elevated pressure in vitro. Arch Ophthalmol. 2001;119:240–245. [PubMed] [Google Scholar]
- 150.Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Geyer O, Almog J, Lupu-Meiri M, Lazar M, Oron Y. Nitric oxide synthase inhibitors protect rat retina against ischemic injury. FEBS Lett. 1995;374:399–402. doi: 10.1016/0014-5793(95)01147-7. [DOI] [PubMed] [Google Scholar]
- 152.Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448–456. doi: 10.1046/j.0022-3042.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- 153.Yoles E, Belkin M, Schwartz M. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. J Neurotrauma. 1996;13:49–57. doi: 10.1089/neu.1996.13.49. [DOI] [PubMed] [Google Scholar]
- 154.Pang IH, Johnson EC, Jia L, Cepurna WO, Shepard AR, Hellberg MR, et al. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Invest Ophthalmol Vis Sci. 2005;46:1313–1321. doi: 10.1167/iovs.04-0829. [DOI] [PubMed] [Google Scholar]
- 155.Libby RT, Howell GR, Pang IH, Savinova OV, Mehalow AK, Barter JW, et al. Inducible nitric oxide synthase, Nos2, does not mediate optic neuropathy and retinopathy in the DBA/2J glaucoma model. BMC Neurosci. 2007;8:108. doi: 10.1186/1471-2202-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kasmala LT, Ransom NL, Conner JR, McKinnon SJ. Oral administration of SC-51, a nitric oxide synthase inhibitor, does not protect optic nerve axons in a hypertensive rat model of glaucoma. Invest Ophthalmol Vis Sci. 2004;45:904. [Google Scholar]
- 157.Park SH, Kim JH, Kim YH, Park CK. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007;47:2732–2740. doi: 10.1016/j.visres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 158.Flammer J, Haefliger IO, Orgül S, Resink T. Vascular dysregulation: A principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 159.Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013;13:43–49. doi: 10.1016/j.coph.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 160.Martinez A, Gonzalez F, Capeans C, Perez R, Sanchez-Salorio M. Dorzolamide effect on ocular blood flow. Invest Ophthalmol Vis Sci. 1999;40:1270–1275. [PubMed] [Google Scholar]
- 161.Boltz A, Schmidl D, Weigert G, Lasta M, Pemp B, Resch H, et al. Effect of latanoprost on choroidal blood flow regulation in healthy subjects. Invest Ophthalmol Vis Sci. 2011;52:4410–4415. doi: 10.1167/iovs.11-7263. [DOI] [PubMed] [Google Scholar]
- 162.McKibbin M, Menage MJ. The effect of once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow in normal tension glaucoma. Eye (Lond) 1999;13(Pt 1):31–34. doi: 10.1038/eye.1999.6. [DOI] [PubMed] [Google Scholar]
- 163.Osborne NN, Cazevieille C, Carvalho AL, Larsen AK, DeSantis L. In vivo and in vitro experiments show that betaxolol is a retinal neuroprotective agent. Brain Res. 1997;751:113–123. doi: 10.1016/s0006-8993(96)01393-5. [DOI] [PubMed] [Google Scholar]
- 164.Osborne NN, DeSantis L, Bae JH, Ugarte M, Wood JP, Nash MS, et al. Topically applied betaxolol attenuates NMDA-induced toxicity to ganglion cells and the effects of ischaemia to the retina. Exp Eye Res. 1999;69:331–342. doi: 10.1006/exer.1999.0706. [DOI] [PubMed] [Google Scholar]
- 165.Gross RL, Hensley SH, Gao F, Yang XL, Dai SC, Wu SM. Effects of betaxolol on light responses and membrane conductance in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2000;41:722–728. [PubMed] [Google Scholar]
- 166.Hirooka K, Kelly ME, Baldridge WH, Barnes S. Suppressive actions of betaxolol on ionic currents in retinal ganglion cells may explain its neuroprotective effects. Exp Eye Res. 2000;70:611–621. doi: 10.1006/exer.2000.0822. [DOI] [PubMed] [Google Scholar]
- 167.Melena J, Stanton D, Osborne NN. Comparative effects of antiglaucoma drugs on voltage-dependent calcium channels. Graefes Arch Clin Exp Ophthalmol. 2001;239:522–530. doi: 10.1007/s004170100312. [DOI] [PubMed] [Google Scholar]
- 168.Dahlmann-Noor A, Vijay S, Jayaram H, Limb A, Khaw PT. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol. 2010;45:333–341. doi: 10.3129/i10-077. [DOI] [PubMed] [Google Scholar]
- 169.Johnson TV, Martin KR. Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr Opin Pharmacol. 2013;13:78–82. doi: 10.1016/j.coph.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 170.Plant GW, Harvey AR, Leaver SG, Lee SV. Olfactory ensheathing glia: Repairing injury to the mammalian visual system. Exp Neurol. 2011;229:99–108. doi: 10.1016/j.expneurol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 171.Sun Y, Williams A, Waisbourd M, Iacovitti L, Katz LJ. Stem cell therapy for glaucoma: Science or snake oil? Surv Ophthalmol. 2015;60:93–105. doi: 10.1016/j.survophthal.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 172.Greco SJ, Rameshwar P. Microenvironmental considerations in the application of human mesenchymal stem cells in regenerative therapies. Biologics. 2008;2:699–705. doi: 10.2147/btt.s2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 174.Tassoni A, Gutteridge A, Barber AC, Osborne A, Martin KR. Molecular mechanisms mediating retinal reactive gliosis following bone marrow mesenchymal stem cell transplantation. Stem Cells. 2015;33:3006–3016. doi: 10.1002/stem.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]


