ABSTRACT
Binding relations among items in the transverse patterning (TP) task is dependent on the integrity of the hippocampus and its extended network. Older adults have impaired TP learning, corresponding to age-related reductions in hippocampal volumes. Unitization is a training strategy that can mitigate TP impairments in amnesia by reducing reliance on hippocampal-dependent relational binding and increasing reliance on fused representations. Here we examined whether healthy older adults and those showing early signs of cognitive decline would also benefit from unitization. Although both groups of older adults had neuropsychological performance within the healthy range, their TP learning differed both under standard and unitized training conditions. Healthy older adults with impaired TP learning under standard training benefited from unitized training. Older adults who failed the Montreal Cognitive Assessment (MoCA) showed greater impairments under standard conditions, and showed no evidence of improvement with unitization. These individuals’ failures to benefit from unitization may be a consequence of early deficits not seen in older adults who pass the MoCA.
KEYWORDS: Cognitive aging, transverse patterning, hippocampus, unitization, relational memory
The creation of associations among distinct elements (relational binding) is a critical function of the hippocampus (Eichenbaum, Otto, & Cohen, 1994; Moses & Ryan, 2006; Ryan & Cohen, 2003), and supports the formation of memories for events or episodes. A hallmark task of relational binding function is the transverse patterning (TP) task in which the relations among items must be learned. TP is akin to the childhood game, “rock-paper-scissors” (RPS) in which the reward value of a given stimulus depends on the identity of another stimulus (e.g., A wins over B, B wins over C, and C wins over A). Learning of novel arbitrary relations in TP is traditionally found to be impaired in amnesic individuals with damage to the hippocampus and its extended network (Rickard & Grafman, 1998; Rickard, Verfaellie, & Grafman, 2006) and in nonhuman animals with hippocampal lesions (Alvarado & Bachevalier, 2005; Alvarado & Rudy, 1995; Driscoll, Howard, Prusky, Rudy, & Sutherland, 2005; but see Bussey, Warburton, Aggleton, & Muir, 1998; Saksida, Bussey, Buckmaster, & Murray, 2006). The ability to form relations among arbitrary items is also impaired in aging (Naveh-Benjamin, 2000), and is thought to reflect age-related changes in hippocampal function (Giovanello, Kensinger, Wong, & Schacter, 2010; Rondina et al., 2015), among other brain regions (Miller et al., 2008; Sperling, 2007). Compared to their younger counterparts, older adults show impaired recognition of associations but intact recognition of the items themselves (Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Old & Naveh-Benjamin, 2008). Germane to the present work, older adults, similar to amnesic individuals, are impaired on TP (Driscoll et al., 2003; Ostreicher, Moses, Rosenbaum, & Ryan, 2010), and these age-related impairments on TP are significantly related to lower hippocampal volumes (Driscoll et al., 2003). Despite impaired learning for arbitrary relations in TP, both amnesic cases (Moses, Ostreicher, Rosenbaum, & Ryan, 2008; Ryan, Moses, Barense, & Rosenbaum, 2013) and older adults (Ostreicher et al., 2010) show intact learning in conditions with semantically rich relations, such as RPS.
Unitization is a training strategy that has recently been shown to help some amnesic cases circumvent their relational binding deficits on versions of TP that require arbitrary relations to be learned (D’Angelo, Kacollja, Rabin, Rosenbaum, & Ryan, 2015; Ryan et al., 2013). Unitization encourages the fusing of distinct items, through an action, into a single unit from which the relations among the items can then be derived. Whereas with relational binding the elements and their respective relations are each stored separately, unitization integrates multiple representations into a single unit (Graf & Schacter, 1989). From this fused representation, the relations among the items may be derived, obviating the need for storage of the relations themselves. With this unitization strategy, two amnesic cases (D.A. in Ryan et al., 2013; N.C. in D’Angelo et al., 2015) were able to learn arbitrary relations in the TP task, and retain them following extended delays (e.g., 1 month). This stands in contrast to their impaired learning under standard training conditions, for which no cognitive strategy was provided and the relations had to be learned through trial and error. Unitization may have supported learning in these cases by reducing reliance on hippocampal-dependent relational binding and increasing reliance on fused representations.
