Abstract
To examine utilization and outcomes in pediatric immune thrombocytopenia (ITP) hospitalizations, we used ICD-9 code 287.31 to identify hospitalizations in patients with ITP in the 2009 HCUP KID, an all-payer sample of pediatric hospitalizations from US community hospitals. Diagnosis and procedure codes were used to estimate rates of ITP-related procedures, comorbidity prevalence, costs, length of stay (LOS), and mortality. In 2009, there were an estimated 4499 hospitalizations in children aged 6 months–17 years with ITP; 43% in children aged 1–5 years; and 47% with emergency department encounters. The mean hospitalization cost was $5398, mean LOS 2.0 days, with 0.3% mortality (n = 13). With any bleeding (15.2%, including gastrointestinal 2.0%, hematuria 1.3%, intracranial hemorrhage [ICH] 0.6%), mean hospitalization cost was $7215, LOS 2.5 days, with 1.5% mortality. For ICH (0.6%, n = 27), mean cost was $40 209, LOS 8.5 days, with 21% mortality. With infections (14%, including upper respiratory 5.2%, viral 4.9%, bacterial 1.9%), the mean cost was $6928, LOS 2.9 days, with 0.9% mortality. Septic shock was reported in 0.3% of discharges. Utilization included immunoglobulin administration (37%) and splenectomies (2.3%). Factors associated with higher costs included age >6 years, ICH, hematuria, transfusion, splenectomy, and bone marrow diagnostics (p < 0.05). In conclusion, of the 4499 hospitalizations with ITP, mortality rates of 1.5%, 21%, and 0.9% were seen with any bleeding, ICH, and infection, respectively. Higher costs were associated with clinically significant bleeding and procedures. Future analyses may reveal effects of the implementation of more recent ITP guidelines and use of additional treatments.
Keywords: Bleeding, costs, infection, inpatient utilization, KID, outcomes
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low circulating platelet counts (thrombocytopenia) caused by both increased platelet destruction and decreased platelet production [1]. Terminology for ITP duration has been standardized to include newly diagnosed (within 3 months of diagnosis), persistent (3–12 months from diagnosis), and chronic (lasting more than 12 months) [2]. Thrombocytopenia places patients at higher risk for minor and more serious bleeding, such as intracranial hemorrhage (ICH) [3]. Estimates of ITP incidence range between 2 and 5 cases per 100 000 per year in children who are younger than 15 years old [4–6]; prevalence estimates range between 4.1 and 12.6 cases per 100 000, depending on age, with a mean prevalence of approximately 7.2 cases per 100 000 in children younger than 18 years old [7–8]. Approximately 75% of pediatric ITP cases are newly diagnosed and persistent, with the remaining cases considered chronic [9–12]. ITP in children is often associated with an antecedent illness or infection and often resolves within 1-6 months [11, 13]. Children with persistent/chronic ITP are more likely to be older and female [6, 14].
While some studies characterize pediatric ITP as benign, describing major bleeding as rare [15–18], other studies have found major bleeding to be more common [19], with rates comparable with those seen in adults [20]. One analysis of 40 cases of ICH in children with ITP found a mortality rate of 25% with ICH and a reported incidence of ICH ranging from 0.19% to 0.78% [21]; other reports have also had varying incidence rates of ICH in pediatric ITP [11, 13, 20]. Regarding infection/sepsis, there are isolated reports of sepsis both with and without splenectomy [19, 22]; the latter may coexist with unrecognized immunodeficiency. Physicians vary in their approaches to children with ITP as found in surveys [23–25] and expert panel discussions [26].
The most common initial therapies for pediatric ITP include intravenous immunoglobulin (IVIg), corticosteroids, or both [12, 27]. Subsequent treatments include corticosteroids, corticosteroids with high-dose IVIg, IV Rho(D) Ig, immunosuppressive agents, and rituximab [3, 28]. These treatments can be associated with side effects that limit repeated administration, potentially leading to in-hospital management of bleeding or reactions [29, 30]. Splenectomy is an effective treatment in children and adult ITP patients; however, the invasive nature of the procedure, the risk of postoperative sepsis, and long-term risks in children limit its use [22, 31].
Pediatric ITP is a rare disease. Notwithstanding the above, there is limited published information regarding its epidemiology, severity, and treatment patterns. To address a part of this data gap, we conducted an observational study utilizing the 2009 Healthcare Cost and Utilization Project (HCUP) Kids’ Inpatient Database (KID), with objectives of describing patient characteristics, length of stay (LOS), medical complications, procedures performed, admission and discharge status, costs, and factors associated with increased costs in hospitalizations in children with ITP in the United States.
Methods
Data source
The KID is one of a family of databases developed as part of the HCUP sponsored by the Agency for Healthcare Research and Quality (AHRQ). It is the only all-payer (including the uninsured) inpatient care database on hospital use, outcomes, and discharges designed to study children’s use of hospital services in the United States [32]. The KID was first made available in 1997, with new data published every 3 years. The 2009 release consists of data collected from 4121 hospitals across 44 states [33], representing a random sample of 7 370 203 pediatric discharges.
Pediatric hospitalizations are defined by KID as all discharges where the patient was aged ≤ 20 years at admission from all community non-rehabilitation hospitals (i.e., short-term, non-federal, general, and specialty hospitals, excluding hospital units of other institutions) [32] in states participating in HCUP. The 80% sample of all non-birth pediatric discharges facilitates analysis of relatively rare conditions such as ITP.
