Abstract
Objective:
Green tea extract (GTE) was shown to be effective in preserving periodontal ligament fibroblasts (PDLFs) of avulsed teeth. This study aimed at determining the potential of GTE in preserving the viability of PDLFs comparing with different storage media.
Materials and Methods:
Periodontal ligament cells were obtained from freshly extracted healthy impacted third molars and cultured in Dulbecco's Modified Eagle Medium (DMEM). Cell viability was determined by storing the cells in seven media; DMEM, tap water, Hank's balanced salt solution (HBSS), whole milk, hypotonic sucrose solution, GTE, and GTE + sucrose for 1, 2, 4, and 24 h at 37°C using tetrazolium salt-based colorimetric (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay. Statistical analysis was performed by one-way analysis of variance and post hoc tests.
Results:
GTE showed significantly higher protective effect than HBSS at 2, 4, and 24 h (P = 0.009, P = 0.02, P = 0.016), DMED at 2 h (P = 0.003), and milk at 4 h (P = 0.039).
Conclusion:
Although with undesirable osmolality and pH, GTE had a good ability in preserving the PDLFs comparing with other studied media.
Keywords: Avulsion, cell viability, Green tea extract, periodontal ligament cell
INTRODUCTION
Tooth avulsion (exarticulation, total luxation) is total displacement of the tooth out of its socket. Avulsion is more frequently happened in the maxillary central incisors while the lower jaw is seldom affected.[1] This prevalence of avulsion was reported 0.5–16%.[2]
Prognosis of tooth replantation depends on the persistence of vital cells in periodontal ligament (PDL), which are able to proliferate on the damaged areas of the external root surface.[3] This issue is affected by two critical factors: (1) The extra-alveolar time and (2) the storage condition.[4,5,6]
The best prognosis of tooth replantation is obtained when the extra-alveolar time does not exceed 5 min.[7,8] However, in situ ation of delayed replantation, preserving the cells in the proper storage medium is recommended.[4,5,6]
An ideal storage medium should keep alive and functional the PDL cells.[9] For this purpose, a medium with physiologic osmolality and pH, containing essential nutrients for cell growth is needed that is also easily available.[9]
Several experiments have been carried out in an attempt to find the ideal storage medium of avulsed tooth, such as milk, saliva, saline solution, water, Viaspan, and Hank's balanced salt solution (HBSS).[10,11] Currently, Green tea extract (GTE) is proposed as a potential storage medium.[12] Green tea (GT), extracted from Camellia sinensis, is a widely consumed beverage throughout the world second to water.[11] GTE contains the main group of polyphenols including catechin, epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG).[13] These polyphenols have anti-inflammatory and antioxidant effects.[13] GTE contains some important elements for cell growth such as calcium (Ca), selenium, zinc, magnesium (Mg), iron, and fluoride as well as carbohydrates and Vitamins B, C, and E.[9] The pH and osmolality of this solution were reported lower than physiologic range.[9,12] However, it was shown that GTE in combination with low-dose cyclosporine A can prolong allograft survival in mice.[14]
The purpose of this study was to evaluate the protective effect of GTE on preserving PDL fibroblasts (PDLFs) comparing with several common storage media in different storage durations.
MATERIALS AND METHODS
This project has been conducted under the Babol University of Medical Sciences Ethics Committee approval to research on human tissue samples.
The GTE was prepared based on the method previously described by Hwang et al. and Nkhili et al.[12,15] Ten grams of the commercial spring GT leaves (Refah Lahijan Co., Iran) harvested in the north of Iran was soaked in 100 mL of distilled water. The temperature of water was gradually increased to 80°C and kept at this level for 45 min. After cooling to the room temperature, the extracts were sterilized using 0.2 µm pressure filters and kept frozen at −20°C until the experiment was initiated. The osmolality and pH of the extracts were measured twice using a standard osmometer (Roebling, Nr. 9610003. Type 13, Germany) and a pH meter (Crison pH-meter Basic 20+, Spain). The total polyphenols content of GTE sample was reported by manufacturer at 24.75%.