In the present study, we examined whether unitization could circumvent relational binding deficits in older adults and support their TP performance, as it does in some amnesic cases. We also hypothesized that the ability of older adults to capitalize on a unitization strategy may depend on cognitive status. In particular, there is accumulating evidence that a subset of nominally healthy, community-dwelling older adults may show significant, early signs of preclinical cognitive decline. Newsome, Duarte, and Barense (2012) compared individuals with mild cognitive impairment (MCI) with two groups of nominally healthy older adults on a perceptual interference task shown to recruit the perirhinal cortex (Barense et al., 2012). The healthy older adults were separated into these two groups based on whether they passed the Montreal Cognitive Assessment (MoCA), which is a brief standardized cognitive assessment tool that is often used to discriminate healthy control participants from clients with MCI (Nasreddine et al., 2005). Critically, Newsome et al. found that individuals with MCI and older adults who failed the MoCA were impaired on the perceptual interference task, relative to the older adults who passed the MoCA. Given that performance in the group of nominally healthy older adults who failed the MoCA did not significantly differ from performance in the patients with MCI, Newsome and colleagues suggested that these otherwise healthy older adults might be at risk for clinically significant cognitive decline. In a subsequent study, Newsome, Pun, Smith, Ferber, and Barense (2013) also found that nominally healthy older adults who failed the MoCA showed electrophysiological signatures similar to those with MCI and AD in an auditory odd-ball task (e.g., reduced P300 amplitude). The vulnerability to interference in those who fail the MoCA has also been shown to extend to memory (Yeung, Ryan, Cowell, & Barense, 2013). Yeung et al. found that relative to those who pass the MoCA, those who fail the MoCA show greater false recognition for novel items that have a significant degree of feature overlap with previously studied items. In these studies, nominally healthy older adults who failed the MoCA tended to fail because of poor performance on the delayed recall section of the MoCA, but generally performed within the average range on neuropsychological tests. In sum, prior work (Newsome et al., 2012; Yeung et al., 2013) has demonstrated that, although the MoCA is not a specific proxy of medial temporal lobe (MTL) function, nominally healthy older adults who fail the MoCA show early signs of cognitive impairment on MTL-dependent tasks, relative to those who pass the MoCA.
In order to determine whether older adults who are cognitively intact and those with early signs of cognitive impairments can similarly capitalize on a unitization strategy, two groups of nominally healthy and community-dwelling older adults were tested on TP under standard conditions for which knowledge regarding the relations among the stimuli could be based on rich semantic information (RPS) and under conditions in which the arbitrary relations among the stimuli had to be learned within the confines of the experiment. Both groups were also tested on a condition for which the unitization strategy was provided to support learning of arbitrary relations among stimuli. As in prior work (Newsome et al., 2012, 2013; Yeung et al., 2013), these two groups differed based on whether the individuals passed the MoCA. Our five predictions for the two groups and the tasks were as follows: (1) Given accumulating evidence that older adults who fail the MoCA show preclinical signs of cognitive decline on MTL-dependent tasks (Newsome et al., 2012), we predicted that older adults who failed the MoCA would show overall greater impairment on TP than those who passed the MoCA. (2) Based on the prior work from our lab (Ostreicher et al., 2010), we predicted that older adults in both groups would show impairments on standard versions of TP with arbitrary relations relative to a semantically rich RPS condition. (3) Given our hypothesis that unitization is a viable strategy to support a relational memory impairment (Ryan et al., 2013), we predicted that both groups would show better performance on TP with unitized training relative to the standard training.
Our fourth and fifth predictions were based on the hypothesis that individuals who failed the MoCA have impairments that are selective for MTL-dependent processes (Newsome et al., 2012; 2013; Yeung et al., 2013), such as relational binding. Therefore, we additionally predicted that, (4) relative to those who passed the MoCA, those who failed the MoCA would show larger TP impairments on a standard TP task with arbitrary relations than in a condition with semantically rich relations (RPS). (5) Similarly, we predicted that relative to those who passed the MoCA, those who failed the MoCA would show larger TP impairments with arbitrary relations under standard training than unitized training. If our predictions are borne out, this would in turn support the hypothesis that individuals who failed the MoCA have selective decline in MTL function including relational binding. However, a failure to find support for these predictions would instead suggest that individuals who failed the MoCA have broader cognitive impairments than has previously been reported. Broad cognitive impairments would cause difficulties in the effective use of cognitive strategies like unitization to mitigate impaired relational binding.