Hospitalization inclusion and exclusion criteria
Because the KID does not contain unique patient identifiers, the unit of analysis was a discharge for a specific hospitalization for patients between 6 months and 17 years of age. All non–birth-related discharges were included if they had an International Classification of Diseases, Clinical Modification, Version 9 (ICD-9) diagnosis code of 287.31 for ITP in any position in the record. The reliability of using administrative code 287.31 to identify ITP has been reported previously [34]. To increase the specificity of identifying ITP, we excluded specific inherited thrombocytopenias (e.g., Wiskott–Aldrich syndrome) and common causes of secondary thrombocytopenia, such as human immunodeficiency virus (HIV; Clinical Classifications Software [CCS] diagnosis code 5), cancer (CCS diagnosis codes 10–45), lupus (CCS diagnosis code 210), and aplastic anemia (ICD-9 code 284.0), or if the record included coding for a bone marrow transplant (CCS procedure code 64) [35]. As medications are not well-coded, discharges in which chemotherapy was given may not have been excluded; however, as discharges containing cancer codes were excluded, the vast majority of these cases should have been removed. In an additional analysis, hemophilia was defined as having ICD-9 codes of 286.0 or 286.1, using the same age restrictions as ITP and excluding HIV, cancer, lupus, anemia, and transplant ICD-9 codes. Only two discharges had codes for both hemophilia and ITP.
Observation period
The 2009 KID database contains individuals discharged between January 1, 2009, and December 31, 2009.
Outcome variables
The key economic variables in this study were hospitalization costs and LOS per discharge. While LOS was directly available in KID, hospitalization costs were estimated by deflating hospitalization charges using cost-to-charge ratios (CCRs) for each hospital, as collected by the Centers for Medicare and Medicaid Services [36]. For example, if a given charge was $10 000 and the CCR was 0.8, the deflated cost would be estimated at $8000. Where hospital-specific CCRs were not available, as occurred with 328 of the 3628 hospitals, the mean value for hospitals in the group (defined by state, urban/rural, investor-owned/other, and bed size) was used, as provided by the KID. For states that did not provide CCRs due to state law, as occurred with 165 hospitals, the national mean of all reported CCRs in the KID was used. Costs include only facility reimbursement, not physician reimbursement. Condition sets and utilization groups were identified using CCS and ICD-9 diagnosis and procedure codes (Supplement Table I).
Table I.
Estimated bleeding and infection hospitalizations in patients with ITP.
| Factor | n (%) | 95% CI | LOS | 95% CI | Cost | 95% CI | Mortality | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Bleeding outcomes | ||||||||||
| Nonbleeding | 3813 (85%) | 3365 | 4261 | 1.9 | 1.8 | 1.9 | $5122 | $4785 | $5482 | 0.07% |
| All bleeding | 686 (15.2%) | 583 | 789 | 2.5 | 2.3 | 2.7 | $7215 | $6355 | $8192 | 1.5% |
| ICH | 27 (0.6%) | 12 | 42 | 8.5 | 4.2 | 17.4 | $40 209 | $23 323 | $69 286 | 20.8% |
| Upper or lower GI bleeding | 20 (0.4%) | 8 | 31 | 2.9 | 2.0 | 4.2 | $12 520 | $7871 | $19 916 | 0.00% |
| Other GI bleeding | 74 (1.6%) | 52 | 97 | 2.7 | 2.1 | 3.4 | $7421 | $5562 | $9902 | 1.95% |
| Hematuria | 60 (1.3%) | 39 | 80 | 3.2 | 2.5 | 4.2 | $10 750 | $7686 | $15 033 | 2.65% |
| Female specific | NA | NA | NA | 2.8 | 1.3 | 6.0 | $11 801 | $3793 | $36 717 | 0.00% |
| Infection outcomes | ||||||||||
| Noninfected | 3849 (86%) | 3395 | 4302 | 1.8 | 1.8 | 1.9 | $5175 | $4817 | $5559 | 0.19% |
| Any infection | 650 (14%) | 557 | 743 | 2.9 | 2.6 | 3.2 | $6928 | $6144 | $7813 | 0.90% |
| URI | 234 (5.2%) | 190 | 277 | 2.2 | 2.0 | 2.5 | $5592 | $4820 | $6489 | 0.00% |
| Skin/SC | 54 (1.2%) | 34 | 74 | 3.7 | 2.6 | 5.4 | $9134 | $5835 | $14 300 | 0.00% |
| Mycosesa | 19 (0.4%) | 8 | 29 | 4.0 | 2.2 | 7.3 | $8276 | $4212 | $16 269 | 0.00% |
| Septicemia | 45 (1.0%) | 27 | 63 | 10.2 | 6.7 | 15.3 | $26 077 | $15 429 | $44 091 | 9.59% |
| Septic shock | 12 (0.3%) | 4 | 20 | 6.6 | 3.6 | 12.0 | $22 825 | $9653 | $54 014 | 11.11% |
| Viral, NEC | 221 (4.9%) | 179 | 262 | 2.5 | 2.2 | 2.9 | $5987 | $5081 | $7054 | 0.00% |
| Bacterial, NEC | 87 (1.9%) | 63 | 110 | 4.8 | 3.6 | 6.4 | $10 824 | $7640 | $15 337 | 0.00% |
aPrimarily dermatophytosis and candidiasis.
Costs calculated from charges by using hospital-specific CCRs where available; mortality based on discharge status.
CCR, cost-to-charge ratio; CI, confidence interval; GI, gastrointestinal; ICH, intracranial haemorrhage; ITP, immune thrombocytopenia; NA, not available; LOS, length of stay; NEC, not elsewhere classified; SC, subcutaneous; URI, upper respiratory infection.
Conditions and comorbidities
Discharges with ICD-9 codes pertaining to bleeding, as identified using the Observational Medical Outcomes Partnership definition for bleeding [37], were categorized into the following: no bleeding, ICH, upper gastrointestinal (GI) bleeding, lower GI bleeding, other GI bleeding, hematuria, hemoglobinuria, female-specific bleeding, pregnancy-related bleeding, and all other bleeding (Supplement Table I).
