Abstract Abstract
The Palaearctic Grayling genus Pseudochazara encompasses a number of petrophilous butterfly species, most of which are local endemics especially in their centre of radiation in SW Asia and the Balkans. Due to a lack of consistent morphological characters, coupled with habitat induced variability, their taxonomy is poorly understood and species delimitation is hampered. We employed a DNA barcoding approach to address the question of separate species status for several European taxa and provide first insight into the phylogeny of the genus. Unexpectedly we found conflicting patterns with deep divergences between presumably conspecific taxa and lack of divergence among well-defined species. We propose separate species status for Pseudochazara tisiphone, Pseudochazara amalthea, Pseudochazara amymone, and Pseudochazara kermana all of which have separate well supported clades, with the majority of them becoming local endemics. Lack of resolution in the ‘Mamurra’ species group with well-defined species (in terms of wing pattern and coloration) such as Pseudochazara geyeri, Pseudochazara daghestana and Pseudochazara alpina should be further explored using nuclear molecular markers with higher genetic resolution.
Keywords: Papilionoidea, Satyrinae, butterflies, phylogeny, barcoding, taxonomy
Introduction
Depending on which systematic order of classification is adhered to, the genus Pseudochazara comprises 27–32 species of Graylings (Gross 1978, Lukhtanov 2007, Savela 2015). It has a wide distribution in the Palaearctic region from North Africa to the Himalayas and Mongolia (Tennent 1996, Tshikolovets 2005, Yakovlev 2012). In addition to vague species delimitation, large intraspecific variation has resulted in the description of over 100 subspecific taxa (Lukhtanov 2007) in this intensively studied taxon.
The main reason for the extensive variation in phenotype can be linked with the specific ecological requirements of these butterflies. They are mostly petrophilous and limited to specific rock substrate to which they are perfectly adapted with their camouflaged underside wing pattern and cryptic coloration. Local adaptation to mimic the coloration of the rock substrate is, therefore, one of the main drivers for such large scale diversification (Lorković 1974, Weiss 1980, Hesselbarth et al. 1995, Tennent 1996, but see Anastassiu et al. 2009).
Trying to resolve the systematics of this genus and its species delimitation has been thwarted by the fact that the genitalia of many Pseudochazara species are virtually identical and their wing shape and coloration, both being partially dependant on environmental conditions (Gross 1978, Hesselbarth et al. 1995), is inconsistent. The last comprehensive taxonomic review which was published by Gross (1978) is already outdated. He recognised 24 species, among which Pseudochazara obscura (Staudinger, 1878) is now considered a subspecies of Pseudochazara lydia (Staudinger, 1878) (see Eckweiler and Rose 1988), Pseudochazara aurantiaca (Staudinger, 1878) and Pseudochazara xerxes Gross & Ebert, 1975 have been reclassified as subspecies of Pseudochazara beroe (Herrich-Schäffer, 1844) (see Lukhtanov 2007), Pseudochazara schahrudensis (Staudinger, 1881) is now considered conspecific with Pseudochazara mamurra (Herrich-Schäffer, 1844) (see Eckweiler 2004) and Pseudochazara pakistana Gross, 1978 is conspecific with either Pseudochazara gilgitica (Tytler, 1926) (see Lukhtanov 2007) or Pseudochazara baldiva (Moore, 1865) (see Wakeham-Dawson et al. 2007). Several members of the Pseudochazara genus from Central Asia that are currently recognised as separate species were considered subspecific taxa in the revision (e.g. Pseudochazara droshica (Tytler, 1926), Pseudochazara gilgitica (Tytler, 1926), Pseudochazara lehana (Moore, 1878)) while Pseudochazara euxina (Kuznetsov, 1909) from Crimea was entirely neglected. Two additional species were described after the revision, Pseudochazara kanishka (Aussem 1980a) and Pseudochazara annieae (Pagès 2007). Following Gross’ revision (1978) the shape of the androconial scales of several Pseudochazara species has proven to be constant, enabling species delimitation (Weiss 1980, Eckweiler and Rose 1989, Wakeham-Dawson and Kudrna 2000, Wakeham-Dawson et al. 2003, Wakeham-Dawson and Kudrna 2005, Wakeham-Dawson 2006, Wakeham-Dawson and Kudrna 2006, Pages 2007, Wakeham-Dawson et al. 2007).
There has been no attempt to reconstruct the phylogeny of the genus or validate species status using molecular markers. Only the taxonomic position within subtribe Satyrina and a sister relationship to Chazara has been established (Peña et al. 2011).
In order to resolve the relationship among Pseudochazara species and re-evaluate their species status, in particular of some European taxa, we employed DNA barcoding – using a standardized gene region (5’ segment of the mitochondrial gene COI) which enabled us to utilize additional Pseudochazara sequences available in the Barcode of Life Database (BOLD 2015). DNA barcodes have been widely and successfully used in Lepidoptera taxonomy and species delimitation as an additional set of characters which are independent of habitat conditions (Hebert et al. 2004, Nazari and Sperling 2007, Nazari et al. 2010, Dinca et al. 2011, Yang et al. 2012, Lukhtanov and Novikova 2015, Pazhenkova et al. 2015). However, there are several limitations of this method (see e.g. Wiemers and Fiedler 2004, Brower 2006, Ritter et al. 2013, Song et al. 2008, Toews and Brelsford 2012) which should be taken into account in the interpretation of the gene tree.
Material and methods
Sample collection, DNA extraction, amplification, sequencing, and alignment
With the aim of achieving consistency, we adopt the nomenclature of the most recent list of Pseudochazara species by Lukhtanov (2007). Following the discovery of Pseudochazara mamurra amymone in Albania (Eckweiler 2012), we initially sampled all the Pseudochazara taxa from the Balkan Peninsula, a hotspot of Pseudochazara diversity in Europe (Verovnik et al. 2014, Gascoigne-Pees et al. 2014). We then broadened the range of our sampling adding additional species from Turkey and the Middle East, the main areas of Pseudochazara diversification. Altogether 27 specimens belonging to 10 species of Pseudochazara, for which the barcoding gene COI was successfully amplified, were included in the study (see Appendix 1). All specimens were dried prior to DNA extraction. In addition, we included COI sequences from 81 individuals belonging to 14 species from the BOLD database (BOLD 2015). Only specimens that could be unambiguously identified by the voucher photos were selected. Following the nomenclature guidelines proposed by Lukhtanov (2007) a total of 34 taxa belonging to 20 species were included in the analysis. As outgroups, we added several sequences of the closely related Satyrine genus Chazara from GenBank, based on the results of the phylogenetic study of Satyrinae by Peña et al. (2011).
Total genomic DNA was extracted from single legs, following the Mammalian tissue preparation protocol (GenElute Mammalian Genomic DNA miniprep kit from Sigma-Aldrich). For each sample a 657 bp fragment of the first subunit of the mitochondrial gene cytochrome c oxidase (COI) was amplified using primers LCO1490 and HCO2198 (Folmer et al. 1994). Amplification followed a standard protocol described in Verovnik et al. (2004). PCR products were visualized on an agarose gel to verify amplification success and sequenced by Macrogen in both directions on an Applied Biosystems 3730xl sequencer.
Phylogenetic analysis
We used Bayesian inference to reconstruct a phylogenetic tree. To achieve more clarity the tree was constructed on a subset of samples including only unique haplotypes belonging to the same taxon. A hierarchical likelihood test was employed in order to test alternative models of evolution, using JModeltest v.0.1.1 (Posada 2008). A GTR (Generalised time reversible) model of nucleotide substitution with gamma distributed rate heterogeneity and a significant proportion of invariable sites was selected in accordance with the Akaike Information Criterion. Bayesian analysis was performed with MrBayes v.3.1.2 implementing the best fit substitution model (Huelsenbeck and Ronquist 2001). Markov chain Monte Carlo search was run with four chains for 4 × 106 generations, taking samples every 100 generations. The approximate number of generations needed to obtain stationarity of the likelihood values (‘‘burn-in’’) of the sampled trees was estimated graphically to 2000 trees. From the remaining trees posterior probabilities were assessed for individual clades based on their observed frequencies. Trees were visualised using Figtree v.1.4.2 (Rambaut 2014). Genetic distances (p-) were calculated with MEGA 6.0 (Tamura et al. 2013). In addition, a statistical parsimony network analysis was performed with TCS 1.21 (Clement et al. 2000).