PDLFs were derived from sterile surgically extracted unerupted third molar teeth, which were planned to be removed due to space management. The PDL of the middle third of the root was scraped with a curette and placed in culture flasks containing Dulbecco's Modified Eagle Medium (DMEM) (Atocel Co., Austria) supplemented with 10% fetal bovine serum (Atocel Co., Austria), penicillin G (100 IU/ml), and streptomycin (100 µg/ml) (Pen-Strep) (Sigma, USA). The flasks were incubated at 37°C in a humidified atmosphere containing 5% CO2, and the medium was exchanged every 3 days. Once confluence of the PDLFs was obtained, the medium was removed and the cells were trypsinized (Trypsin-EDTA; Sigma, USA) and after washing, they were transmitted to another flask (first passage). Cells from 3 to 7 passages were used for the experiments.
In a 96-well tissue culture plate (Jet biofil, Sorfa, Germany), 12 × 103 cells were seeded in each well and incubated. After 48 h, the media were exchanged with 100 µl of one of the seven test media (DMEM as positive control, distilled water as negative control, HBSS, pasteurized long-life whole milk [Haraz, Tehran, Iran], hypotonic sucrose solution [HSS] [213 mOsm], GTE + sucrose [87 mOsm + 213 mOsm], and GTE) for 1, 2, 4, and 24 h.
After 1, 2, 4, and 24 h, the storage medium was removed, the cells were washed 3 times with Ca/Mg-free phosphate buffered saline (PBS), and then 50 µl of 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) solution (MTT, Sigma) (5 mg/ml) was added to each well and plates were incubated at 37°C. After 4 h, the precipitated formazan dyes were dissolved in 150 µl of acidified isopropanol (HCl 0.04 N) (Sigma, USA).
Cell viability was determined by measuring the differential optical densities at 570 nm and 630 nm as reference wavelength using a spectrophotometer (Rayto, microplate reader RT-2100C, China). The cells stored in DMEM were used as positive control for cell growth.
Data were analyzed using the statistical software SPSS (version 22, IBM Co. USA). One-way analysis of variance and post hoc tests were used and P < 0.05 was considered statistically significant.
RESULTS
Acidity and osmolality of DMEM were estimated 7.3 and 305 mosmol, tap water: 4.8 and 15 mosmol; HBSS: 7.2 and 280 mosmol; whole milk: 4.6 and 286 mosmol; HSS: 5.7 and 213 mosmol; GTE: 5.4 and 87 mosmol; and GTE + sucrose: 5.2 and 300 mosmol, respectively.
The viability of PDLF cells cultured in different storage media at 37°C was determined using MTT assay after 1, 2, 4, 24 h and the results are presented in Figure 1.
Figure 1.
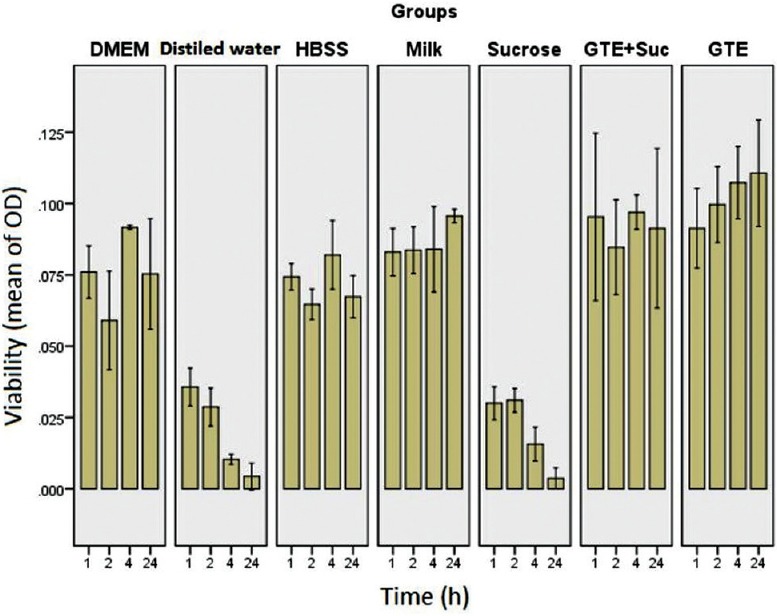
Viability of preserving periodontal ligament fibroblast cells (mean of optical density) in term of different storage media and time intervals
The viability of PDLFs in DMEM, HBSS, whole milk, GTE, and GTE + sucrose was significantly higher than HSS and distilled water after 1, 2, 4, and 24 h.