Because immunosuppressive interventions were used in some patients, we identified infections using the following CCS categories for diagnosis codes: tuberculosis, septicemia, bacterial infection, hepatitis, viral infection, other infections including parasitic infection, sexually transmitted infection, central nervous system infection, inflammation or infection of the eye, other upper respiratory infections (URIs), urinary tract infections, and skin or subcutaneous tissue infections (Supplement Table I). Septic shock was separately identified using ICD-9 codes. Sepsis was defined as having a septicemia or septic shock code.
A composite variable, called major complications, was created to identify hospitalizations of greater severity and included GI bleeding, ICH, hematuria, female-specific bleeding, septicemia or septic shock, and ICD-9 diagnosis codes 459.0 (hemorrhage, unspecified) and 596.7 (hemorrhage into bladder wall; Supplement Table I). A discharge containing codes for more than one of these complications was only counted once.
Therapeutic and diagnostic utilization
ICD-9 procedure codes were used to identify use of Ig (both anti-D Ig and IVIg) and IV steroid use. Medication details were incomplete, as ICD-9 procedure codes are limited in scope. Bone marrow diagnostics, splenectomy, removal of an accessory spleen, and other spleen-related procedures were also identified using ICD-9 procedure codes. Hospitalizations with asplenia or other splenic conditions were identified using ICD-9 diagnosis code 759.0.
Analyses
This cross-sectional study of 1 year of pediatric inpatient discharges was descriptive in nature. Estimates were calculated using SAS 9.4 (SAS Institute, Inc., Cary, NC). KID provides nationally representative weights for each hospitalization, which are used to sum across each category and obtain national estimates of hospitalizations. The Taylor series linearization method was used for variance calculations (95% CIs). Summary statistics for discharges in ITP patients were weighted to yield population-based estimates with appropriate 95% CIs. As the data contain 80% of pediatric discharges, there is some uncertainty regarding the total number of discharges and related comorbidities in the US population and 95% CIs. Mean LOS and total cost were compared between discharges in patients with ITP and all other discharges in KID. Mean LOS and total cost were also reported by age, gender, and key variables. Mortality rates were calculated by taking the total weighted in-hospital deaths (i.e., death discharge status) divided by the total weighted discharges.
Additionally, multivariate least-squares regression was performed to understand factors associated with total costs in ITP-related discharges. Due to the small number of deaths, risk factors for in-patient mortality were not evaluated. All table cells with 10 or fewer observations were not populated to minimize patient confidentiality concerns in accordance with the data-use agreement for the KID.
Results
All ITP discharges
In 2009, there were an estimated 4499 (95% confidence interval [CI]: 3983–5014) hospital discharges in the United States in children aged 6 months to 17 years with ITP (Supplement Table II). Half (51%) of ITP hospitalizations occurred in males and 45% occurred in children aged 6 months to 5 years. The mean hospitalization cost (excluding physician reimbursement) was $5398, the mean LOS was 2.0 days, and the mortality rate was 0.3%; costs by sex and age are shown in Figure 1A and Supplement Table II. Hospitalizations ending in death were associated with an average cost of $43 151 (95% CI: $16 732–$111 286) and LOS of 7.8 days (95% CI: 2.7–22.6).
Table II.
Other utilization.
| Factor | n (%) | 95% CI | LOS | 95% CI | Costs | 95% CI | Mortality | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ig infusion | 1686 (37%) | 1389 | 1982 | 1.9 | 1.8 | 1.9 | $6107 | $5480 | $6806 | 0.17% |
| Anti-D | 111 (2.5%) | 69 | 153 | 1.9 | 1.7 | 2.2 | $4161 | $3415 | $5070 | 0.00% |
| IVIg | 1582 (35%) | 1299 | 1866 | 1.9 | 1.8 | 2.0 | $6275 | $5612 | $7016 | 0.18% |
| IV corticosteroids | 29 (0.6%) | 12 | 46 | 3.7 | 1.8 | 7.6 | $9590 | $4085 | $22 516 | 4.99% |
| Splenectomy | 105 (2.3%) | 77 | 133 | 3.1 | 2.5 | 3.8 | $14 595 | $12 076 | $17 641 | 0.00% |
| BM diagnostics | 330 (7.3%) | 251 | 409 | 4.0 | 3.5 | 4.6 | $12 172 | $10 615 | $13 961 | 0.92% |
| ED services | 2095 (47%) | 1801 | 2389 | 2.0 | 1.9 | 2.1 | $5658 | $5258 | $6089 | 0.47% |
| Transfusions | 337 (7.5%) | 276 | 399 | 4.0 | 3.5 | 4.6 | $11 929 | $10 239 | $13 891 | 2.95% |
Costs calculated from charges by using hospital-specific CCRs where available; mortality based on discharge status; transfusions include all transfusion types, including platelet transfusions.
BM, bone marrow; CCR, cost-to-charge ratio; CI, confidence interval; ED, emergency department; Ig, immunoglobulin; IV, intravenous; IVIg, intravenous Ig; LOS, length of stay.
Figure 1.