Results
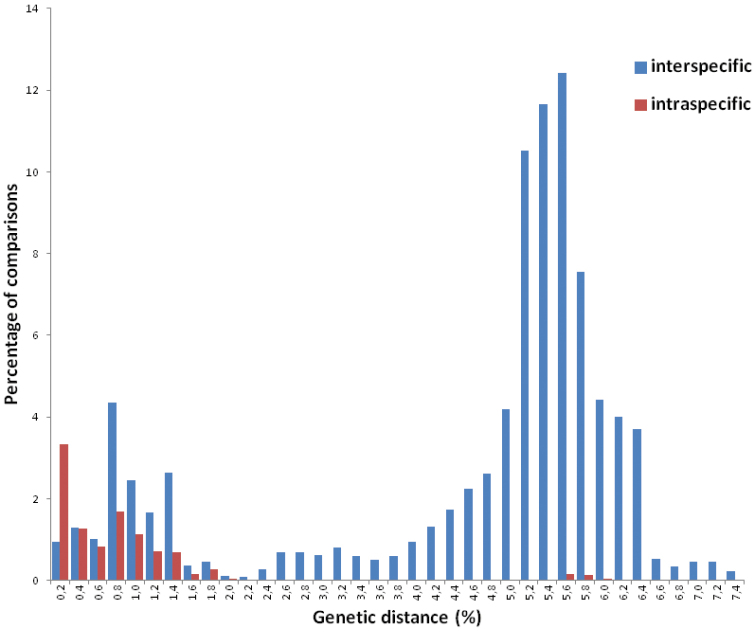
No insertions or deletions were observed in the mitochondrial COI gene and therefore the alignment was unambiguous. For the COI dataset 63 unique haplotypes among 108 Pseudochazara sequences were detected. 114 (17.5%) sites were variable and 95 (14.6%) were parsimony informative. The average interspecific genetic distance was 4.9%, but in the case of Pseudochazara mniszechii the intraspecific diversity ranged from 0 to 6.7% with highly distinct divergent sequences of Pseudochazara mniszechii tisiphone. No evident barcoding gap was observed separating intraspecific from interspecific pairwise genetic distances (Fig. 1). On the contrary, sharing of identical haplotypes was observed in the following taxa: Pseudochazara graeca / Pseudochazara mamurra amymone, Pseudochazara mamurra mamurra / Pseudochazara daghestana, and Pseudochazara beroe aurantiaca / Pseudochazara alpina. On the other hand, 82% of species comparisons showed high (≥2%) interspecific distances.
Figure 1.
Frequency distribution of pairwise intra- and interspecific p-distances of the COI sequences in the genus Pseudochazara. No “barcoding gap” exists between these two data series.
The calculated maximum connection for parsimony networks at the default 95% limit was 11 steps, and resulted in 9 separate networks within Pseudochazara. 6 of them contain only single species (Pseudochazara atlantis, Pseudochazara turkestana, Pseudochazara thelephassa, Pseudochazara lehana, Pseudochazara kanishka, and Pseudochazara anthelea), whereas the remaining 3 comprise several closely related species (Figs 2–4). Outgroups were contained in 2 distinct networks (Chazara enervata and Chazara briseis/Chazara heydenreichi).
Figure 2.
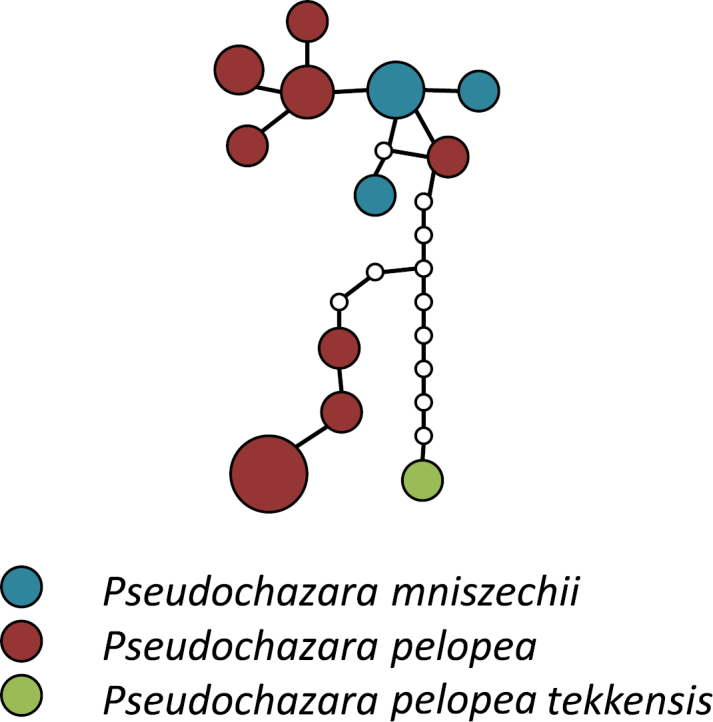
Statistical Parsimony network of the ‘pelopea’ species group. Coloured circles represent COI haplotypes and their size corresponds to the number of samples per haplotype. Small white circles represent unsampled haplotypes.
Figure 4.
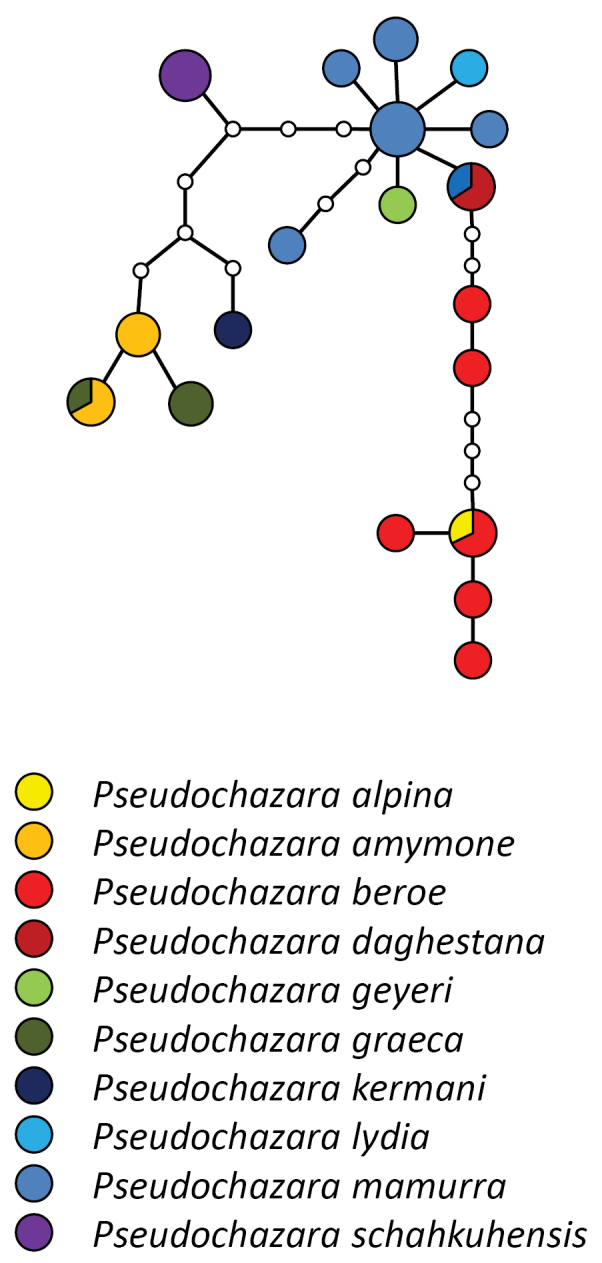
Statistical Parsimony network of the ‘mamurra’ species group. Coloured circles represent COI haplotypes and their size corresponds to the number of samples per haplotype. Small white circles represent unsampled haplotypes.
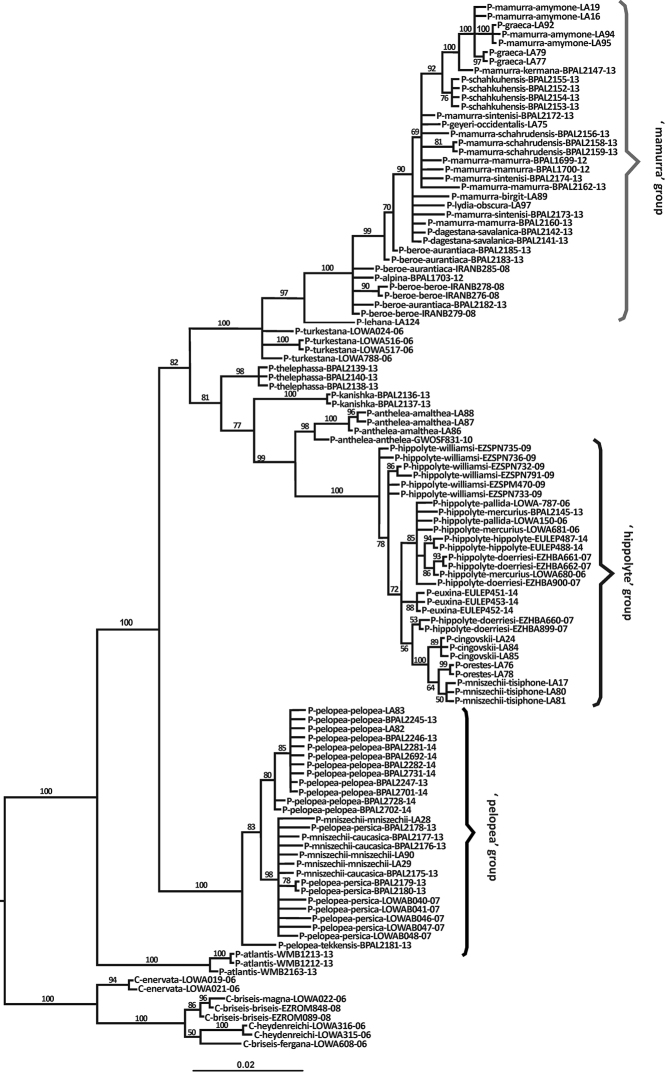
The topology of the Bayesian Inference tree of all Pseudochazara samples, including the selected outgroup species (Fig. 5), confirms the monophyly of the genus. High posterior probability values support a basal position of Pseudochazara atlantis, the only species of the genus present in (and confined to) North Africa. This is somewhat surprising as Pseudochazara anthelea and Pseudochazara thelephassa are considered to be morphologically the most distinct and separate species within the genus (Gross 1978). Pseudochazara atlantis has tentatively been placed into two groups, the ‘mamurra’ species group (Brown 1976), based on androconia shape, and the ‘pelopea’ species group (Wakeham-Dawson and Dennis 2001), on account of the shape of male genitalia. Pseudochazara atlantis is also distinctive according to the TCS analysis and forms a separate network. In addition, the second basal split within Pseudochazara is well supported, and, apart from some single species clades, three species groups tentatively named as the ‘pelopea’, ‘hippolyte’ and ‘mamurra’ clades received high support. We present the results for these clades separately:
Figure 5.
