Abstract
Objective:
This study is aimed to establish the microtensile bond strength of enamel following exposure to an aerated drink at various time intervals with/without application of remineralization agent. In addition, degree of remineralization and demineralization of tooth enamel has been assessed using polarized light microscopy.
Materials and Methods:
Seventy extracted human incisors split into two halves were immersed in aerated beverage (cola drink) for 5 min and stored in saliva until the time of microtensile bond testing. Prepared specimens were divided randomly into two study groups; remineralizing group (n = 70): specimens were treated for remineralization using casein phosphopeptides and amorphous calcium phosphate (CPP-ACP) remineralization agent (Recaldent™; GC Europe) and control group (n = 70): no remineralization treatment; specimens were kept in artificial saliva. All specimens were tested for microtensile bond strength at regular intervals (1 h, 1 days, 2 days, 1 week, and 2 weeks) using a universal testing machine. The results statistically analyzed (P = 0.05) using two-way ANOVA test.
Results:
Results showed statistically significant increase in bond strength in CPP-ACP tested group (P < 0.05) at all-time intervals. The bond strength of remineralizing group samples at 2 days (~13.64 megapascals [MPa]) is comparable to that of control group after 1 week (~12.44 MPa).
Conclusions:
CPP-ACP treatment of teeth exposed to an aerated drink provided significant increase in bond strength at a shorter interval compared to teeth exposed to saliva alone.
Keywords: Aerated drinks, microtensile bond strength, remineralization-demineralization, remineralizing agent
INTRODUCTION
Tooth enamel is highly mineralized and the hardest tissue of the body.[1,2,3] The loss of tooth enamel and dentin may occur due to various pathologies such as caries, tooth wear, and trauma.[4] In addition to dental caries, acidic beverages also cause demineralization of tooth enamel leading to tooth wear in the form of erosion.[5] Dental erosion is the loss of dental hard tissues by chemical process without the involvement of cariogenic bacteria.[6,7] Several investigations have been conducted to evaluate the effects of aerated drinks and citrus juices on dental hard tissues.[5] Dental enamel is a crystalline lattice of various minerals the principle component of which is hydroxyapatite.[1] Saliva contains supersaturated solution of calcium and phosphate which maintains the pH of the oral environment. The shift of saliva pH to a critically low value (5 or low) either due to citreous drinks or due to cariogenic results in the demineralization of tooth enamel and leaching of mineral ions.[8] The important factors responsible for the balance of remineralization and demineralization are salivary pH, buffering capacity, and salivary flow rate, with pH and buffering capacity increases as flow rate increases. Buffering capacity and bicarbonate contents of saliva resist the change in pH.[9] Saliva plays an important role by neutralizing the acids and providing calcium and phosphate ions to aid in remineralization. Aerated drinks such as colas (pH = 2.6) and noncolas (pH = 3.5) lower the pH of oral environment thus resulting in leaching out of calcium and phosphate ions from the enamel because of the dissolution of hydroxyapatite crystals.[8]
Since the introduction of adhesive resin composites in 1955,[10] these materials have gained popularity due to better physical and esthetic properties. Although the performance of resin composites has improved by incorporation of nanomaterials,[11] the long-term success depends on the bond strength of these materials to tooth tissues. The bond strength of adhesive materials is directly related to minerals in tooth structures. The acidic treatment (from carbonated beverages or any other source) results in loss of minerals from enamel and alters the surface properties compromising the bond strength.[12,13] It has been hypothesized that teeth exposed to carbonated drinks will often yield lower bond strength corresponding to demineralization of enamel. This study is aimed to establish the microtensile bond strength of enamel following exposure to an aerated drink at various time intervals with/without application of remineralization agent. In addition, degree of remineralization and demineralization of tooth enamel has been assessed using polarized light microscopy.
MATERIALS AND METHODS
Sample preparation
The current study was conducted using seventy permanent first premolars freshly extracted during orthodontic treatment. All teeth were cleared of debris ultrasonically and polished for 30 s using nonfluoridated and oil-free pumice slurry. Teeth were washed using deionized water to remove any residual debris or tissue remnants. Each tooth was sectioned into two identical halves longitudinally in buccolingually direction using a microtome. Each tooth section was treated with sand paper (600 grit; A-797455; Foredom's Blackstone Industries Bethel, CT 06801) to get homogeneously uniform experimental surfaces. All specimens were treated with chilled (5–7°C) carbonated soft drink (Coca-Cola; Atlanta, GA 30301, USA) for 5 min followed by storage in deionized distilled water (pH, 6.5;37°C). The storage medium was changed every day until further experimentation.