After 1 h, DMEM, HBSS, whole milk, GTE, and GTE + sucrose were equally effective to protect the cells. From the 2nd h, GTE showed significantly higher protective effect than HBSS (P = 0.009) and DMED (P = 0.003), but there was no significant difference between GTE and whole milk and GTE + sucrose. The viability of PDLFs in GTE was significantly higher than HBSS (P = 0.023) and whole milk (P = 0.039) after 4 h while no significant difference was observed between GTE and DMEM and GTE + sucrose. At the time of 24 h, GTE was significantly more effective than HBSS (P = 0.016), but there was no significant difference between GTE, DMEM, GTE + sucrose, and milk. There was no significant difference between whole milk DMEM, HBSS, GTE, and GTE + sucrose in all time intervals, except at 4 h between milk and GTE. The dual comparison of the groups has shown that there was no significant difference between water and HSS as well as GTE and GTE + sucrose groups (P > 0.05). Figure 2 illustrates the concentration of viable cells after 2 h immersion in different media.
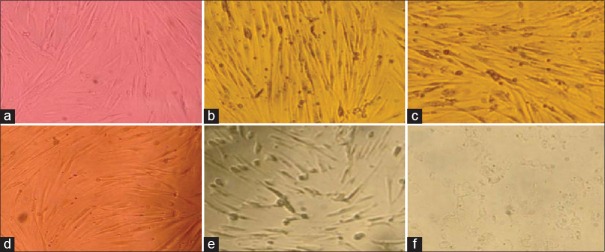
Figure 2.
Concentration of viable cells after 2 h immersion in different media: (a-f) Dulbecco's Modified Eagle Medium, Green tea extract + sucrose, Green tea extract, Hank's balanced salt solution, hypotonic sucrose solution, water, respectively
DISCUSSION
The primary outcome of this study was the confirmation of the equality or even the superiority of GTE rather than other common studied media. GTE as an accessible worldwide consumed drink contains high amounts of protective nutrients including antioxidative polyphenols and is a potential good storage medium for avulsed teeth. This result was in agreement with the other studies.[9,12]
In the present assay, DMEM (positive control), distilled water (negative control), HBSS, whole milk, GTE, isotonic GTE + sucrose, and HSS (control for GTE + sucrose) were tested for preserving the viability of PDLFs. The HBSS, as a well-known standard storage solution for avulsed teeth, has been recommended by the International Association of Dental Traumatology,[16] which we also used in this study. The present results showed that GTE and HBSS can keep the PDLF cells alive up to 24 h, and the effect of GTE at the 2nd, 4th, and 24th h is significantly superior to HBSS. This is consistent with the results of the study performed by Hwang et al., in which it was concluded that most of the PDL cells survive in both GTE and HBSS up to 24 h, but their difference is not significant.[12]
Furthermore, Ghasempour et al. have shown that GT and HBSS equally preserve the viability of PDLs in 1, 3, and 15 h.[9]
Another studied medium was whole milk which is a physiologically compatible liquid (pH and osmolality) containing nutrients and growth factors.[17,18] Whole milk was reported as one of the most practical transport media for storage of avulsed teeth because of its availability in almost all situations.[19] It contains amino acids and vitamins able to deactivating harmful enzymes to the PDL cells.[19] Milk was suggested as an appropriate transport medium for avulsed teeth; however, the ability of milk to preserve the cells decreases after 2–3 h.[20] Blomlof reported that only 50% of PDL cells survive after 12 h.[21] In the present assay, GTE and whole milk were equally effective to protect the cells at all times except at 4 h that GTE was more able.