Phylogeny of Pseudochazara species derived from the barcoding gene COI using Bayesian inference analysis. Values on major branches are Bayesian posterior probabilities. Branches with support lower than 50% were collapsed manually. Branch names combine taxon name and sample ID (see Appendix 1). Nomenclature follows Lukhtanov (2007).
‘Pelopea’ group
This group, which forms a distinct network in the TCS analysis (Fig. 2), includes two species, Pseudochazara pelopea and Pseudochazara mniszechii. However, there is no genetic differentiation between them, with Pseudochazara pelopea persica and Pseudochazara pelopea caucasica intermixed with Pseudochazara mniszechii. Two well supported clades pertain to geographically isolated subspecies of Pseudochazara pelopea, the Levant region (nominotypic Pseudochazara pelopea pelopea) and Kopet Dhag in NE Iran (Pseudochazara pelopea tekkensis). Both subspecies are morphologically distinct from Pseudochazara pelopea persica, in particular the latter, with much wider and more pronounced orange submarginal bands on their forewings. Pseudochazara pelopea tekkensis is considered a separate species by Nazari (2003). Pseudochazara mniszechii is also polyphyletic due to the separate position of the subspecies tisiphone from the southern Balkans, which is clearly not closely related, and belongs to the ‘hippolyte’ group.
‘Hippolyte’ group
The ‘hippolyte’ clade sensu stricto includes the widely distributed Pseudochazara hippolyte complex which has a vast range from southern Spain to central China (Tshikolovets 2011) together with a number of local endemics from the southern Balkan Peninsula: Pseudochazara cingovskii in the Republic of Macedonia, Pseudochazara orestes from north-eastern Greece and the neighbouring part of Bulgaria, Pseudochazara mniszechii tisiphone from north-western Greece and southern Albania and Pseudochazara euxina from the Crimean Peninsula. Both, the haplotype network analysis (Fig. 3) and the phylogeny (Fig. 5) show that Pseudochazara mniszechii tisiphone is not a subspecies of Pseudochazara mniszechii despite superficial resemblance in wing patterns and coloration. In fact, it is closely related to two other local endemics from the Balkan Peninsula, Pseudochazara cingovskii and Pseudochazara orestes. The presence of Pseudochazara mniszechii tisiphone in the western part of Turkey, near Bursa (Hesselbarth et al. 1995) remains to be verified. The single haplotype of Pseudochazara euxina is nestled among samples of Pseudochazara hippolyte, so our preliminary results do not support its current status as a separate species. Within this clade Pseudochazara hippolyte williamsi from southern Spain appears basally, however with low posterior probability and it is not monophyletic. All other described subspecies (Pseudochazara hippolyte pallida, Pseudochazara hippolyte doerriesi, Pseudochazara hippolyte mercurius) are less distinct from the nominotypical subspecies, with two Central Asiatic subspecies (Pseudochazara hippolyte pallida, Pseudochazara hippolyte mercurius) sharing haplotypes.
Figure 3.
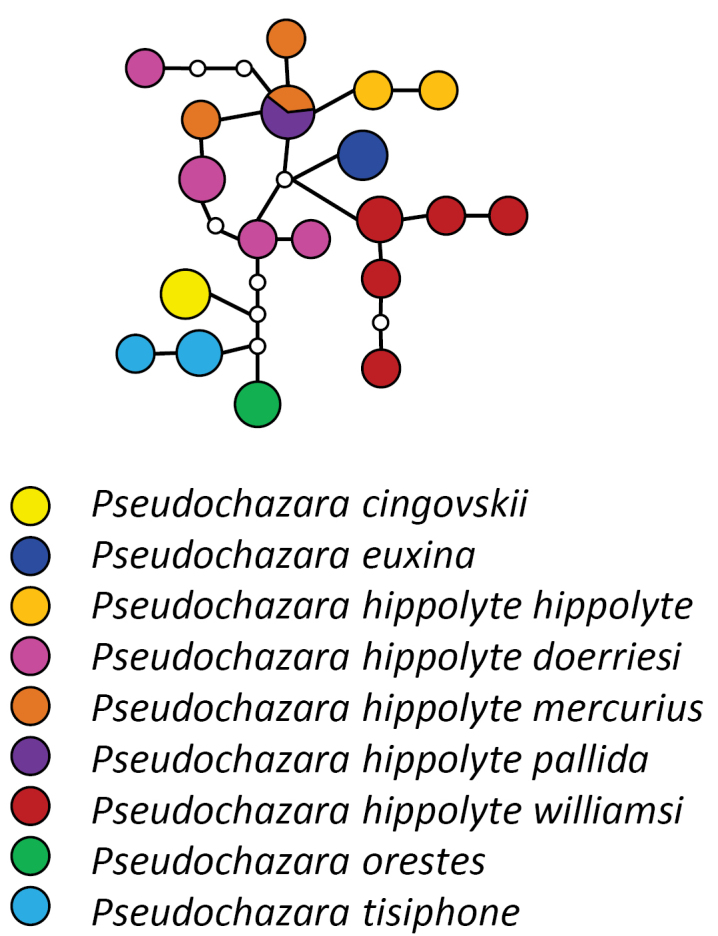
Statistical Parsimony network of the ‘hippolyte’ species group. Coloured circles represent COI haplotypes and their size corresponds to the number of samples per haplotype. Small white circles represent unsampled haplotypes.
The sister relationship of Pseudochazara thelephassa and Pseudochazara anthelea, which is indicated by genital morphology (the presence of a distinct costal process on the dorsal side of the valve) and wing pattern (the presence of a well-defined black area in the forewing discal cell) (Aussem 1980b, Hesselbarth et al. 1995, Wakeham-Dawson and Dennis 2001), could not be corroborated as Pseudochazara anthelea appears to be a sister clade to the ‘hippolyte’ group sensu strictu with high posterior probability. Pseudochazara kanishka from Tajikistan is a sister species of the anthelea-hippolyte clade, while Pseudochazara thelephassa is sister taxon to the anthelea-hippolyte-kanishka clade, however, with low support. These results concur with wing pattern, i.e. a well-defined black area in the forewing discal cell, also present in specimens of Pseudochazara kanishka.
It is important to note that the average genetic distance between two geographically separated subspecies, Pseudochazara anthelea anthelea from Asia Minor and neighbouring islands, and Pseudochazara anthelea amalthea from the Balkan Peninsula was 1.5%. This result is indicative for differentiation into distinct species as predicted by Kudrna et al. (2011).
In the TCS analysis, this group is split into 3 networks: a) the hippolyte clade sensu stricto (Fig. 3), b) Pseudochazara anthelea, and c) Pseudochazara thelephassa.
‘Mamurra’ group
The only two entirely Central Asian species available for analysis, Pseudochazara turkestana and Pseudochazara lehana, form a well-supported clade together with the ‘mamurra’ group, indicating their close relationship, but with a separate network for each in the TCS analysis. All other sequences form a single network (Fig. 4). Although the species sampling in Central Asia is incomplete, there is no evidence of a deep split between Asiatic and European/African taxa as predicted by Wakeham-Dawson and Dennis (2001). The ‘mamurra’ group is monophyletic, and includes several well-defined species (in terms of wing patterns, androconia and genitalia) with identical or very similar haplotypes. The following taxa could not be distinguished based on COI haplotypes as they do not form separate monophyletic clades: Pseudochazara mamurra, Pseudochazara beroe, Pseudochazara geyeri, Pseudochazara daghestana, Pseudochazara alpina, and Pseudochazara lydia. Only a single sequence was obtained for Pseudochazara geyeri and Pseudochazara lydia, so their position within this group is tentative. However, it is clear that Pseudochazara lydia is closely related to Pseudochazara mamurra with which it shares similarities e.g. the shape of the androconia (Wakeham-Dawson 2005). Pseudochazara alpina shares the haplotype with Pseudochazara beroe and they appear closely related, however, this is again based on the inclusion of a single sequence.
Within the ‘mamurra’ group the only well supported clade includes the taxa Pseudochazara schahkuhensis, Pseudochazara mamurra kermana, Pseudochazara graeca and Pseudochazara mamurra amymone. While Pseudochazara schahkuhensis is sympatric in part of its range with Pseudochazara mamurra, all other taxa have geographically isolated ranges. Pseudochazara graeca and Pseudochazara mamurra amymone are present in the southern part of the Balkan Peninsula with partial range overlap (Pamperis 2009). Both species are clearly morphologically distinct, but genetically not identifiable in COI haplotypes. Clearly this relationship puts in question the status of Pseudochazara mamurra amymone as a subspecies of Pseudochazara mamurra. The same conclusion can be drawn for Pseudochazara mamurra kermana from Iran (Kerman province), which is also well placed within this clade as a sister species to both southern Balkan Peninsula taxa.