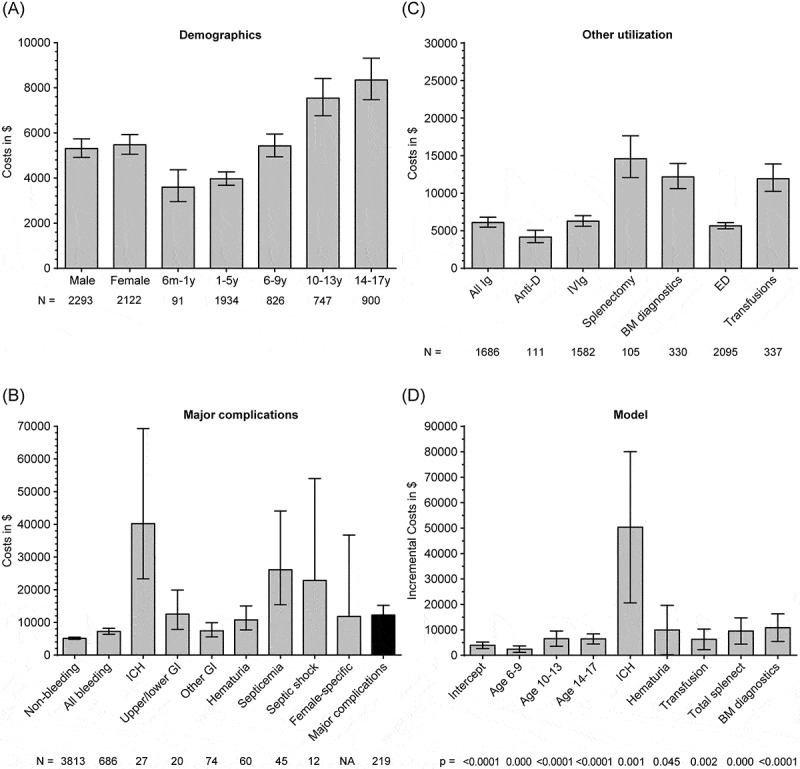
Mean ITP hospitalization costs by (A) demographic characteristics of sex and age, (B) whether various complications occurred, (C) whether various treatments were given or interventions were performed, and (D) multivariate analysis, with incremental costs shown for factors significantly (p < 0.05) associated with higher costs. BM, bone marrow; ED, emergency department; GI, gastrointestinal; ICH, intracranial hemorrhage; Ig, immunoglobulin; ITP, immune thrombocytopenia; IVIg, intravenous Ig; m, month; y, year; NA, not available; splenect, splenectomy.
Bleeding and infection discharges
There were 686 discharges in patients with ITP with any bleeding diagnosis (15.2% of all ITP discharges; Table I): mean facility cost was $7215, mean LOS was 2.5 days, and mortality rate was 1.5%. There were an estimated 27 cases of ICH (3.9% of 686 bleeding hospitalizations), with mean facility cost of $40 209, mean LOS of 8.5 days, and mortality rate of 20.8%. The majority of ICH cases were non-fatal; these had mean facility costs of $47 903 and LOS of 11.9 days.
Infections occurred in 650 hospitalizations [14% of ITP discharges (Table I)]; these discharges had mean costs of $6928, mean LOS of 2.9 days, and a mortality rate of 0.90%. The most common infections were URIs (5.2%) and viral (4.9%) or bacterial (1.9%) infections not elsewhere classified. Splenectomy may have a role in the development and outcome of sepsis. For the 1875 hospitalizations in the 2009 KID (N = 7 370 203) in which the patients did not have a spleen, 100 (5.3%) had sepsis; the mortality rate in those 100 hospitalizations was 16%.
Major complications, defined as GI bleeding, ICH, hematuria, female-specific bleeding, and septicemia or septic shock (component breakdown in Table I), occurred in 5.2% of ITP hospitalizations, with mean LOS of 3.9 days, mean cost of $12 236, and a mortality rate of 5.0% (Figure 1B).
Healthcare utilization in discharges
Rates of interventions are detailed in Table II, with costs shown in Figure 1C. Use of ITP treatments (i.e., IV anti-D, IVIg, IV steroids, and platelet transfusions) was seen in 1845 (41%) of ITP discharges, with Ig administered in 1686 (37%) hospitalizations (111 discharges received anti-D and 1582 IVIg). Rates of Ig use by age were: 27.3% for ages 6–12 months, 41.9% for ages 1–5 years, 36.3% for ages 6–9 years, 36.8% for ages 10-13 years, and 30.8% for ages 14–17 years. The relative rates of IVIg vs. anti-D use for all ages (35.2% vs. 2.5%) were similar in the various age groups. Of note, the 0.6% (n = 29) discharges with recorded use of IV corticosteroids were characterized by high mean costs ($9590). Splenectomies were performed in 105 discharges (2.3%) and bone marrow diagnostics in 330 ITP discharges (7.3%). A large portion of ITP hospitalizations (47%) began with an encounter in the emergency department (ED).
Cost model
In a multivariate analysis, factors significantly (p < 0.05) associated with higher costs in hospitalizations with ITP included age > 6 years, ICH, hematuria, transfusion (separately coded from IVIg), splenectomy, and bone marrow diagnostics (Table III). The intercept, or reference group, was defined as the cost when all other variables in the model were 0, i.e., in this case $3912 for a boy aged 6 months to 5 years who was not admitted through the ED and who had no other factors during his hospitalization. Various incremental costs can be added to $3912 depending on the characteristics of a given hospitalization (intercept and incremental costs in Figure 1D). For example, a boy aged 6–9 years with GI bleeding who received an Ig infusion would be expected to incur a hospital cost of $3912 (intercept) + $2415 (for age 6–9 years) + $2164 (for GI bleeding) + $1629 (for Ig infusion) = $10 120.
Table III.