Methods
Participants
For the present study, older adults were recruited to fill two groups of 20 participants, where the groups differed based on whether participants passed the MoCA (i.e., scored 26/30 or higher; Newsome et al., 2012). Individuals who reported English as their second language were excluded from this study, as the MoCA has only been standardized on monolingual speakers (Nasreddine et al., 2005). Inclusion criteria included no known neurological conditions, and no history of concussions. Seven older adults (four who failed the MoCA and three who passed the MoCA) participated in the study but were excluded for the following reasons. Six participants (four who failed the MoCA and two who passed the MoCA) only participated in the TP tests and did not return for later neuropsychological testing and elemental testing, and one participant (who passed the MoCA) did not finish the training portion of the elemental task due to a computer problem.
The final sample consisted of 20 older adults who had MoCA scores below the cutoff of 26 (Mscore = 23.1, Range = 18–25), who formed the lower MoCA group (13 female, Mage = 71.6 years, SD = 6.8, Meducation = 15.4 years, SD = 2.8, 14 right-handed), and 20 older adults who had MoCA scores above 26 (Mscore = 27.8, Range = 26–30), who formed the higher MoCA group (17 female, Mage = 71.4 years, SD = 5.6, Meducation = 15.0 years, SD = 2.9, 18 right-handed). Notably, individuals in the lower MoCA group tended to fail the MoCA because of poor performance on the delayed recall portion of the MoCA (M lower MoCA = 1.7 vs. M higher MoCA = 4.0). Participants were recruited from the Adult Volunteer Pool at the University of Toronto and completed testing in two or three testing sessions. The study was approved by the University of Toronto Ethics Review Board. All participants gave informed written consent and received monetary compensation. At the time of testing, none of the participants had a formal diagnosis of MCI or any other cognitive impairment, and were all nominally healthy, community-dwelling, older adults. We note one participant in the lower MoCA group had a MoCA score of 18, which might suggest that he/she has significant impairment. However, as this and all individuals in the lower MoCA group were nominally healthy and had no clinical diagnoses at the time of testing, more work is needed to determine the clinical significance of low MoCA scores in nominally healthy older adults.
Apparatus and stimuli
The apparatus and stimuli were similar to those used in Ryan et al. (2013). The experiment was programmed using E-prime, and the stimuli were presented on a Dell laptop computer. Three stimuli (A, B, C) were used in each of the three TP conditions: RPS, Shapes-Standard, and Shapes-Unitized (Figure 1). The conditions differed in the extent to which the relations were semantically rich versus arbitrary and in terms of how training was conducted. The RPS condition contained known objects with semantically rich relations. For example, we believe that it is fair to assume that the knowledge that “scissors cut paper” is a commonly held semantic association. RPS was included in the present study as amnesic individuals traditionally demonstrate intact performance in this condition, and performance on this condition is thought to rely on access to semantic information (Moses et al., 2008; Ryan et al., 2013) as mediated by extra-hippocampal structures including left prefrontal and temporal cortical structures (Moses et al., 2009). RPS was used then as a contrast to standard TP with other known objects for which arbitrary relations had to be acquired within the experimental session. The RPS stimuli depicted the hand game where rock crushes scissors, scissors cut paper, and paper covers rock. Unlike our prior work (Moses et al., 2008; Ostreicher et al., 2010; Ryan et al., 2013), the majority of participants in the present study were unfamiliar with the rules of RPS (13 participants from the higher MoCA group and 18 participants from the lower MoCA group). Prior to beginning the RPS condition, the experimenter asked participants if they were familiar with the rules of RPS, and asked the participants to reproduce the hand signals and rules. If participants were not familiar with the rules of RPS, the experimenter described the rules and had the participants reproduce the hand signals for them prior to beginning the training phase. The experimenter used semantically rich descriptions (e.g., scissors cuts paper) to ensure that the 31 participants unfamiliar with RPS understood the rules prior to the experimental session. 1 Note that although fewer individuals in the lower MoCA group had a priori knowledge of the rules of RPS, prior knowledge of RPS was not a significant predictor of accuracy on RPS (t(36) = −0.48, p = .633), while group status was a significant predictor (t(36) = 3.53, p = .001).