In the study of Jung et al., 0, 10, and 100 µM concentrations of EGCG at 3 times intervals were examined. This study revealed that viability of PDLFs of Beagle dogs is promoted by increasing concentration of EGCG and the efficacy of GTE in maintaining the viability of human PDL cells was similar to that of HBSS and milk.[22]
Two main factors to select a good storage medium are physiologic pH and osmolality (6.6–7.8 and 290–330 mOsm, respectively),[12] and any deviation from this normal condition could be fatal to the cells. GTE is a hypotonic solution used in this experiment with an osmolality about 87 mOsm. To compensate this osmolality gap (213 mOsm), we calculated the proper amount of sucrose (a highly abundant naturally found disaccharide) to be added to the GTE (GTE + sucrose). Furthermore, to exclude any additional effects induced by sucrose, we used sucrose solution as another group. Surprisingly, GTE and GTE + sucrose did not show any significant difference at all times. It seems that the correction of osmolality had not the main role in preserving potential of GTE. In addition, only the pH of HBSS and DMEM was in the physiologic range. It seems that the lower pH had no adverse effect on protective capacity of GTE and milk.
Many studies have proven that storage time could affect dramatically the effectiveness of storage media in maintaining the PDLFs cell viability.[8,23,24] Most avulsed teeth receive dental cares during the first few hours after avulsion; hence, we chose storage times of 1, 2, 4, and 24 h.
Furthermore, there are different methods to assay cell viability with own advantages or limitations. MTT assay is one of the most common viability tests which measure the cells biomass by evaluating the reductive NADPH production by mitochondria. The yellowish MTT is a water soluble tetrazolium derivative that easily incorporates into the viable cells. It is reduced to purple formazan crystals, in the viable cells proportional to the cells biomass. Then, the formazan precipitate is later solubilized either by acidic isopropanol or dimethylsulfoxide and the cell viability (proportionate to the metabolic activity) is quantified by measuring the amount of formazan solution optical density using a spectrophotometer The accuracy, timesaving, and no need use of radioisotopes are the main advantages of MTT assay.[25] Since GTE has high antioxidative potentials, the cells were washed 3 times with Ca/Mg-free PBS before adding the MTT solution to abolish any nonspecific formazan formation. Further studies to clarify the role of chemical composition of GTE on cell viability with other methods and to compare with other sources of polyphenols such as propolis have recommended by authors.
CONCLUSION
Although with undesirable osmolality and pH, GTE had a good ability in preserving the PDLFs comparing with other studied media.
Financial support and sponsorship
Babol University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Andreasen JO, Andreasen FM, Andersson L. Copenhagen, Denmark: Blackwell Munksgaard; 2007. Textbook and Color Atlas of Traumatic Injuries to the Teeth. [Google Scholar]
- 2.Ozer S, Yilmaz EI, Bayrak S, Tunc ES. Parental knowledge and attitudes regarding the emergency treatment of avulsed permanent teeth. Eur J Dent. 2012;6:370–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes MC, Westphalen VP, Westphalen FH, Silva Neto U, Fariniuk LF, Carneiro E. Study of storage media for avulsed teeth. Braz J Dent Traumatol. 2009;1:69–76. [Google Scholar]
- 4.Gopikrishna V, Thomas T, Kandaswamy D. A quantitative analysis of coconut water: A new storage media for avulsed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e61–5. doi: 10.1016/j.tripleo.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Goswami M, Chaitra T, Chaudhary S, Manuja N, Sinha A. Strategies for periodontal ligament cell viability: An overview. J Conserv Dent. 2011;14:215–20. doi: 10.4103/0972-0707.85789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gramza A, Korczak J, Amarowicz R. Tea polyphenols-their antioxidant properties and biological activity-a review. Pol J Food Nutr Sci. 2005;14:219. [Google Scholar]