Factors associated with hospitalization cost.
| Factor | Incremental cost | 95% CI | p value | |
|---|---|---|---|---|
| Intercept | $3912 | $2632 | $5192 | <0.0001 |
| Age 6–9 years | $2415 | $1155 | $3676 | 0.000 |
| Age 10–13 years | $6553 | $3576 | $9530 | <0.0001 |
| Age 14–17 years | $6427 | $4425 | $8429 | <0.0001 |
| Girl | ($60) | ($1457) | $1338 | 0.933 |
| ED admission | ($526) | ($2011) | $959 | 0.488 |
| Septic shock | $45 663 | ($23 804) | $115 131 | 0.198 |
| Ig infusion | $1629 | ($398) | $3656 | 0.115 |
| ICH | $50 328 | $20 605 | $80 051 | 0.001 |
| Any GI bleeding | $2164 | ($1719) | $6047 | 0.275 |
| Hematuria | $9939 | $234 | $19 643 | 0.045 |
| Transfusion | $6265 | $2245 | $10 285 | 0.002 |
| Total splenectomy | $9560 | $4411 | $14 710 | 0.000 |
| Bone marrow diagnostics | $10 866 | $5453 | $16 279 | <0.0001 |
Incremental cost shows the independent effect of each factor on the total hospitalization costs.
Parentheses indicate negative costs.
The intercept reflects the cost for a boy aged 6 months to 5 years who was not admitted through the ED and who had no other factors during his hospitalization.
CI, confidence interval; ED, emergency department; GI, gastrointestinal; ICH, intracranial haemorrhage; Ig, immunoglobulin.
We further examined to what extent costs for hospitalizations varied by age, bleeding, and IVIg use. As shown in Supplement Table III, the costs for hospitalizations with bleeding were greater for all age groups, with a steadily increasing difference in costs from + $459 for ages 6–12 months to + $5714 for age 14–17 years. Likewise, hospitalization costs were greater with IVIg use, except for patients aged 6–12 months, with an increase ranging from + $865 for those 1–5 years of age to + $4608 for those 14–17 years of age.
Non-ITP discharges
For reference, we examined hospital discharges without an ITP diagnosis code. In 2009, there were a total of 7 365 704 non-ITP discharges (95% CI: 7 124 087–7 608 321), of which roughly half (47%) occurred in male patients; 71.1% were in patients aged 6 months to 5 years. The mean hospitalization cost was $1964, the mean LOS was 2.5 days, and the mortality rate was 0.36%. Results were generally similar for ITP and non-ITP for bleeding discharges (non-ITP: mean cost $7667, mean LOS 3.8 days, mortality rate 3.3%), infection discharges (non-ITP: mean cost $4707, mean LOS 3.3 days, mortality rate 0.65%), and major complications (non-ITP: mean cost $11 103, mean LOS 5.3 days, mortality rate 4.8%). However, when ICH occurred in non-ITP discharges (n = 24 710, 0.3% of discharges, or 21% of all 117 884 bleeding hospitalizations), the mean cost was $13 108 (vs. $40 209 for ICH with ITP discharges), the mean LOS was 4.4 days (vs. 8.5 days for ICH with ITP discharges), and the mortality rate was 7.9% (vs. 20.8% for ICH with ITP discharges).
Discussion
This report analyzes resource utilization, costs, and clinical outcomes during hospitalizations of children with ITP in the United States. It is one of the first studies utilizing a publically available, all-payer, nationally representative database to examine ITP-related hospitalizations in children. Hospitalizations of children with ITP with clinically significant bleeding and procedures were associated with higher costs. For example, hospitalizations with the most serious bleeding event (ICH), while uncommon, were the most expensive (mean cost of $40 209). The number of splenectomies performed in ITP discharges was approximately 100 per year. Key factors associated with hospitalizations having higher costs were age > 6 years, ICH, hematuria, transfusion, splenectomy, and bone marrow diagnostics (p < 0.05). This study also adds to the sparse data on sepsis in pediatric ITP. Not surprisingly with these findings, hospitalizations of ITP patients with septic shock and/or septicemia had higher associated mean hospital costs.
It therefore is clear that in contrast to the commonly held assumption that ITP is a relatively benign disease, major complications, including various bleeding and infection outcomes, occurred in 5.2% of pediatric ITP hospitalizations with an associated mortality rate of 5.0%. The overall mortality rate with ITP was 0.3% (13 deaths of 4499 discharges) as compared with 0% (no deaths of 1974 discharges, data not shown) in hemophilia, which highlights the risk of death in ITP relative to another rare but serious bleeding condition. The mortality rate associated with any bleeding in ITP-related hospitalizations was 1.5%; the mortality with any infection was 0.9%. Hospitalizations with ICH, while fortunately relatively rare (27 cases or 0.6%), were associated with a mortality rate of 21%, in keeping with a previously published mortality rate of 25% [21].
A comparison with the recent study by Kime et al. highlights how the nature of the databases examined, hospitals included, and analyses can shape the findings of key clinical and utilization measures [38]. Kime et al. reported clinical and financial data, including pharmacy files, from the Pediatric Health Information System (PHIS) of 2314 children with ITP who were discharged from 40 free-standing children’s hospitals from 2008 to 2010. The data presented in this manuscript, from KID, were based on inpatient facility claims of 4499 discharges of children with ITP in 2009 from an 80% sample of over 4000 community hospitals. Thus, while with KID, there are facility claims only from community hospitals for discharges, with PHIS, there are comprehensive clinical and financial data from children’s hospitals for individual patients.
Some key findings were similar. Both analyses found that half of patients/discharges were male and half were ≤6 years of age, with bleeding being fairly common (KID: 15.2% vs. PHIS: 12%) and ICH uncommon (both 0.6%). The rate of bleeding reported in both studies may reflect that children with ITP could have been hospitalized for other conditions and conversely that less severe bleeding may have been handled on an outpatient basis. Hospital stays had similar mean LOS (KID: 2.0 days vs. PHIS: 2.2 days). Once adjusted for CCR, the mean costs in KID (~$5400) were comparable with the ~$9000 in median charges seen with PHIS.