Discussion
Our study supports the monophyly of the genus Pseudochazara with high posterior probability values of the COI gene tree. Within the genus, however, two conflicting patterns appear with, unexpectedly, deep divergences between presumably conspecific taxa on the one hand and lack of divergence among well-defined species on the other. This is to some extent concordant with similar studies in related genera in the subfamily Satyrinae (Kodandaramaiah and Wahlberg 2009, Nazari et al. 2010, Kreuzinger et al. 2014). The basal position of Pseudochazara atlantis from North-western Africa as sister group to all remaining Pseudochazara species falls into the first category. Based on distinct male genitalia morphology and wing shape/patterns Pseudochazara anthelea and Pseudochazara thelephassa were considered to form the basal split within the genus (Gross 1978, Aussem 1980b, Hesselbarth et al. 1995, Wakeham-Dawson and Dennis 2001). The basal position of Pseudochazara atlantis is difficult to explain in terms of biogeography, as it indicates a North African origin of the genus, which has its centre of divergence much further eastwards in the Middle East (Hesselbarth et al. 1995, Tshikolovets 2011). Pseudochazara atlantis is an alpine species distributed only in the Atlas Mountains of Morocco (Tennent 1996), therefore its isolation from the main distribution of the genus could possibly have preceded the last land bridge connections with Europe at the end of the Miocene (Garcia-Castellanos et al. 2009). Hence, its basal position could be an artefact of long-branch attraction (Bergsten 2005) and/or incomplete sampling of the entirely Asiatic species. Therefore, confirmation with additional genetic markers and additional sampling is required.
Another unexpected result is a deep split between Pseudochazara mniszechii and Pseudochazara mniszechii tisiphone, species which are very similar in wing patterns/coloration and considered conspecific in current literature (Hesselbarth et al. 1995, Kudrna et al. 2011, Tshikolovets 2011, Eckweiler 2012) and databases (Lukhtanov 2007, Savela 2015, Fauna Europaea 2016). Based on the COI gene tree Pseudochazara tisiphone Brown, 1980 (stat. n.) is a separate species closely related to two local endemics from the southern part of the Balkan Peninsula, Pseudochazara orestes and Pseudochazara cingovskii. Actually Pseudochazara tisiphone was originally described as a subspecies of Pseudochazara cingovskii (Brown 1980) and its close relationship was hypothesised also by Wakeham-Dawson and Dennis (2001) based on the similarity of the male genitalia. The low level of genetic differentiation between Pseudochazara tisiphone, Pseudochazara orestes, and Pseudochazara cingovskii indicates a relatively recent speciation, however, we are inclined towards supporting their separate species status based on constant differences in wing patterns/coloration and also their ecological specialization (Pamperis 2009, Verovnik et al. 2013).
A split between Pseudochazara anthelea anthelea from Asia Minor and Pseudochazara anthelea amalthea from the Balkan Peninsula has been suggested based on minor differences in male genitalia and consistent differences in female wing coloration between both taxa (Olivier 1996, Wakeham-Dawson and Dennis 2001). They are considered separate morphospecies by Kudrna et al. (2011). We can agree with separate species status as the split between the two taxa is much older compared to almost no differentiation in three morphologically and ecologically well defined species: Pseudochazara tisiphone, Pseudochazara orestes, and Pseudochazara cingovskii. Following this reasoning, Pseudochazara pelopea tekkensis from NE Iran could also be considered a distinct species, however, inclusion of more samples is needed to confirm this status.
Given the high resolution of the basal clades within the COI gene tree, the lack of differentiation between taxa within the ‘mamurra’ and ‘pelopea’ group was unexpected. In particular, species like Pseudochazara geyeri and Pseudochazara daghestana are among the most easily recognisable species in the genus with uniform and very distinct wing patterns/coloration. There are several possible hypotheses to explain this lack of differentiation:
– Incomplete lineage sorting: recent speciation could result in unresolved relationships among these closely related species; however, well-defined species borders in terms of constant wing pattern differentiation coupled with broad overlaps in species ranges challenges this hypothesis.
– Recent gene flow: gene flow between closely related taxa is a known phenomenon (Descimon and Mallet 2009) and masks relationships among species especially with mitochondrial DNA (Gompert et al. 2008). The species involved have broadly overlapping ranges and could sometimes be found syntopic (Aussem 1980c, Hesselbarth et al. 1995), so hybridization is possible. Actually hybridization is documented even among the most distantly related species such as Pseudochazara anthelea and Pseudochazara geyeri (Aussem, 1980c). Nuclear markers with higher genetic resolution (e.g. microsatellites, SNPs) would be required to study the contact zones between these taxa to confirm ongoing gene flow. It must be noted that partial exclusion is evident when two or more Pseudochazara species are syntopic, as one is always dominant, while the others appear in very low frequencies (Hesselbarth et al. 1995, Verovnik et al. 2014).
– Pseudogenes or Wolbachia infections: both are common in invertebrates, particularly in arthropods (Bensasson et al. 2011, Gerth et al. 2014, Leite 2012, Ritter et al. 2013). As the vast majority of the haplotypes in the ‘mamurra’ and ‘pelopea’ clades originate from the BOLD database it is impossible to check or correct for this potential error.
The most enigmatic taxon among the 'mamurra' group is Pseudochazara mamurra amymone from northern Greece and Albania (Eckweiler 2012, Verovnik et al. 2014). Apart from the author’s original description (Brown 1976) little has been published regarding this elusive taxon for a long time. Failed attempts to locate the vaguely described type locality (Cuvelier 2010) have led to several misleading hypotheses, resulting in speculation that it may even be a rare hybrid between Pseudochazara tisiphone and Pseudochazara anthelea (Wakeham-Dawson and Dennis 2001, Kudrna et al. 2011). Somewhat surprisingly, the COI gene tree suggests it has a close relationship with Pseudochazara graeca, another species from the southern Balkan Peninsula. These two taxa have distinct and constant wing patterns and differ in their habitat requirements, with Pseudochazara mamurra amymone inhabiting steep and hot rocky gorges at lower elevations (Gascoigne-Pees et al. 2014) while Pseudochazara graeca is predominantly a montane (high elevation) species endemic to Greece (Anastassiu et al. 2009). Thus, despite paraphyly of Pseudochazara amymone Brown, 1976 (stat. n.) in relation to Pseudochazara graeca, we believe they both represent valid species within the ‘mamurra’ group. Consequently Pseudochazara kermana Eckweiler, 2004 (stat. n.), sister species to Pseudochazara amymone and Pseudochazara graeca combined, should also be elevated to species rank, although additional populations of Pseudochazara mamurra in Iran should be examined to confirm this status. Alternatively, all the taxa within the ‘mamurra’ group, including the monophyletic Pseudochazara schakuhensis, a sister species to the amymone-graeca-kermana clade, should be treated as a single very polymorphic species, a rather more destructive approach given the current taxonomy.
Although we are aware of the pitfalls of using single gene trees in the interpretation of phylogenetic patterns (Nichols 2001), we believe that strongly supported basal branching and splits between taxa, considered conspecific, represent valid insights into speciation in the Pseudochazara genus and together with distinct morphology and ecology allows species delimitation. Hence, we propose separate species status for the following taxa: Pseudochazara tisiphone, Pseudochazara amalthea, Pseudochazara amymone, and Pseudochazara kermana. This has important conservation implications, as most of these species are local endemics and therefore potentially threatened (Verovnik et al. 2014). Wider taxon sampling and inclusion of nuclear markers would undoubtedly help to a better understanding of the taxonomy of this fascinating butterfly genus.
Acknowledgments
We would like to express our gratitude to Wolfgang Eckweiler for his identification of several specimens from voucher photos housed in the BOLD database and we thank Evgeny V. Zakharov, Vlad Dinca and Axel Hausmann for their agreement to use unpublished DNA sequences from their projects in the BOLD database. We are thankful to our colleagues Tarkan Soyhan, Filip Franeta, Dubi Benyamini and Joseph Verhulst for providing additional samples of Pseudochazara for DNA analysis and Martin Gascoigne-Pees for checking the English. We also thank Niklas Wahlberg and an anonymous reviewer for helpful comments to improve the manuscript.
Appendix 1
Table 1.
List of samples of the genus Pseudochazara included in the barcoding analysis (either own samples with “LA” ID or from BOLD).