- 7.Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors 4. Factors related to periodontal ligament healing. Endod Dent Traumatol. 1995;11:76–89. doi: 10.1111/j.1600-9657.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 8.Tezel H, Atalayin C, Kayrak G. Replantation after traumatic avulsion. Eur J Dent. 2013;7:229–32. doi: 10.4103/1305-7456.110192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghasempour M, Moghadamnia AA, Abedian Z, Amir MP, Feizi F, Gharekhani S. In vitro viability of human periodontal ligament cells in green tea extract. J Conserv Dent. 2015;18:47–50. doi: 10.4103/0972-0707.148894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litwin J, Lundquist G, Söder PO. Studies on long-term maintenance of teeth and viable associated cells in vitro. Europ J Dent Res. 1971;79:536–9. doi: 10.1111/j.1600-0722.1971.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JY, Choi SC, Park JH, Kang SW. The use of green tea extract as a storage medium for the avulsed tooth. J Endod. 2011;37:962–7. doi: 10.1016/j.joen.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea – A review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi S, Bruch D, Gatto LA, Kittur DS. Green tea extract prolongs allograft survival as an adjunctive therapy along with low dose cyclosporine A. J Surg Res. 2009;154:85–90. doi: 10.1016/j.jss.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Nkhili E, Tomao V, El Hajji H, El Boustani ES, Chemat F, Dangles O. Microwave-assisted water extraction of green tea polyphenols. Phytochem Anal. 2009;20:408–15. doi: 10.1002/pca.1141. [DOI] [PubMed] [Google Scholar]
- 16.Flores MT, Andersson L, Andreasen JO, Bakland LK, Malmgren B, Barnett F, et al. Guidelines for the management of traumatic dental injuries. II. Avulsion of permanent teeth. Dent Traumatol. 2007;23:130–6. doi: 10.1111/j.1600-9657.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- 17.Chamorro MM, Regan JD, Opperman LA, Kramer PR. Effect of storage media on human periodontal ligament cell apoptosis. Dent Traumatol. 2008;24:11–6. doi: 10.1111/j.1600-9657.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos CL, Sonoda CK, Poi WR, Panzarini SR, Sundefeld ML, Negri MR. Delayed replantation of rat teeth after use of reconstituted powdered milk as a storage medium. Dent Traumatol. 2009;25:51–7. doi: 10.1111/j.1600-9657.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier SF, Pouliot Y, Maubois JL. Growth factors from bovine milk and colostrum: Composition, extraction and biological activities. Lait. 2006;86:99–125. [Google Scholar]
- 20.Nozari A, Esmaeilpour T, Fijan S. Investigation of the capability of the new storage media in keeping the periodontal ligament cells viability. Sadra Med Sci J. 2013;1:103–12. [Google Scholar]
- 21.Blomlöf L. Storage of human periodontal ligament cells in a combination of different media. J Dent Res. 1981;60:1904–6. doi: 10.1177/00220345810600111301. [DOI] [PubMed] [Google Scholar]
- 22.Jung IH, Yun JH, Cho AR, Kim CS, Chung WG, Choi SH. Effect of (-)-epigallocatechin-3-gallate on maintaining the periodontal ligament cell viability of avulsed teeth: A preliminary study. J Periodontal Implant Sci. 2011;41:10–6. doi: 10.5051/jpis.2011.41.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomlöf L, Otteskog P. Viability of human periodontal ligament cells after storage in milk or saliva. Scand J Dent Res. 1980;88:436–40. doi: 10.1111/j.1600-0722.1980.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 24.de Souza BD, Lückemeyer DD, Felippe WT, Alves AM, Simões CM, Felippe MC. Effect of milk renewal on human periodontal ligament fibroblast viability in vitro. Dent Traumatol. 2012;28:214–6. doi: 10.1111/j.1600-9657.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- 25.Bharath MJ, Sahadev CK, Ramachandra PK, Rudranaik S, George J, Thomas A. Comparative evaluation of four transport media for maintaining cell viability in transportation of an avulsed tooth – An in vitro study. J Int Soc Prev Community Dent. 2015;5:69–73. doi: 10.4103/2231-0762.151981. [DOI] [PMC free article] [PubMed] [Google Scholar]