Pharmacy charges were half of charges seen in PHIS; the proportion of costs accounted for by pharmacy in KID was not available. Pharmacy costs may well have made up a smaller proportion of total costs in KID, given that rates of IVIg use (KID: 35% vs. PHIS: >78%) and anti-D use (KID: 2.5% vs. PHIS: 10.6%) were much less with KID than PHIS. However, the true rates of IVIg and anti-D use in KID may be underestimated, because reimbursement procedures may not require coding IVIg or anti-D use on medical claims. Rates of IVIg use within KID in children’s hospitals (N = 41, 1135 discharges) and non-children’s hospitals (N = 4080, 2880 discharges) were similar (36% vs. 35%), so the difference in IVIg use rates between KID and PHIS is likely not due to a difference between children’s and non-children’s hospitals. Future analyses focusing on medication use would benefit from pharmacy records, as available in PHIS and used in Kime et al., whereas analyses of costs associated with a wide range of clinical outcomes would best be performed with a database such as KID.
IVIg use likely contributed to increased costs across the board, given that they were used in 37% of KID discharges. The use of IVIg may have reduced bleeding. If recent treatment guidelines [3, 39] are implemented, use of Ig may decrease; it will be of interest to see if there is any corresponding increase in bleeding or any changes in medical costs (e.g., for LOS). As might be expected since IVIg dosing is weight based, the increased costs with bleeding or IVIg use were most pronounced for the oldest patients (14–17 years of age). The relatively low reported use of anti-D in the inpatient setting was likely due to its preferential use in the outpatient setting; in any case, the predominant use of IVIg (vs. anti-D) would not have significantly affected the cost analyses described here.
Regarding children who did not receive specific treatments for ITP, such as IVIg or anti-D, we cannot discern from KID whether that was because they received treatments that did not have procedure codes (such as oral corticosteroids), because they were admitted for observation, because they were transferred or admitted after receiving front-line therapy elsewhere, or because they were admitted for a reason not directly related to ITP. Further, we were not able to discern to what extent past medical history, past responses to or adverse events associated with various treatments, or comorbidities may have affected treatment choices. The relatively large proportion of patients without ITP-specific treatment in KID as compared with PHIS may reflect differences in practice patterns and/or patient populations. Patients with more difficult-to-manage disease may be more likely to be directed to a children’s hospital as opposed to a general community hospital. Another possibility is that more admissions to community hospitals are the admission at which ITP was diagnosed; this is consistent with the 7.3% rate of bone marrow diagnostics.
Assessment of infection in this study (14%) used a comprehensive set of ICD-9 codes as categorized by CCS, with established algorithms, thus capturing a broad range of infections. Most infections were not serious (e.g., URI in 5.2% of patients, unspecified viral infections in 4.9%). The rates of serious infections, such as septicemia (1.0%), septic shock (0.3%), and mycoses (0.4%), while lower, were not negligible. To capture the most clinically serious outcomes, we created a composite endpoint that included septicemia and septic shock as well as several of the more serious bleeding outcomes (such as GI bleeding, ICH, and hematuria). It is likely that the more serious, clinically relevant adverse events are more likely to be coded and reimbursed; thus, this composite measure is more likely to accurately reflect clinical outcomes.
Compared with all other hospitalizations without ITP in the 2009 KID (N = 7 365 704), ITP-related hospitalizations (N = 4499) generally had comparable cost, LOS, and mortality. However, several factors make comparing these hospitalizations problematic. First, these hospitalizations differ in severity of symptoms and extent of utilization. For example, when patients who do not have ITP are hospitalized with bleeding, those hospitalizations last longer (mean LOS of 3.8 days for non-ITP vs. 2.5 days for ITP, 95% CI non-overlapping) and are associated with greater mortality rates (3.3% non-ITP vs. 1.5% ITP, p < 0.05) and higher rates of ICH (21% vs. 4.0%, p < 0.0001), traumatic ICH (17% vs. 0.2%, p < 0.0001), and sepsis (4.2% vs. 1.5%, p < 0.005). Second, many factors could influence outcomes that are likely different between ITP and non-ITP hospitalizations, such as platelet counts, comorbidities, past medical history, and past treatments (none of which were available in this database). Last, parents and clinicians may well have varying thresholds for hospitalization and interventions for children with ITP as compared with children who do not have ITP. Thus, while the data from ITP as compared with non-ITP hospitalizations are of interest, direct comparison may be misleading.
In addition to the above, there are several additional limitations of this study. First, costs are approximate, since they are based on CCR ratios, and physician charges were not included. Second, because patient history was not included in the database, it was impossible to determine the duration or severity of the ITP. Other patient-level data besides age and gender were limited to the information available on the discharge record, so it was not possible to adjust for many factors that could influence outcomes in ITP (e.g., platelet counts, comorbid conditions, and other risk factors) or discern any relationship between platelet counts and bleeding or thrombosis. Third, it should be emphasized that the KID is a database of hospital discharges, not unique patients (i.e., the same patient with two hospitalizations could potentially be sampled twice), and that hospitalized children with ITP may differ from the overall pediatric ITP population. As a result, extrapolation to the general pediatric ITP population may not be fully accurate or appropriate. Finally, these analyses by nature were limited to inpatient utilization; much of the management of ITP occurs in the outpatient setting.
In all, however, these data provide a detailed picture of resource utilization and costs during hospitalizations of children with ITP. In particular, in contrast to the perception that pediatric ITP is a disease not generally requiring treatment, our results highlight the real and tangible risks that hospitalized children with ITP face: serious bleeding including ICH, infection including sepsis, and death. Knowledge of what outcomes are associated with greater utilization, longer stays, and increased mortality may indicate areas of clinical practice for which changes are indicated, either by individualization of patient care or by revamping standard treatment guidelines. Finally, as the data described in this manuscript were from 2009 hospital discharge records, the resource use, LOS, and mortality data are representative of clinical practice 7 years ago. Analyses of the recently released 2012 KID data may illustrate to what extent changing clinical practice affects these outcomes, particularly in light of more recent guidelines on ITP treatment in children.