| ID | GenBank | Species | Location | Lat | Long | Date | Legit |
|---|---|---|---|---|---|---|---|
| LA16 | KU499958 | Pseudochazara mamurra amymone | Baboshtice, Körce, Albania | 40°31.038'N | 20°47.647'E | 11.vii.2012 | Rudi Verovnik |
| LA17 | KU499959 | Pseudochazara mniszechii tisiphone | Baboshtice, Körce, Albania | 40°31.038'N | 20°47.647'E | 11.vii.2012 | Rudi Verovnik |
| LA19 | KU499960 | Pseudochazara mamurra amymone | Devoll Gorge, Körce, Albania | 40°42.576'N | 20°31.446'E | 10.vii.2012 | Rudi Verovnik |
| LA24 | KU499961 | Pseudochazara cingovskii | Pletvar Pass, Prilep, Macedonia | 41°22.456'N | 21°38.805'E | 14.vii.2010 | Rudi Verovnik |
| LA28 | KU499962 | Pseudochazara mniszechii | Sivas, Turkey | 39°41.519'N | 36°59.877'E | 22.vii.2009 | Tarkan Soyhan |
| LA29 | KU499963 | Pseudochazara mniszechii | Eskişehir, Turkey | 39°43.801'N | 30°31.428'E | 16.vi.2007 | Tarkan Soyhan |
| LA75 | KU499964 | Pseudochazara geyeri occidentalis | Galičica Pass, Macedonia | 40°57.379'N | 20°48.961'E | 30.vii.2013 | Filip Franeta |
| LA76 | KU499965 | Pseudochazara orestes | Falakro Mt., Greece | 41°16.138'N | 24°3.947'E | 7.vii.2013 | Filip Franeta |
| LA77 | KU499966 | Pseudochazara graeca | Katara Pass, Metsova, Greece | 39°47.580'N | 21°12.272'E | 22.vii.2012 | Filip Franeta |
| LA78 | KU499967 | Pseudochazara orestes | Granitis, Drama,Greece | 41°18.533'N | 23°54.862'E | 27.vii.2013 | Rudi Verovnik |
| LA79 | KU499968 | Pseudochazara graeca | Katara Pass, Metsova, Greece | 39°47.580'N | 21°12.272'E | 26.vii.2013 | Rudi Verovnik |
| LA80 | KU499969 | Pseudochazara mniszechii tisiphone | Drenovë, Korcë, Albania | 40°35.352'N | 20°48.508'E | 21.vii.2013 | Rudi Verovnik |
| LA81 | KU499970 | Pseudochazara mniszechii tisiphone | Drenovë, Korcë, Albania | 40°35.352'N | 20°48.508'E | 21.vii.2013 | Rudi Verovnik |
| LA82 | KU499971 | Pseudochazara pelopea | Mt. Hermon, Israel | 33°19.766'N | 35°47.243'E | 2013 | Dubi Benyamini |
| LA83 | KU499972 | Pseudochazara pelopea | Mt. Hermon, Israel | 33°19.766'N | 35°47.243'E | 2013 | Dubi Benyamini |
| LA84 | KU499973 | Pseudochazara cingovskii | Pletvar Pass, Prilep, Macedonia | 41°22.456'N | 21°38.805'E | 2013 | Filip Franeta |
| LA85 | KU499974 | Pseudochazara cingovskii | Pletvar Pass, Prilep, Macedonia | 41°22.456'N | 21°38.805'E | 2013 | Filip Franeta |
| LA86 | KU499975 | Pseudochazara anthelea amalthea | Veles, Topolka, Macedonia | 41°41.915'N | 21°46.927'E | 2010 | Filip Franeta |
| LA87 | KU499976 | Pseudochazara anthelea amalthea | Mt. Parnassos, Greece | 38°31.233'N | 22°36.566'E | 2010 | Filip Franeta |
| LA88 | KU499977 | Pseudochazara anthelea amalthea | Drenovë, Korcë, Albania | 40°35.352'N | 20°48.508'E | 2013 | Filip Franeta |
| LA89 | KU499978 | Pseudochazara mamurra birgit | Mt. Aladaglar, Turkey | 37°47.568'N | 35°9.242'E | 2006 | Filip Franeta |
| LA90 | KU499979 | Pseudochazara mniszechii | Mt. Aladaglar, Turkey | 37°47.568'N | 35°9.242'E | 2006 | Filip Franeta |
| LA92 | KU499980 | Pseudochazara graeca | Mt. Iti, Greece | 38°49.333'N | 22°16.635'E | 1999 | Filip Franeta |
| LA94 | KU499981 | Pseudochazara mamurra amymone | Drenovë, Korcë, Albania | 40°35.352'N | 20°48.508'E | 2013 | Filip Franeta |
| LA95 | KU499982 | Pseudochazara mamurra amymone | Devoll Gorge, Körce, Albania | 40°42.576'N | 20°31.446'E | 2013 | Filip Franeta |
| LA97 | KU499983 | Pseudochazara lydia obscura | Mersin, Turkey | 36°57.017'N | 34°23.019'E | 12.vii.2010 | Tarkan Soyhan |
| LA124 | KU499984 | Pseudochazara lehana | Saabo Digur La, Ladakh, India | 34°10.554'N | 77°39.529'E | 15.vii.2013 | Joseph Verhulst |
| BPAL1699–12 | Pseudochazara mamurra | Azerbaijan: near Shamkir, 1300 m | 40.6989 | 45.8697 | 31.vii.2011 | Tikhonov V. | |
| BPAL1700–12 | Pseudochazara mamurra | Azerbaijan: near Shamkir, 1300 m | 40.6989 | 45.8697 | 31.vii.2011 | Tikhonov V. | |
| BPAL1703–12 | Pseudochazara alpina | Russia: North Ossetia-Alania, rv. Ardon, Skasan, 1850 m | 42.6956 | 43.9989 | 12.viii.2011 | Tikhonov V. | |
| BPAL2136–13 | Pseudochazara kanishka | Tajikistan: Khodra-Mumin Mnt. | 26.v.2001 | A. Petrov | |||
| BPAL2137–13 | Pseudochazara kanishka | Tajikistan: Khodra-Mumin Mnt. | 26.v.2001 | A. Petrov | |||
| BPAL2138–13 | Pseudochazara thelephassa | Iran: Char Mahall-o-Bahtiyari, Sahr-e-Kord, 2000 m | 28.v.2002 | P. Hofmann | |||
| BPAL2139–13 | Pseudochazara thelephassa | Iran: Kerman, Kuh-e-Madvar, 5 km S Jowzan, 2400–2600 m | 24.v.2002 | P. Hofmann | |||
| BPAL2140–13 | Pseudochazara thelephassa | Iran: Kerman, Kuh-e-Segoch, Mahan Pass, 2400–2600 m | 21.v.2002 | P. Hofmann | |||
| BPAL2141–13 | Pseudochazara dagestana savalanica | Iran: Azarbayjan-e-Sharqi, N Taran, Kuh-e-Sabalan, 2900–3000 m | 10.vii.2001 | Westphal | |||
| BPAL2142–13 | Pseudochazara dagestana savalanica | Iran: Azarbayjan-e-Sharqi, N Taran, Kuh-e-Sabalan, 2900–3000 m | 10.vii.2001 | Westphal | |||
| BPAL2145–13 | Pseudochazara hippolyte mercurius | China: Xinjiang, Tian Shan, Borohoro Shan, 40 km SSW Kytun, 1850–2050 m | 44.0939 | 84.7942 | 08.vii.2006 | Grieshuber | |
| BPAL2147–13 | Pseudochazara mamurra kermana | Iran: Kerman, Kuh-e-Madvar, 5 km S Jowzan, 2200–2400 m | 28.v.1999 | P. Hofmann | |||
| BPAL2152–13 | Pseudochazara schahkuhensis | Iran: Khorasan, Kopet Dagh, 15 km E Emam Qoli, N Quchan, 2100–2200 m | 19.vi.2001 | P. Hofmann | |||
| BPAL2153–13 | Pseudochazara schahkuhensis | Iran: Khorasan, Kopet Dagh, Qoucan, 1800 m | 13.vii.2000 | Hacz-Köszegi | |||
| BPAL2154–13 | Pseudochazara schahkuhensis | Iran: Khorasan, Kopet Dagh, Qoucan, 1800 m | 14.vii.2000 | Hacz-Köszegi | |||
| BPAL2155–13 | Pseudochazara schahkuhensis | Iran: Khorasan, Kopet Dagh, Qoucan, 1800 m | 15.vii.2000 | Hacz-Köszegi | |||
| BPAL2156–13 | Pseudochazara mamurra schahrudensis | Iran: Tehran, Elburs, Tuchal, 2400–2600 m | 16.vi.2001 | P. Hofmann | |||
| BPAL2158–13 | Pseudochazara mamurra schahrudensis | Iran: Tehran, Elburs, Tuchal, 2400–2600 m | 16.vi.2001 | P. Hofmann | |||
| BPAL2159–13 | Pseudochazara mamurra schahrudensis | Iran: Tehran, Elburs, Tuchal, 2400–2600 m | 16.vi.2001 | P. Hofmann | |||
| BPAL2160–13 | Pseudochazara mamurra mamurra | Turkey: Artvin, Kilickaya, 1100–1200 m | 01.vi.1998 | P. Hofmann | |||
| BPAL2162–13 | Pseudochazara mamurra mamurra | Turkey: Erzurum, Dikmen, SW Üzundere, 1300 m | 16.vii.1998 | P. Hofmann | |||
| BPAL2172–13 | Pseudochazara mamurra sintenisi | Turkey: Bayburt, 5 km N Bayburt, 1500 m | 10.vii.1998 | P. Hofmann | |||
| BPAL2173–13 | Pseudochazara mamurra sintenisi | Turkey: Erzincan, 5 km SE Caglayan, 1400 m | 08.vii.1998 | P. Hofmann | |||
| BPAL2174–13 | Pseudochazara mamurra sintenisi | Turkey: Gümüshane, Demirkaynak, 13 km SW Torul, 1100 m | 06.vii.1998 | P. Hofmann | |||
| BPAL2175–13 | Pseudochazara mniszechii caucasica | Turkey: Bayburt, 5 km N Bayburt, 1500 m | 10.vii.1998 | P. Hofmann | |||
| BPAL2176–13 | Pseudochazara mniszechii caucasica | Turkey: Erzincan, 5 km SE Caglayan, 1400 m | 08.vii.1998 | P. Hofmann | |||
| BPAL2177–13 | Pseudochazara mniszechii caucasica | Turkey: Erzurum, road Bayburt-Ispir, Laleli, 1300–1400 m | 11.vii.1998 | P. Hofmann | |||
| BPAL2178–13 | Pseudochazara pelopea persica | Iran: Char Mahall-o-Bahtiyari, Sahr-e-Kord, 2000 m | 28.v.2002 | P. Hofmann | |||
| BPAL2179–13 | Pseudochazara pelopea persica | Iran: Kerman, Kuh-e-Madvar, 5 km S Jowzan, 2400–2600 m | 24.v.2002 | P. Hofmann | |||
| BPAL2180–13 | Pseudochazara pelopea persica | Iran: Kerman, Kuh-e-Madvar, 5 km S Jowzan, 2400–2600 m | 24.v.2002 | P. Hofmann | |||