Prepared samples were randomly divided into following study groups [Figure 1]:
Figure 1.
Study groups and sample distribution for surface characterization of dental hard tissues
Remineralizing group (n = 70): Specimens treated for remineralization using casein phosphopeptides and amorphous calcium phosphate (CPP-ACP) remineralization agent (Recaldent™; GC Europe)
Control group (n = 70): No remineralization treatment; specimens were kept in artificial saliva.
For the remineralization group, CPP-ACP was applied to all specimens (3 min every day) following manufacturer's instructions. All specimens were tested for microtensile bond strength at regular intervals (1 h, 1 day, 2 days, 1 week, and 2 weeks) using the protocol described below.
Acid etching treatment and bonding
Conventional acid etching technique following the manufacturer's instructions was used. Briefly, the labial surface of all specimens was etched using 37% orthophosphoric acid (Total Etch, Ivoclar Vivadent, Schaan) for 15 s followed by washing and air drying. The adhesive bonding agent (Prime and Bond NT, Dentsply, USA) was applied and cured for 20 s using a QTH lamp (Astralis, Ivoclar Vivadent, Schaan). An attachment was built (4 mm diameter) on etched enamel surface using a resin composite (Filtek Z350; 3M ESPE) and cured for 20 s. The prepared specimens were used to measure the microtensile bonding strength.
Microtensile bond strength measurement
Universal mechanical tester (Controls groups; Milan, Italy) was used to test the tensile bonding strength. Specimens were trimmed using a hard tissue microtome and a custom made jig with specific slot dimensions (1 mm × 2 mm × 5 mm). The tensile testing was performed by moving the crosshead at an acceleration of 0.5 mm/min until the failure of specimen.
The extent of demineralization and remineralization was observed using a polarized light microscope (Omano OM349P, Omano, UK). The data was collected and analyzed statistically using SPSS computer software (version 22, IBM Corporation, New York, USA). The results were statistically evaluated using a dependent variable (tensile bond strength) and two-way ANOVA test; P < 0.05 was considered statistically significant.
RESULTS
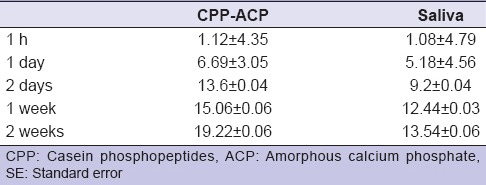
This in vitro study assessed the effects of using remineralizing agent CPP-ACP on tooth enamel and microtensile bond strength using adhesive composite materials. The microscopic structure of untreated structure [Figure 2a] was remarkably changed upon exposure to chilled carbonated drink [Figure 2b] for a very short period (5 min). The remineralization treatment of enamel surface using CPP-ACP (2 days) exhibited significant changes suggesting good penetration of remineralizing agent into the enamel [Figure 2c]. The microtensile bond strength between enamel and adhesive resin was calculated in megapascals (MPa) [Table 1]. The microtensile bond strength was significantly affected by treatment with carbonated drink and remineralizing CPP-ACP. The tensile bond strength of remineralization (CPP-ACP) and saliva (control) groups after 1 h was recorded as 1.12± 4.35 MPa and 1.082 ± 4.79 MPa, respectively. The bond strength was observed to increase in both groups with time. For CPP-ACP group, bond strengths were 6.69 ± 3.05 MPa, 13.64 ± 0.04 MPa, 15.06 ± 0.06 MPa, and 19.22 ± 0.06 MPa, respectively for day 1, day 2, week 1, and week 2. Similar trend of increasing the bond strength with time was observed in case of control group; however, values remain lower than corresponding value in the CPP-ACP group [Table 1]. The differences of bond strength were not significant for measurement at 1 h and 1 day (P > 0.05). In contrast, the bond strength of CPP-ACP group was significantly increased at day 2, week 1, and week 2 compared to control group (P < 0.05). These findings suggested that remineralizing tooth tissues using CPP-ACP may improve the bonding strength of restorative dental materials.
Figure 2.