Supplementary Material
Acknowledgments
Susanna Mac, MD, PhD, of Amgen Inc. provided medical writing assistance. The authors made decisions regarding submission of the manuscript. M.D. and J.D. wrote the first draft.
Funding Statement
Amgen Inc. funded these analyses, which were directed by the authors.
Funding
Amgen Inc. funded these analyses, which were directed by the authors.
Declaration of interest statement
M.T. is an advisor for Baxalta, Biogen, Grifols, Novo Nordisk, and Pfizer; received research funding from Grifols and Novo Nordisk; is on the speakers’ bureau with Biogen and Grifols. M.D. and J.D. are consultants for and received research funding from Amgen Inc. as part of Outcomes Insights. R.K. received research funding support from Cangene Corp. and Novartis, Canada, and is an advisor for Celgene Corp. M.E. is an employee and stockholder of Amgen Inc. J.B. received research funding from Amgen Inc., Cangene, GlaxoSmithKline (GSK), Genzyme, IgG of America, Immunomedics, Ligand, Eisai, Shionogi, and Sysmex; is on advisory boards for Amgen Inc., GSK, Ligand, Shionogi, and Eisai; and is a consultant for Portola.
References
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N. Blood. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B. Blood. International consensus report on the investigation and management of primary immune thrombocytopenia. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- Chu YW, Korb J, Sakamoto KM. Idiopathic thrombocytopenic purpura. Pediatr Rev. 2000;21:95–104. doi: 10.1542/pir.21-3-95. [DOI] [PubMed] [Google Scholar]
- Zeller B, Helgestad J, Hellebostad M, Kolmannskog S, Nystad T, Stensvold K, Wesenberg F. Immune thrombocytopenic purpura in childhood in Norway: A prospective, population-based registration. Pediatr Hematol Oncol. 2000;17:551–558. doi: 10.1080/08880010050122816. [DOI] [PubMed] [Google Scholar]
- Glanz J, France E, Xu S, Hayes T, Hambidge S. A population-based, multisite cohort study of the predictors of chronic idiopathic thrombocytopenic purpura in children. Pediatrics. 2008;121:e506–e512. doi: 10.1542/peds.2007-1129. [DOI] [PubMed] [Google Scholar]
- Segal JB, Powe NR. Prevalence of immune thrombocytopenia: Analyses of administrative data. J Thromb Haemost. 2006;4:2377–2383. doi: 10.1111/j.1538-7836.2006.02147.x. [DOI] [PubMed] [Google Scholar]
- Feudjo-Tepie MA, Robinson NJ, Bennett D. Prevalence of diagnosed chronic immune thrombocytopenic purpura in the US: Analysis of a large US claim database: A rebuttal. J Thromb Haemost. 2008;6:711–713. doi: 10.1111/j.1538-7836.2008.02911.x. [DOI] [PubMed] [Google Scholar]
- Walker RW, Walker W. Idiopathic thrombocytopenia, initial illness and long term follow up. Arch Dis Child. 1984;59:316–322. doi: 10.1136/adc.59.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor AH, Harms A, Kaufmehl K. Acute immune thrombocytopenia (ITP) in childhood: Retrospective and prospective survey in Germany. Semin Thromb Hemost. 2001;27:253–267. doi: 10.1055/s-2001-15255. [DOI] [PubMed] [Google Scholar]
- Kühne T, Imbach P, Bolton-Maggs PH, Berchtold W, Blanchette V, Buchanan GR. Newly diagnosed idiopathic thrombocytopenic purpura in childhood: An observational study. Lancet. 2001;358:2122–2125. doi: 10.1016/S0140-6736(01)07219-1. [DOI] [PubMed] [Google Scholar]
- Tamminga R, Berchtold W, Bruin M, Buchanan GR, Kühne T. Possible lower rate of chronic ITP after IVIG for acute childhood ITP an analysis from registry I of the Intercontinental Cooperative ITP Study Group (ICIS) Br J Haematol. 2009;146:180–184. doi: 10.1111/j.1365-2141.2009.07743.x. [DOI] [PubMed] [Google Scholar]
- Kühne T, Buchanan GR, Zimmerman S, Michaels LA, Kohan R, Berchtold W, Imbach P. Intercontinental Childhood ITP Study Group, Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605–608. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- Donato H, Picón A, Martinez M, Rapetti MC, Rosso A, Gomez S, Rossi N, Bacciedoni V, Schvartzman G, Riccheri C. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: A multicentered study from Argentina. Pediatr Blood Cancer. 2009;52:491–496. doi: 10.1002/pbc.21872. [DOI] [PubMed] [Google Scholar]
- Imbach P, Kühne T, Müller D, Berchtold W, Zimmerman S, Elalfy M, Buchanan GR. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS) Pediatr Blood Cancer. 2006;46:351–356. doi: 10.1002/pbc.20453. [DOI] [PubMed] [Google Scholar]
- Neunert CE, Buchanan GR, Imbach P, PHB Bolton-Maggs, Bennett CM, Neufeld EJ, Vesely SK, Adix L, Blanchette VS, Kühne T. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008;112:4003–4008. doi: 10.1182/blood-2008-03-138487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunert CE, Buchanan GR, Imbach P, PHB Bolton-Maggs, Bennett CM, Neufeld E, Vesely SK, Adix L, Blanchette VS, Kühne T. Bleeding manifestations and management of children with persistent and chronic immune thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group (ICIS) Blood. 2013;121:4457–4462. doi: 10.1182/blood-2012-12-466375. [DOI] [PubMed] [Google Scholar]
- Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. 2002;141:683–688. doi: 10.1067/mpd.2002.128547. [DOI] [PubMed] [Google Scholar]
- Medeiros D, Buchanan GR. Major hemorrhage in children with idiopathic thrombocytopenic purpura: Immediate response to therapy and long-term outcome. J Pediatr. 1998;133:334–339. doi: 10.1016/s0022-3476(98)70265-3. [DOI] [PubMed] [Google Scholar]