| BPAL2181–13 | Pseudochazara pelopea tekkensis | Iran: Khorasan, Kopet Dagh, 15 km E Emam Qoli, N Quchan, 2100–2200 m | 19.vi.2001 | P. Hofmann | |||
| BPAL2182–13 | Pseudochazara beroe aurantiaca | Iran: Tehran, Elburs, 15 km NE Firuzkuh pass, 1300–2400 m | 24.vii.2000 | P. Hofmann | |||
| BPAL2183–13 | Pseudochazara beroe aurantiaca | Iran: Mazandaran, Khosh-Yeylaq, 65 km NE Shahrud, 2000–2100 m | 23.vi.2001 | P. Hofmann | |||
| BPAL2185–13 | Pseudochazara beroe aurantiaca | Iran: Khorasan, Kopet Dagh, 15 km E Emam Qoli, N Quchan, 2100–2200 m | 19.vi.2001 | P. Hofmann | |||
| BPAL2245–13 | Pseudochazara pelopea pelopea | Israel | 22.vi.2013 | V.A.Lukhtanov & A.V.Novikova | |||
| BPAL2246–13 | Pseudochazara pelopea pelopea | Israel | 22.vi.2013 | V.A.Lukhtanov & A.V.Novikova | |||
| BPAL2247–13 | Pseudochazara pelopea pelopea | Israel | 22.vi.2013 | V.A.Lukhtanov & A.V.Novikova | |||
| BPAL2281–14 | Pseudochazara pelopea pelopea | Syria: Bloudan, 1500 m | 16.vii.1999 | A, Salk | |||
| BPAL2282–14 | Pseudochazara pelopea pelopea | Syria: Bloudan, 1500 m | 16.vii.1999 | A, Salk | |||
| BPAL2692–14 | Pseudochazara pelopea pelopea | Israel | 03.vii.2014 | V.Lukhtanov & A. Novikova | |||
| BPAL2701–14 | Pseudochazara pelopea pelopea | Israel | 03.vii.2014 | V.Lukhtanov & A. Novikova | |||
| BPAL2702–14 | Pseudochazara pelopea pelopea | Israel | 03.vii.2014 | V.Lukhtanov & A. Novikova | |||
| BPAL2728–14 | Pseudochazara pelopea pelopea | Israel | 04.vii.2014 | V.Lukhtanov | |||
| BPAL2731–14 | Pseudochazara pelopea pelopea | Israel | 04.vii.2014 | V.Lukhtanov | |||
| EULEP451–14 | Pseudochazara euxina | Ukraine | 11.vii.2007 | local collector | |||
| EULEP452–14 | Pseudochazara euxina | Ukraine | 11.vii.2007 | local collector | |||
| EULEP453–14 | Pseudochazara euxina | Ukraine | 11.vii.2007 | local collector | |||
| EULEP487–14 | Pseudochazara hippolyte hippolyte | Russia | 52.65 | 59.5667 | 23.vii.1998 | K. Nupponen | |
| EULEP488–14 | Pseudochazara hippolyte hippolyte | Russia | 51.8 | 57.0833 | 14.vii.1998 | K. Nupponen | |
| EZHBA660–07 | Pseudochazara doerriesi | Russia | 51.717 | 94.4 | 17.vii.2000 | Oleg Kosterin | |
| EZHBA661–07 | Pseudochazara doerriesi | Russia | 51.717 | 94.4 | 17.vii.2000 | Oleg Kosterin | |
| EZHBA662–07 | Pseudochazara doerriesi | Russia | 51.717 | 94.4 | 17.vii.2000 | Oleg Kosterin | |
| EZHBA899–07 | Pseudochazara doerriesi | Russia | 51.7667 | 91.9333 | 30.vi.2004 | Oleg Kosterin | |
| EZHBA900–07 | Pseudochazara doerriesi | Russia | 51.7667 | 91.9333 | 30.vi.2004 | Oleg Kosterin | |
| EZROM089–08 | HQ004207 | Chazara briseis | Romania: Transylvania: Suatu | 46.783 | 23.95 | 16.viii.2006 | Dinca Vlad |
| EZROM848–08 | HQ004205 | Chazara briseis | Romania: Transylvania: Suatu | 46.799 | 23.959 | 16.viii.2006 | Dinca Vlad |
| EZSPM470–09 | GU676107 | Pseudochazara hippolyte | Spain: Granada: San Juan (Sierra Nevada) | 37.094 | -3.115 | 16.vii.2009 | Dinca V. |
| EZSPN732–09 | GU676410 | Pseudochazara hippolyte | Spain: Granada: Laguna Seca, Hueneja | 37.097 | -2.97 | 18.vii.2008 | S. Montagud , J. A. Garcia-Alama & J. Garcia |
| EZSPN733–09 | GU676411 | Pseudochazara hippolyte | Spain: Granada: Laguna Seca, Hueneja | 37.097 | -2.97 | 18.vii.2008 | S. Montagud , J. A. Garcia-Alama & J. Garcia |
| EZSPN735–09 | GU676413 | Pseudochazara hippolyte | Spain: Granada: Laguna Seca, Hueneja | 37.097 | -2.97 | 18.vii.2008 | S. Montagud , J. A. Garcia-Alama & J. Garcia |
| EZSPN736–09 | GU676406 | Pseudochazara hippolyte | Spain: Granada: Laguna Seca, Hueneja | 37.097 | -2.97 | 18.vii.2008 | S. Montagud , J. A. Garcia-Alama & J. Garcia |
| EZSPN791–09 | GU676354 | Pseudochazara hippolyte | Spain: Granada: North-East Granada province | 37.097 | -2.97 | 23.vii.2008 | Gil, Felipe |
| GWOSF831–10 | JF850408 | Pseudochazara anthelea anthelea | Cyprus | 34.9559 | 32.9951 | 05.vi.2010 | M. Seizmair |
| IRANB276–08 | Pseudochazara beroe beroe | Iran | 38.583 | 44.367 | 29.vii.2002 | Vazrick Nazari | |
| IRANB278–08 | Pseudochazara beroe beroe | Iran | 38.583 | 44.367 | 29.vii.2002 | Vazrick Nazari | |
| IRANB279–08 | Pseudochazara beroe beroe | Iran | 37.776 | 46.445 | 22.vi.2001 | Vazrick Nazari | |
| IRANB285–08 | Pseudochazara beroe aurantiaca | Iran | 36.12 | 51.2 | 16.viii.2000 | Vazrick Nazari | |
| IRANB292–08 | Pseudochazara pelopea persica | Iran | 34.603 | 47.055 | 01.vii.2001 | Vazrick Nazari | |
| LOWA019–06 | FJ663351 | Chazara enervata | Kazakhstan: Tienschan: Kurdai Pass | 43.333 | 74.95 | 11.vi.2000 | V.Lukhtanov |
| LOWA021–06 | FJ663349 | Chazara enervata | Kazakhstan: Tienschan: Kurdai Pass | 43.333 | 74.95 | 11.vi.2000 | V.Lukhtanov |
| LOWA022–06 | FJ663347 | Chazara briseis magna | Kazakhstan: Tienschan: Kurdai Pass | 43.333 | 74.95 | 11.vi.2000 | V.Lukhtanov |
| LOWA024–06 | FJ664025 | Pseudochazara turkestana turkestana | Kazakhstan: Tienschan: Kurdai Pass | 43.333 | 74.95 | 11.vi.2000 | V.Lukhtanov |
| LOWA150–06 | FJ664021 | Pseudochazara hippolyte pallida | Russia | 50.1 | 88.417 | 07.vii.1999 | V.Lukhtanov |
| LOWA315–06 | FJ663353 | Chazara heydenreichi | Kazakhstan: Ust-Kamenogorsk Region: Kendyrlik | 47.5 | 85.183 | 14.vii.1997 | V. Lukhtanov |
| LOWA316–06 | FJ663352 | Chazara heydenreichi | Kazakhstan: Ust-Kamenogorsk Region: Kendyrlik | 47.5 | 85.183 | 14.vii.1997 | V. Lukhtanov |
| LOWA516–06 | FJ664024 | Pseudochazara turkestana turkestana | Kyrgyzstan: Gultcha distr.: Chiitala | 39.85 | 73.333 | 29.vii.1995 | V. Lukhtanov |
| LOWA517–06 | FJ664023 | Pseudochazara turkestana turkestana | Kyrgyzstan: Gultcha distr.: Chiitala | 39.85 | 73.333 | 29.vii.1995 | V. Lukhtanov |
| LOWA608–06 | FJ663348 | Chazara briseis maracandica | Uzbekistan: Kashkardarinskaya obl.: Tamshush | 38.967 | 67.4 | 20.vi.1994 | V. Lukhtanov |
| LOWA680–06 | FJ664020 | Pseudochazara hippolyte mercurius | Kazakhstan: Dzhambulskaya obl.: Kurdai Pass | 43.333 | 74.95 | 28.vi.1993 | V. Lukhtanov |
| LOWA681–06 | FJ664019 | Pseudochazara hippolyte mercurius | Kazakhstan: Dzhambulskaya obl.: Kurdai Pass | 43.333 | 74.95 | 28.vi.1993 | V. Lukhtanov |
| LOWA787–06 | FJ664018 | Pseudochazara hippolyte hippolyte | Kazakhstan | 47.4 | 83.917 | 22.vi.1997 | V. Lukhtanov |
| LOWA788–06 | FJ664022 | Pseudochazara turkestana tarbagata | Kazakhstan | 47.4 | 83.917 | 22.vi.1997 | V. Lukhtanov |
| LOWAB040–07 | Pseudochazara pelopea persica | Armenia | 40.083 | 44.917 | Andrei Sourakov | ||
| LOWAB041–07 | Pseudochazara pelopea persica | Armenia | 40.083 | 44.917 | Andrei Sourakov | ||
| LOWAB046–07 | Pseudochazara pelopea persica | Armenia | 40.083 | 44.917 | Andrei Sourakov | ||
| LOWAB046–07 | Pseudochazara pelopea caucasica | Armenia | 40.083 | 44.917 | Andrei Sourakov | ||
| LOWAB047–07 | Pseudochazara pelopea persica | Armenia | 40.083 | 44.917 | Andrei Sourakov | ||
| LOWAB048–07 | Pseudochazara pelopea persica | Armenia | 40.083 | 44.917 | Andrei Sourakov | ||
| WMB1212–13 | Pseudochazara atlantis | Morocco | 33.025 | -5.071 | 01.vii.2011 | Vila, R., Dinca, V. & Voda, R. | |
| WMB1213–13 | Pseudochazara atlantis | Morocco | 33.025 | -5.071 | 01.vii.2011 | Vila, R., Dinca, V. & Voda, R. | |
| WMB2163–13 | Pseudochazara atlantis | Morocco | 31.09 | -7.915 | 15.vii.2012 | Tarrier, Michel |
Citation
Verovnik R, Wiemers M (2016) Species delimitation in the Grayling genus Pseudochazara (Lepidoptera, Nymphalidae, Satyrinae) supported by DNA barcodes. ZooKeys 600: 131–154. doi: 10.3897/zookeys.600.7798