Figure 1.
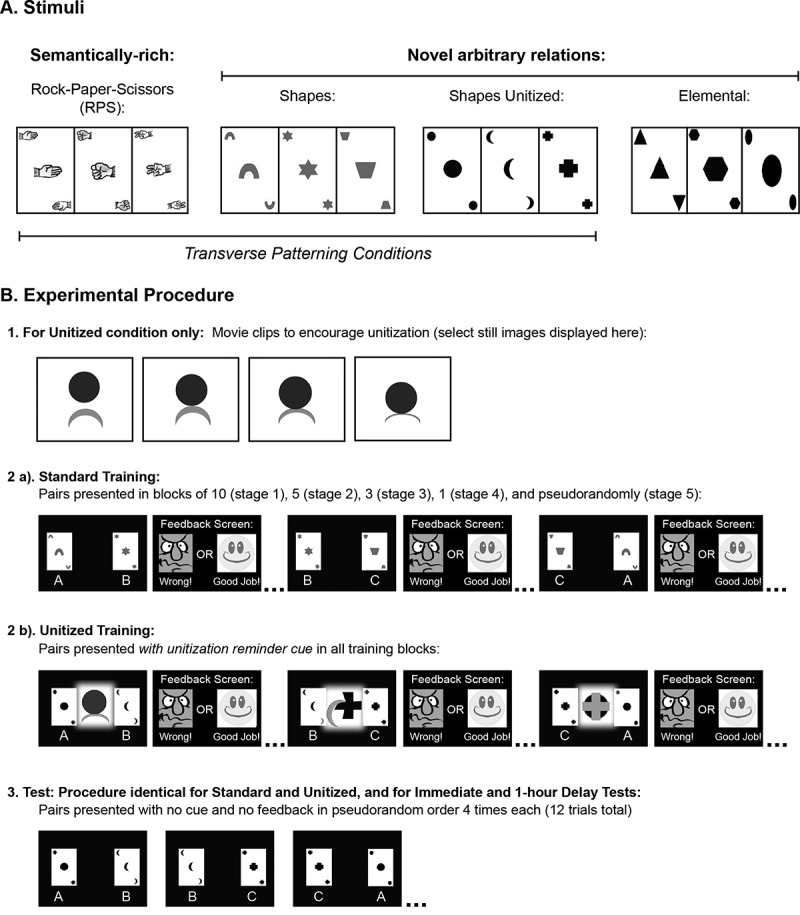
A. Stimuli used in the three transverse patterning conditions and elemental condition. B. Experimental procedures. B-1. Example stills from the flash animations that were shown before training for the unitized condition. Flash animations depicted one object physically interacting with the other object with the relations of squish, pierce, or cover. B-2. Training procedures. B-2a. Standard training (RPS, Shapes, and Elemental) presented two stimuli, one on each side of the screen, and participants were required to select the correct item that “wins”. Responses were self-paced and feedback was provided. B-2b. Unitized training was identical to standard training except that a still image from the animations was included in the center of the stimulus display (“U”) to serve as a “hint” for which stimulus was correct. B-3. Test procedures. All test blocks, regardless of whether training was standard or unitized, and regardless if the test was immediate or after an hour delay, followed the same procedure and the same stimulus arrangement. Note that for ease of illustration, the stimuli are shown by their corresponding letters (A–C for the stimulus elements, U for the unitization cue); however, such letters were not presented to the participants.
The Shapes-Standard and Shapes-Unitized conditions also contained known objects (e.g., star, trapezoid, arch, circle, crescent, cross; see Figure 1), but the relations among the objects were arbitrary and expected to be unknown before the experimental session. In all conditions, participants were trained on the relations A + B–, B + C–, and C + A–.
Participants were also given an Elemental task for which relational binding is not required. In the elemental task, performance can be guided by the associative strength of the individual items (i.e., one item always wins and another always loses), and is not dependent on hippocampal function; amnesic cases with damage to the hippocampus and its extended system show intact performance on the elemental task, but impaired performance on TP (Rickard & Grafman, 1998). Three additional, known geometric shapes with arbitrary, previously unknown relations were used as stimuli in the Elemental task and participants were trained on a hierarchy of relations: (A > B > C; see Figure 1).