- Kühne T, Berchtold W, Michaels LA, Wu R, Donato H, Espina B, Tamary H, Rodeghiero F, Chitlur M, Rischewski J. Newly diagnosed immune thrombocytopenia in children and adults: A comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96:1831–1837. doi: 10.3324/haematol.2011.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): Study of 40 cases. Blood. 2009;114:4777–4783. doi: 10.1182/blood-2009-04-215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühne T, Blanchette V, Buchanan GR, Ramenghi U, Donato H, Tamminga RYJ, Rischewski J, Berchtold W, Imbach P. Intercontinental Childhood ITP Study Group. Splenectomy in children with idiopathic thrombocytopenic purpura. A prospective study of 134 children from the Intercontinental Childhood ITP Study Group. Pediatr Blood Cancer. 2007;49:829–834. doi: 10.1002/pbc.21108. [DOI] [PubMed] [Google Scholar]
- Neunert CE, Bright BC, Buchanan GR. Severe chronic refractory immune thrombocytopenic purpura during childhood: a survey of physician management. Pediatr Blood Cancer. 2008;51:513–516. doi: 10.1002/pbc.21621. [DOI] [PubMed] [Google Scholar]
- SK Vesely, GR Buchanan, Adix L, JN George, AR Cohen, VS Blanchette, Kühne T. American Society of Pediatric Hematology/Oncology, 2001. Self-reported initial management of childhood idiopathic thrombocytopenic purpura. Results of a survey of members of the American Society of Pediatric Hematology/Oncology. 2001;2003(25):130–133. doi: 10.1097/00043426-200302000-00009. J Pediatr Hematol Oncol. [DOI] [PubMed] [Google Scholar]
- Vesely SK, Buchanan GR, Cohen A, Raskob G, George J. Self-reported diagnostic and management strategies in childhood idiopathic thrombocytopenic purpura: Results of a survey of practicing pediatric hematology/oncology specialists. J Pediatr Hematol Oncol. 2000;22:55–61. doi: 10.1097/00043426-200001000-00011. [DOI] [PubMed] [Google Scholar]
- Bolton-Maggs P, Tarantino MD, Buchanan GR, Bussel JB, George JN. The child with immune thrombocytopenic purpura: Is pharmacotherapy or watchful waiting the best initial management? A panel discussion from the 2002 meeting of the American Society of Pediatric Hematology/Oncology. J Pediatr Hematol Oncol. 2004;26:146–151. doi: 10.1097/00043426-200402000-00020. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Klima J, Despotovic JM, O’Brien SH. Anti-D immunoglobulin therapy for pediatric ITP: Before and after the FDA’s black box warning. Pediatr Blood Cancer. 2013;60:E149–E151. doi: 10.1002/pbc.24633. [DOI] [PubMed] [Google Scholar]
- Ancona KG, Parker RI, Atlas MP, Prakash D. Randomized trial of high-dose methylprednisolone versus intravenous immunoglobulin for the treatment of acute idiopathic thrombocytopenic purpura in children. J Pediatr Hematol Oncol. 2002;24:540–544. doi: 10.1097/00043426-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Intravenous immune globulin therapy for neurologic diseases. Ann Intern Med. 1997;126:721–730. doi: 10.7326/0003-4819-126-9-199705010-00008. [DOI] [PubMed] [Google Scholar]
- Ji X. Treatment progress of idiopathic thrombocytopenic purpura. Chin J Thromb Haemost. 2004;10:41–45. [Google Scholar]
- Aronis S, Platokouki H, Avgeri M, Pergantou H, Keramidas D. Retrospective evaluation of long-term efficacy and safety of splenectomy in chronic idiopathic thrombocytopenic purpura in children. Acta Paediatr. 2004;93:638–642. [PubMed] [Google Scholar]
- Introduction to the KID Healthcare Cost Utilization Project (HCUP) [Internet] Rockiville (MD): Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) 2009 http://www.hcup-us.ahrq.gov/db/nation/kid/KID_2009_Introduction.pdf - [cited 2015 Sep 24] Available from. [PubMed]
- HCUP KID Database Documentation [Internet] Rockiville (MD): Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) 2014 http://www.hcup-us.ahrq.gov/db/nation/kid/kiddbdocumentation.jsp - [cited 2015 Sep 24] Available from.
- Danese MD, Lindquist K, Gleeson M, Deuson R, Mikhael J. Cost and mortality associated with hospitalizations in patients with immune thrombocytopenic purpura. Am J Hematol. 2009;84:631–635. doi: 10.1002/ajh.21500. [DOI] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Palmer L, Clinical Classifications Software (CCS . Available from: Rockville (MD): Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP; 2012. http://www.hcup-us.ahrq.gov/tools_software.jsp [Google Scholar]
- HCUP-US Cost-to-Charge Ratio Files [Internet]. Rockiville (MD): Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP 2014 http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp Available from:
- Health Outcomes of Interest [Internet] Observational Medical Outcomes Partnership. 2013 http://omop.org/HOI Available from:
- Kime C, Klima J, Rose MJ, O’Brien SH. Patterns of inpatient care for newly diagnosed immune thrombocytopenia in US children’s hospitals. Pediatrics. 2013;131:880–885. doi: 10.1542/peds.2012-2021. [DOI] [PubMed] [Google Scholar]
- Neunert C, Lim W, Crowther M, Cohen A, Jr Solberg L, Crowther MA. American Society of Hematology. The American Society of Hematology. evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;2011(117):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