References
- Anastassiu HT, Coutsis JG, Ghavalas N. (2009) New data regarding the geographical distribution of Pseudochazara graeca in Greece, with notes about its wing coloration, the status of its ssp. coutsisi (= zagoriensis), as well as the supposed correlation between the HW underside ground colour and the geological character of the habitat in both P. graeca and Hyponephele lycaon (Lepidoptera: Nymphalidae, Satyrinae). Phegea 37: 135–145. http://www.phegea.org/Phegea/2009/Phegea37-4_135-145.pdf [Google Scholar]
- Aussem B. (1980a) Eine neue Satyride der Gattung Pseudochazara de Lesse, 1951 aus Afghanistan (Satyridae). Nota lepidopterologica 3: 5–15. [Google Scholar]
- Aussem B. (1980b) Zur Kenntnis der Androkonienfelder von Pseudochazara thelephassa (Geyer, 1827) und Pseudochazara anthelea (Hübner, 1824) (Lepidoptera, Satyridae). Entomofauna – Zeitschrift für Entomologie 17: 354–358. [Google Scholar]
- Aussem B. (1980c) Ein Freiland-Hybrid der Gattung Pseudochazara (Lep., Satyridae). Entomologische Zeitschrift mit Insektenbörse 90: 161–165. [Google Scholar]
- Bensasson D, Zhang X, Hartl DL, Hewitt GM. (2011) Mitochondrial pseudogenes: evolution’s misplaced witnesses. Trends in Ecology and Evolution 16: 314–321. doi: 10.1016/S0169-5347(01)02151-6 [DOI] [PubMed] [Google Scholar]
- Bergsten J. (2005) A review of long-branch attraction. Cladistics 21: 163–193. doi: 10.1111/j.1096-0031.2005.00059.x [DOI] [PubMed] [Google Scholar]
- BOLD (2015) Barcoding Life. http://www.barcodinglife.com [accessed 15.10.2015]
- Brower AVZ. (2006) Problems with DNA barcodes for species delimitation: ‘ten species’ of Astraptes fulgerator reassessed (Lepidoptera: Hesperiidae). Systematics and Biodiversity 4: 127–132. doi: 10.1017/S147720000500191X [Google Scholar]
- Brown J. (1976) A review of the genus Pseudochazara de Lesse, 1951 (Lepidoptera, Satyridae) in Greece. Entomologist’s Gazette 27: 85–90. [Google Scholar]
- Brown J. (1980) On the status of a little known satyrid butterfly from Greece. Entomologist’s Record and Journal of Variation 92: 280–281. [Google Scholar]
- Clement M, Posada D, Crandall KA. (2000) TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x [DOI] [PubMed] [Google Scholar]
- Descimon H, Mallet J. (2009) Bad species. In: Settele J, Shreeve TG, Konvicka M, Van Dyck H. (Eds) Ecology of Butterflies in Europe. Cambridge University Press, Cambridge, 219–249. [Google Scholar]
- Dinca V, Zakharov EV, Hebert PDN, Vila R. (2011) Complete DNA barcode reference library for a country’s butterfly fauna reveals high performance for temperate Europe. Philosophical Transactions of the Royal Society B: Biological Sciences 278: 347–355. doi: 10.1098/rspb.2010.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckweiler W. (2004) Die Verbreitung und subspezifische Gliederung von Pseudochazara mamurra (Herrich-Schäffer, [1846]) (Lepidoptera: Nymphalidae, Satyrinae). Nachrichten des Entomologischen Vereins Apollo, N.F. 25: 9–14. [Google Scholar]
- Eckweiler W. (2012) New discoveries of Pseudochazara mamurra amymone Brown, 1976 (Lepidoptera: Nymphalidae, Satyrinae). Nachrichten des Entomologischen Vereins Apollo, N.F. 33: 1–4. [Google Scholar]
- Eckweiler W, Rose K. (1988) Identität, Verbreitung und subspezifische Gliederung von Pseudochazara lydia (Staudinger, 1878) (Lepidoptera, Satyridae). Nachrichten des Entomologischen Vereins Apollo, N.F. 9: 213–223. [Google Scholar]
- Fauna Europaea. (2016) Fauna Europaea version 2.5. Web Service available online at http://www.faunaeur.org [accessed on 6.3.2016]
- Folmer OM, Black M, Hoeh R, Lutz R, Vrijehoek R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 5: 304–313. http://www.mbari.org/staff/vrijen/PDFS/Folmer_94MMBB.pdf [PubMed] [Google Scholar]
- Garcia-Castellanos D, Estrada F, Jiménez-Munt I, Gorini C, Fernàndez M, Vergés J, De Vicente R. (2009) Catastrophic flood of the Mediterranean after the Messinian salinity crisis. Nature 462: 778–781. doi: 10.1038/nature08555 [DOI] [PubMed] [Google Scholar]
- Gascoigne-Pees M, Verovnik R, Franeta F, Popović M. (2014) The lifecycle and ecology of Pseudochazara amymone (Brown, 1976) (Lepidoptera: Nymphalidae, Satyrinae). Nachrichten des Entomologischen Vereins Apollo, N.F. 35: 129–138. [Google Scholar]
- Gerth M, Gansauge MT, Weigert A, Bleidorn C. (2014) Phylogenomic analyses uncover origin and spread of the Wolbachia pandemic. Nature Communications 5: . doi: 10.1038/ncomms6117 [DOI] [PubMed] [Google Scholar]
- Gompert Z, Forister ML, Fordyce JA, Nice CC. (2008) Widespread mito-nuclear discordance with evidence for introgressive hybridization and selective sweeps in Lycaeides. Molecular Ecology 17: 5231–5244. doi: 10.1111/j.1365-294X.2008.03988.x [DOI] [PubMed] [Google Scholar]
- Gross FJ. (1978) Beitrag zur Systematik von Pseudochazara-Arten (Lep., Satyridae). Atalanta 9: 41–103. http://www.zobodat.at/pdf/Atalanta_9_0041-0103.pdf [Google Scholar]
- Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. (2004) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America 101: 14812–14817. doi: 10.1073/pnas.0406166101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbarth G, van Oorschot H, Wagener S. (1995) Die Tagfalter der Türkei unter Berücksichtigung der angrenzenden Länder. Selbstverlag Sigbert Wagener, Bocholt, Germany, 2201 pp. [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. doi: 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Kodandaramaiah U, Wahlberg N. (2009) Phylogeny and biogeography of Coenonympha butterflies (Nymphalidae: Satyrinae) – patterns of colonization in the Holarctic. Systematic Entomology 34: 315–323. doi: 10.1111/j.1365-3113.2008.00453.x [Google Scholar]
- Kreuzinger AJ, Fiedler K, Letsch H, Grill A. (2014) Tracing the radiation of Maniola (Nymphalidae) butterflies: new insights from phylogeography hint at one single incompletely differentiated species complex. Ecology and Evolution 18: 1153–1161. doi: 10.1002/ece3.1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrna O, Harpke A, Lux K, Pennerstorfer J, Schweiger O, Settele J, Wiemers M. (2011) Distribution atlas of butterflies in Europe. Gesellschaft für Schmetterlingsschutz, Halle, 576 pp. [Google Scholar]
- Lorković Z. (1974) Die Verteilung der Varibilität von Hipparchia statilinus Hufn. (Lepid., Satyridae) in Beziehung zum Karstboden des ostadratischen Küstenlandes. Acta entomologica Jugoslavica 10: 41–53. [Google Scholar]
- Leite LAR. (2012) Mitochondrial pseudogenes in insect DNA barcoding: differing points of view on the same issue. Biota Neotropica 12: 301–308. doi: 10.1590/S1676-06032012000300029 [Google Scholar]
- Lukhtanov VA. (2007) Nymphalidae: Satyrinae. In: Global Butterfly Names Project. Global Butterfly Names – http://www.ucl.ac.uk/taxome/gbn/ [accessed 16.1.2015]
- Lukhtanov VA, Novikova AV. (2015) Interpretation of mitochondrial diversity in terms of taxonomy: a case study of Hyponephele lycaon species complex in Israel (Lepidoptera, Nymphalidae, Satyrinae). ZooKeys 538: 21–34. doi: 10.3897/zookeys.538.6689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari V. (2003) Butterflies of Iran. Dayereye Sabz publications, Tehran, 564 pp. [Google Scholar]
- Nazari V, Sperling FAH. (2007) Mitochondrial DNA divergence and phylogeography in western Palaearctic Parnassiinae (Lepidoptera: Papilionidae): How many species are there? Insect Systematics & Evolution 38: 121–138. doi: 10.1163/187631207788783996 [Google Scholar]