Procedure
Participants completed two or three testing sessions depending on their availability. In the first session, participants were trained and tested on the three TP conditions, which were presented in the following order: RPS, Shapes, and Shapes-Unitized. RPS was always administered first based on prior work showing that semantically rich conditions such as RPS can support TP learning in older adults (Ostreicher et al., 2010). Shapes-Unitized was always administered last so as to not influence strategies on the Shapes-Standard condition.
In a subsequent session, participants completed the elemental task and, if needed, neuropsychological testing; however, some participants completed the elemental task and neuropsychological testing in two separate sessions. Three participants completed the neuropsychological testing prior to the TP session as part of other studies in the laboratory. The elemental task was completed on a separate day from the TP conditions to minimize interference regarding the organization of response rules. In all three TP conditions and the elemental condition, participants completed a training phase, followed by two test phases: one test phase was given immediately following training and a second test phase was given following a one-hour delay in order to minimize the influence of working memory/online maintenance strategies on test performance (see D’Angelo et al., 2015; Ryan et al., 2013).
Training phase
Training procedures are shown in Figure 1B and were identical to those used in our prior work (Moses et al., 2008; Ostreicher et al., 2010; Ryan et al., 2013). With the exception of the RPS condition, participants were not informed of the relations among the stimuli and were required to learn the relations by trial and error. Participants were shown two objects on every trial, and their task was to select one of the objects. Participants responded using the keys “P” and “Q” on the laptop keyboard to indicate the left and right stimulus, respectively. Feedback was provided on every trial to indicate whether or not they were performing the task correctly: a happy-face cartoon and the caption “Good Job!” and an angry-face cartoon and the caption “Wrong!” were displayed following correct and incorrect responses, respectively. Mean accuracy was presented at the end of each block of trials.
As in our prior work (Moses et al., 2008; Ostreicher et al., 2010; Ryan et al., 2013), training unfolded over five stages. In the first stage, participants completed a block of 10 trials of one pair (e.g., AB × 10), followed by a block of 10 trials of the next pair (e.g., BC × 10), and, lastly, a block of 10 trials of the third pair (e.g., CA × 10). In the second stage, participants completed a block consisting of five trials of each of the pairs in a consecutive order (e.g., [AB, BC, CA] × 5). The third stage consisted of three blocks; for each block, participants completed three presentations of each of the pairs in a consecutive order (e.g., [AB, BC, CA] × 3). In the fourth stage, participants completed a block of trials in which the three pairs were presented nine times (e.g., [AB, BC, CA] × 9). Lastly, in the fifth stage, participants were presented with two blocks in which each of the pairs appeared 18 times in a pseudorandom order. If a participant’s accuracy was less than 50% for a block of trials, the block was repeated. The participants completed a minimum of 207 trials across the five stages during the training phase.
Training in the Shapes-Unitized condition was identical to training in the Shapes-Standard condition with the following exceptions. Prior to commencing the training phase, participants watched three animations that highlighted the relations among the three stimuli. Each animation depicted two items interacting such that a winner was made clear. The interactions were as follows: the circle squished the crescent, the crescent stabbed the plus sign, and the plus sign covered the circle. For each animation, the experimenter verbally described the animation without labeling the objects and asked participants to point to the object they thought would be the winner (e.g., “this object is squishing this other object, if you had to pick, which one do you think would be the winner?”). Participants were corrected if they chose the incorrect object. The training phase was identical to the other conditions, with the exception that a final still from each of the animations was presented centrally between the two items to remind the participants of the animations and to encourage the formation of a fused or unitized representation. One unitized image (U) was presented on each trial and corresponded to the animation depicting the two items presented on the current trial (Figure 1B-2). Note that participants were never explicitly given the TP rule (A + B–, B + C–, C + A–) in either the Shapes-Standard or Shapes-Unitized conditions. The unitization strategy merely highlighted the winner in each pair using the animations.
Test phase
Participants completed both an immediate and 1-hour delay test for all conditions (Figure 1B-3). Both tests consisted of 12 trials, in which each pair was shown four times. Test phases were identical in all conditions—the central unitized images were not presented in the test phase in the Shapes-Unitized condition. Throughout training and test phases, each object was presented equally often on each side of the display (left/right).