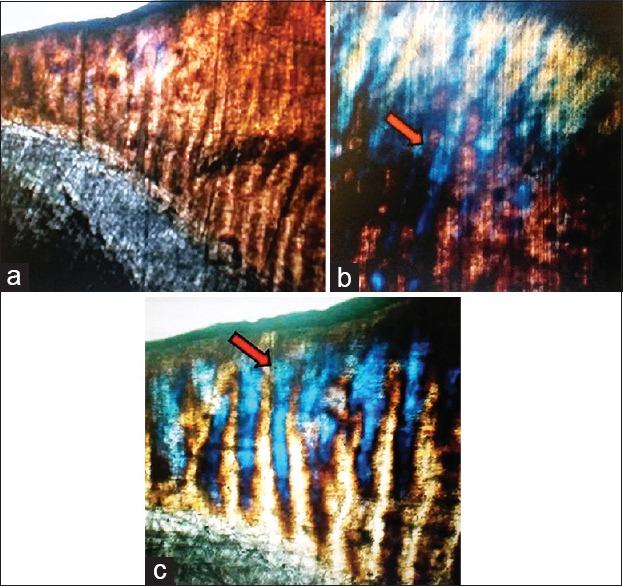
Polaroid microscopic images showing effects of various surface treatments (a) buccolingual section of premolar surface enamel, inner enamel and dentin (b) treatment of enamel specimen with carbonated drink for 5 min (c) tooth specimen from casein phosphopeptides and amorphous calcium phosphate group assessed 2 days after remineralization treatment; penetration of casein phosphopeptides and amorphous calcium phosphate in enamel and corresponding changes (arrows)
Table 1.
Microtensile bond strength (MPa) with/without application of remineralizing agents (mean±SE)
DISCUSSION
We have determined the effect of carbonated beverage on microtensile bond strength of enamel as function of time. The tensile bond strength was improved in response to both saliva and CPP-ACP; however, CPP-ACP treatment showed better results compared to saliva regardless of time interval. The structural integrity of the dental hard tissues (enamel/dentin) depends on the hierarchy and dynamic balance between demineralization and remineralization. Saliva is a dynamic medium that is supersaturated with minerals. Under physiological conditions, saliva has the ability to remineralize tooth using bio-available calcium and phosphates.[14] The action of salivary phosphor-proteins (statherin) causes precipitation of calcium and phosphorus salt.[8] Aerated drinks contain acids (carbonic acid, citric/phosphoric acid) that are known to cause dental erosion. These acids are next stronger to battery acids with a pH of 2.52 and strong enough to demineralize enamel.[15]
In the previous investigations,[5,15,16] the influence of time interval in relation to microtensile bond strength after exposure to an aerated drink was not taken into account. Reynolds[17] showed that exposure of inset enamel plaque to solution containing tryptic peptides of casein remarkably reduced the enamel subsurface demineralization using a caries model in situ. The CPP-ACP is a sticky protein that binds calcium/phosphorus and maintains the amorphous state. CPP stabilizes the nanoclusters of amorphous calcium/phosphate in the meta-stable solution. It contains the sequence: Ser (P)-Ser (P)-Ser (P)-Glu-Glu; Pse is phosphoryl residue that stabilizes calcium/phosphorus ions in aqueous solution, hence confirming their bioavailability.[18] The CPP-ACP is a well proven remineralizing agent.[17,18] The alkaline CPP-ACP when added to sports drinks decreases the acidity and erosive effects. The role of fluoride in inhibiting tooth demineralization and prevention of caries is well known.[4,19,20,21,22] The fluoride incorporated CPP-ACP complex are also available where ACP has been replaced by amorphous calcium-fluoride-phosphate.[23] This fluoride incorporated CPP-ACP may have additional benefits of releasing fluoride in the oral cavity and anticarcinogenic properties.
Microtensile bond strength testing gives more accurate values than conventional tensile strength because the small size of the specimens allows better stress distribution during the test loading. The lower variance of data results could be attributed to the reduction in flow density at the interface.[24] In the present study, it was found that samples treated with CPP-ACP have shown better bond strength at all-time intervals. The decreased bond strength of samples not treated by CPP-ACP can be linked to the increased demineralization due to acidic drinks as well as etching agent.[12] It is well-known that increasing the etching time leads to reduction in bond strength. The bond strength of CPP-ACP applied teeth after 2 days following exposure to an aerated drink was equal to 7 days without application of remineralizing agent. The 1-week bond strength of teeth that were not exposed to remineralizing agent was statistically similar to that of 2 days bond strength achieved with CPP-ACP treated teeth. There are a few limitations; this study was conducted in in vitro and the experimental conditions did not reflect the dynamic conditions of oral environment. A number of factors (temperature, pH, saliva chemistry, food interaction, presence of microorganism) may interfere the remineralization process and bond strength. This study has provided the baseline results hinting that demineralization treatment before adhesive restorations has the potentials to improve the bond strength. However, in vivo and clinical studies are required to validate these claims.