- Nazari V, Ten Hagen W, Bozano GC. (2010) Molecular systematics and phylogeny of the ‘Marbled Whites’ (Lepidoptera: Nymphalidae, Satyrinae, Melanargia Meigen). Systematic Entomology 35: 132–147. doi: 10.1111/j.1365-3113.2009.00493.x [Google Scholar]
- Nichols R. (2001) Gene trees and species trees are not the same. Trends in Ecology & Evolution 16: 358–364. doi: 10.1016/S0169-5347(01)02203-0 [DOI] [PubMed] [Google Scholar]
- Olivier A. (1996) Notes on the taxonomic status and supposed biogeographic affinity of the Pseudochazara anthelea (Hübner, [1824]) populations from Kípros (Cyprus) and from the Greek island of Kós (Lepidoptera: Nymphalidae Satyrinae). Phegea 24: 5–12. http://uahost.uantwerpen.be/vve/Phegea/1996/Phegea24-1_5-12.pdf [Google Scholar]
- Pagès J. (2007) Une nouvelle espèce de Pseudochazara du Pakistan (Nymphalidae, Satyrinae). Nota lepidopterologica 30: 361–365. http://www.soceurlep.eu/uploads/nota/bd30_2/08_Pag%E8s.pdf [Google Scholar]
- Pazhenkova EA, Zakharov EV, Lukhtanov VA. (2015) DNA barcoding reveals twelve lineages with properties of phylogenetic and biological species within Melitaea didyma sensu lato (Lepidoptera, Nymphalidae). ZooKeys 538: 35–46. doi: 10.3897/zookeys.538.6605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña C, Nylin S, Wahlberg N. (2011) The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods. Zoological Journal of the Linnean Society 161: 64–87. doi: 10.1111/j.1096-3642.2009.00627.x [Google Scholar]
- Pamperis LN. (2009) The Butterflies of Greece. Editions Pamperis, Athens, 768 pp. [Google Scholar]
- Posada D. (2008) jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. doi: 10.1093/molbev/msn083 [DOI] [PubMed] [Google Scholar]
- Rambaut A. (2014) Figtree v1.4.2. Computer program and documentation distributed by the author. http://tree.bio.ed.ac.uk/software [accessed 15.10.2014]
- Ritter S, Michalski SG, Settele J, Wiemers M, Fric ZF, Sielezniew M, Šašić M, Rozier Y, Durka W. (2013) Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS ONE 8: 1–13. doi: 10.1371/journal.pone.0078107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savela M. (2015) Lepidoptera and some other life forms. FUNET database – http://www.nic.funet.fi/pub/sci/bio/life/insecta/lepidoptera/ [accessed 6.1.2015]
- Song H, Buhay JE, Whiting MF, Crandall KA. (2008) Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudo-genes are coamplified. Proceedings of the National Academy of Sciences of the United States of America 105: 13486–13491. doi: 10.1073/pnas.0803076105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution 30: 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent J. (1996) The Butterflies of Morocco, Algeria and Tunisia. Gem Publishing Company, Oxfordshire, 217 pp. [Google Scholar]
- Toews DPL, Brelsford A. (2012) The biogeography of mitochondrial and nuclear discordance in animals. Molecular Ecology 21: 3907–3930. doi: 10.1111/j.1365-294X.2012.05664.x [DOI] [PubMed] [Google Scholar]
- Tshikolovets VV. (2005) The Butterflies of Ladak (N.-W. India) (Lepidoptera, Rhopalocera). Tshikolovets publications, Brno–Kyiv, 176 pp. [Google Scholar]
- Tshikolovets VV. (2011) Butterflies of Europe & the Mediterranean area. Tshikolovets publications, Pardubice, Czech Republic, 544 pp. [Google Scholar]
- Verovnik R, Micevski B, Maes D, Wynhoff I, van Swaay C, Warren M. (2013) Conserving Europe’s most endangered butterfly: the Macedonian Grayling (Pseudochazara cingovskii). Journal of Insect Conservation 17: 941–947. doi: 10.1007/s10841-013-9576-6 [Google Scholar]
- Verovnik R, Popović M, Šašić M, Cuvelier S, Maes D. (2014) Wanted! Dead or alive: the tale of the Brown’s Grayling (Pseudochazara amymone). Journal of Insect Conservation 18: 675–682. doi: 10.1007/s10841-014-9674-0 [Google Scholar]
- Verovnik R, Sket B, Trontelj P. (2004) Phylogeography of subterranean and surface populations of water lice Asellus aquaticus (Crustacea: Isopoda). Molecular Ecology 13: 1519–1532. doi: 10.1111/j.1365-294X.2004.02171.x [DOI] [PubMed] [Google Scholar]
- Wakeham-Dawson A. (2005) Further descriptions of androconia from Staudinger’s Pseudochazara de Lesse, 1951 (Lepidoptera: Nymphalidae, Satyrinae) type specimens in the Zoologisches Museum der Humboldt-Universität zu Berlin. Entomologist’s Gazette 56: 139–146. [Google Scholar]
- Wakeham-Dawson A. (2006) Descriptions of wing androconia from some Pseudochazara de Lesse, 1951(Lepidoptera: Nymphalidae, Satyrinae) type specimens in The Natural History Museum, London. Entomologist’s Gazette 57: 99–107. [Google Scholar]
- Wakeham-Dawson A, Kudrna O. (2000) A quantitative description of androconia from Staudinger’s Pseudochazara de Lesse, 1951 (Lepidoptera: Nymphalidae, Satyrinae) type specimens in the Zoological Museum of the Humboldt University of Berlin. Entomologist’s Gazette 51: 75–81. [Google Scholar]
- Wakeham-Dawson A, Dennis RLH. (2001) A quantitative description of the male genitalia of 23 taxa of Pseudochazara de Lesse, 1951 (Lepidoptera: Nymphalidae, Satyrinae). Entomologist’s Gazette 52: 227–250. [Google Scholar]
- Wakeham-Dawson A, Parker R, John E, Dennis RLH. (2003) Comparison of the male genitalia and androconia of Pseudochazara anthelea acamanthis (Rebel, 1916) from Cyprus, Pseudochazara anthelea anthelea (Hübner, 1924) from mainland Turkey and Pseudochazara anthelea amalthea (Frivaldsky, 1845) from mainland Greece (Lepidoptera: Nymphalidae, Satyrinae). Nota lepidopterologica 25: 251–263. [Google Scholar]
- Wakeham-Dawson A, Kudrna O. (2005) Further descriptions of androconia from Staudinger’s Pseudochazara de Lesse, 1951 (Lepidoptera: Nymphalidae, Satyrinae) type specimens in the Zoologisches Museum der Humboldt-Universität zu Berlin. Entomologist’s Gazette 56: 139–146. [Google Scholar]
- Wakeham-Dawson A, Kudrna O. (2006) Description of wing androconia from the lectotype of Pseudochazara caucasica (Lederer, 1864) (Lepidoptera: Nymphalidae, Satyrinae), with notes on the topotype wing androconia of related taxa. Entomologist’s Gazette 57: 137–141. [Google Scholar]
- Wakeham-Dawson A, Kudrna O, Dennis RLH. (2007) Description of androconia in the Palaearctic Asian Pseudochazara baldiva (Moore, 1865) butterfly species-group (Nymphalidae: Satyrinae) with designation of two lectotypes and reference to type and other material in the Natural History Museum, London. Nota lepidopterologica 30: 211–223. http://www.soceurlep.eu/uploads/nota/bd30_2/01_Wakeham-Dawson.pdf [Google Scholar]
- Weiss JC. (1980) Le genre Pseudochazara de Lesse en Europe et en Afrique du Nord. Description d’une sous-espèce nouvelle de Ps. hippolyte Esper (Lep.: Satyridae). Linneana Belgica 8: 98–108. [Google Scholar]
- Wiemers M, Fiedler K. (2007) Does the DNA barcoding gap exist? – a case study in blue butterflies (Lepidoptera: Lycaenidae). Frontiers in Zoology 4: . doi: 10.1186/1742-9994-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev RV. (2012) Checklist of Butterflies (Papilionoidea) of the Mongolian Altai Mountains, including descriptions of new taxa. Nota lepidopterologica 35: 51–96. http://www.soceurlep.eu/uploads/nota/bd35_1/07_Yakovlev.pdf [Google Scholar]
- Yang Z, Landry J-F, Handfield L, Zhang Y, Solis MA, Handfield D, Scholtens BG, Mutanen M, Nuss M, Hebert PDN. (2012) DNA barcoding and morphology reveal three cryptic species of Anania (Lepidoptera: Crambidae: Pyraustinae) in North America, all distinct from their European counterpart. Systematic Entomology 37: 686–705. doi: 10.1111/j.1365-3113.2012.00637.x [Google Scholar]