Post-experimental questionnaires were administered to each participant for each condition to assess awareness of the relationships and perceived learning strategies (see Ostreicher et al., 2010); however, due to experimenter error, the first 8 participants in the lower MoCA group and first 9 participants in the higher MoCA group did not receive the full questionnaires. Therefore, issues of awareness will not be considered for the present purposes.
Neuropsychological testing
Cognitive profiles for the two participant groups were obtained via neuropsychological assessment. All participants completed the neuropsychological assessment within 1 year of experimental testing (see Newsome et al., 2012, 2013; Yeung et al., 2013). The battery consisted of the Logical Memory subtest from the Wechsler Memory Scale IV (WMS) (Wechsler, 2009), Trails A & B (Reitan & Wolfson, 1985), the Digit Span subtest of the Wechsler Adult Intelligence Scale (WAIS) (Wechsler, Coalson, & Raiford, 2008), the Rey–Osterrieth Complex Figure (Osterrieth, 1944), the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999), and the Visual Object and Space Perception Battery (VOSP) (Warrington & James, 1991).
Results from the neuropsychological testing are shown in Table 1. The neuropsychological tests were used to characterize differences between the two groups using Cohen’s d, as it is not expected to alter in a systematic way with changes in sample size. Cohen’s d was used to show on which tests the two groups showed small, medium, large, and very large differences. Recall that d values of 0.2, 0.5, and 0.8 are considered small, medium, and large effects, respectively (Cohen, 1988). Additionally, we have labeled d values over 1.0 to highlight “very large” effects. Scaled scores are presented for all measures with the following exceptions. Given the age range of the participants, the two groups were compared only on raw scores on Story B from the WMS, as it was common to all participants. The WASI scores presented are t-scores and the VOSP scores are raw scores. Overall, the neuropsychological testing revealed that mean performance in the two groups fell within the normal range (high average to low average-borderline). Both groups had high-average to average performance on the WASI and on Trails A & B. Participants had average to low-average performance on the Rey–Osterrieth Complex Figure test; low-average performance was observed only for the lower MoCA group. Participants in both groups had average performance on the forward digit span subtest of the WAIS. The higher MoCA group and lower MoCA group, respectively, had low-average and low-average/borderline performance on the backward digit span subtest.
Table 1.
Mean (SD) and range of performance for each test in the neuropsychological assessment for each group.
| Higher MoCA group | Lower MoCA group | Effect size (Cohen’s d) | ||
|---|---|---|---|---|
| Age in years | 71.2 (5.5) | 71.6 (6.8) | 0.03 | |
| 60–79 | 59–82 | |||
| Education | 14.8 (2.9) | 15.4 (2.8) | 0.14 | |
| 10–22 | 12–24 | |||
| MoCA | 27.8 (1.3) | 23.1 (1.8) | 3.03 | |
| 26–30 | 18–25 | |||
| Visuospatial/executive | 4.2 (0.9) | 3.5 (0.9) | 0.76 | |
| 2–5 | 1–5 | |||
| Naming | 2.9 (0.4) | 2.6 (0.6) | 0.52 | |
| 2–3 | 1–3 | |||
| Attention | 5.9 (0.4) | 5.2 (0.9) | 1.03 | |
| 5–6 | 3–6 | |||
| Language | 2.8 (0.4) | 2.6 (0.8) | 0.25 | |
| 2–3 | 1–4 | |||
| Abstraction | 2.0 (0.2) | 1.8 (0.4) | 0.60 | |