CONCLUSIONS
The remineralization treatment using ACP accelerates remineralization of enamel compared to the saliva. The mineralization ability of saliva is not enough to cope with the dimerization caused by aerated drinks. The rapid remineralization of enamel in response to remineralizing agent increased the bond strength significantly at a shorter interval compared to saliva. In clinical prospects, the remineralizing agents can be applied on adherent tooth surface to improve the bond strength.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nanci A. St. Louis, MO, London: Mosby; 2012. Ten Cate's Oral Histology: Development, Structure, and Function; p. 411. [Google Scholar]
- 2.Zafar MS, Ahmed N. Nano-mechanical evaluation of dental hard tissues using indentation technique. World Appl Sci J. 2013;28:1393–9. [Google Scholar]
- 3.Zafar MS, Ahmed N. Nanomechanical characterization of exfoliated and retained deciduous incisors. Technol Health Care. 2014;22:785–93. doi: 10.3233/THC-140852. [DOI] [PubMed] [Google Scholar]
- 4.Kidd EA. Oxford, New York: Oxford University Press; 2005. Essentials of Dental Caries. [Google Scholar]
- 5.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006;85:226–30. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 6.Roberson T, Heymann HO, Swift EJ., Jr . St. Louis, Missouri, USA: Elsevier Health Sciences; 2006. Sturdevant's Art and Science of Operative Dentistry. [Google Scholar]
- 7.Cawson RA. Edinburgh: Churchill Livingstone; 2002. Cawson's Essentials of Oral Pathology and Oral Medicine; p. 402. [Google Scholar]
- 8.Wefel JS, Donly KJ. Philadelphia, USA: WB Saunders; 1999. Cariology. [Google Scholar]
- 9.Dixon M, Jones Y, Mackie IE, Derwent SK. Mandibular incisal edge demineralization and caries associated with Twin Block appliance design. J Orthod. 2005;32:3–10. doi: 10.1179/146531205225020724. [DOI] [PubMed] [Google Scholar]
- 10.Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–53. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 11.Khurshid Z, Zafar M, Qasim S, Shahab S, Naseem M, AbuReqaiba A. Advances in nanotechnology for restorative dentistry. Materials. 2015;8:717–31. doi: 10.3390/ma8020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafar MS, Ahmed N. The effects of acid etching time on surface mechanical properties of dental hard tissues. Dent Mater J. 2015;34:315–20. doi: 10.4012/dmj.2014-083. [DOI] [PubMed] [Google Scholar]
- 13.Colak H, Ercan E, Hamidi MM. Shear bond strength of bulk-fill and nano-restorative materials to dentin. Eur J Dent. 2016;10:40. doi: 10.4103/1305-7456.175697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar MS. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm J. 2015 doi: 10.1016/j.jsps.2015.02.015. doi:10.1016/j.jsps.2015.02.015. E-published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain PA, Nihill P, Sobkowski J, Agustin MZ. Commercial soft drinks: pH and in vitro dissolution of enamel. Gen Dent. 2007;55:150–4. [PubMed] [Google Scholar]
- 16.Seow WK, Thong KM. Erosive effects of common beverages on extracted premolar teeth. Aust Dent J. 2005;50:173–8. doi: 10.1111/j.1834-7819.2005.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds EC. The prevention of sub-surface demineralization of bovine enamel and change in plaque composition by casein in an intra-oral model. J Dent Res. 1987;66:1120–7. doi: 10.1177/00220345870660060601. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds EC, Cai F, Shen P, Walker GD. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res. 2003;82:206–11. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 19.Ullah R, Zafar MS. Oral and dental delivery of fluoride: A review. Fluoride. 2015;48:195–204. [Google Scholar]
- 20.Zafar MS, Ahmed N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride. 2015;48:184–94. [Google Scholar]
- 21.Niazi F, Naseem M, Khurshid Z, Zafar MS, Almas K. Role of salvadora persica chewing stick (miswak): A natural toothbrush for holistic oral health. Eur J Dent. 2016;10:301. doi: 10.4103/1305-7456.178297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zafar MS. Effects of surface pre-reacted glass particles on fluoride release of dental restorative materials. World Appl Sci J. 2013;28:457–62. [Google Scholar]
- 23.Cross KJ, Huq NL, Reynolds EC. Casein phosphopeptides in oral health – Chemistry and clinical applications. Curr Pharm Des. 2007;13:793–800. doi: 10.2174/138161207780363086. [DOI] [PubMed] [Google Scholar]
- 24.Botelho MG. The microtensile bond strength of Fuji IX glass ionomer cement to antibacterial conditioned dentin. Oper Dent. 2005;30:311–7. [PubMed] [Google Scholar]