| 1–2 | 1–2 | |||
| Delayed recall | 4.0 (1.0) | 1.7 (1.2) | 2.03 | |
| 2–5 | 0–4 | |||
| Orientation | 6.0 (0.0) | 5.6 (0.8) | 0.97 | |
| 6–6 | 3–6 | |||
| Wechsler Memory Scale IV – Logical Memory | ||||
| Immediate recall | 14.2 (3.2) | 12.6 (4.9) | 0.39 | |
| 8–20 | 4–22 | |||
| Delayed recall | 12.2 (4.2) | 8.4 (4.6) | 0.86 | |
| 3–20 | 0–16 | |||
| Recognition | 12.2 (2.1) | 10.8 (1.8) | 0.76 | |
| 7–15 | 7–13 | |||
| Rey–Osterrieth Complex Figure | ||||
| Copy | 10.1 (3.9)A | 9.6 (4.5)A | 0.13 | |
| 3–18 | 3–18 | |||
| Immediate recall | 10.0 (3.6)A | 8.3 (2.7)ALA | 0.54 | |
| 3–18 | 4–14 | |||
| Delayed recall | 9.5 (3.3)A | 7.0 (3.8)LA | 0.71 | |
| 3–14 | 2–13 | |||
| Wechsler Abbreviated Scale of Intelligence | ||||
| Vocabulary | 57.9 (8.8)HA | 51.1 (14.7)A | 0.58 | |
| 44–75 | 20–74 | |||
| Similarities | 57.8 (7.4)HA | 56.1 (8.8)A | 0.20 | |
| 35–70 | 35–67 | |||
| Matrix reasoning | 59.8 (11.7)HA | 56.0 (8.2)A | 0.38 | |
| 24–74 | 42–69 | |||
| Block design | 53.4 (9.8)A | 48.8 (9.9)A | 0.47 | |
| 34–72 | 30–68 | |||
| Trails | ||||
| Trail A | 10.6 (2.8)A | 8.9 (2.8)A | 0.60 | |
| 6–16 | 4–14 | |||
| Trail B | 12.3 (2.5)AHA | 9.5 (2.0)A | 1.30 | |
| 8–16 | 7–13 | |||
| Wechsler Adult Intelligence Scale | ||||
| Forward digit span | 10.2 (3.4)A | 9.1 (2.8)A | 0.35 | |
| 4–19 | 4–15 | |||
| Backward digit span | 7.9 (1.9)LA | 6.3 (3.2)LAB | 0.62 | |
| 5–11 | 2–14 | |||
| Visual Object and Space Perception Battery | ||||
| Shape detection (/20) | 19.1 (1.0) | 18.9 (1.3) | 0.18 | |
| (cutoff score < 15) | 17–20 | 17–20 | ||
| Incomplete letter (/20) | 19.6 (0.5) | 18.9 (1.2) | 0.87 | |
| (cutoff score < 16) | 19–20 | 16–20 | ||
| Silhouettes (/30) | 20.3 (4.6) | 20.0 (5.5) | 0.05 | |
| (cutoff score < 15) | 10–27 | 9–30 | ||
| Object decision (/20) | 17.2 (2.4) | 16.9 (1.5) | 0.15 | |
| (cutoff score < 14) | 11–20 | 14–20 | ||
| Progressive Silhouettes (/20) | 10.0 (2.6) | 11.0 (3.9) | 0.31 | |
| (cutoff score > 15) | 6–15 | 4–18 | ||
| Dot counting (/10) | 10.0 (0.0) | 9.9 (0.3) | 0.65 | |
| (cutoff score < 8) | 10–10 | 9–10 | ||
| Position discrimination (/20) | 19.8 (0.6) | 18.8 (2.0) | 0.76 | |
| (cutoff score < 18) | 18–20 | 12–20 | ||
| Number location (/10) | 9.5 (0.8) | 8.4 (1.9) | 0.84 | |
| (cutoff score < 7) | 8–10 | 2–10 | ||
| Cube analysis (/10) | 9.6 (0.5) | 9.0 (1.3) | 0.66 | |
| (cutoff score < 6) | 9–10 | 5–10 | ||
Cohen’s d of the difference characterizes the differences between the two groups on each measure. Grey filling on the far right represents effect sizes (light to dark shades represent small to very large effects). For standardized scores, superscripts indicate normed labels: HA, high average; AHA, average-high average; A, average; ALA, average-low average; LA, low average; LAB, low average-borderline.
The two groups showed medium to very large difference on delayed tests of memory (WMS delayed recall, WMS delayed recognition, Rey–Osterrieth delayed recall), tests of executive function (Trails A & B), working memory (WAIS backwards digit span), visual perceptual processing (VOSP incomplete letters, position discrimination, number location, cube analysis), and semantic memory (WASI vocabulary), each of which may be an important component process of unitization. Note that these group differences occur within the context of performance that generally falls within age norms. The fact that performance fell within age norms further suggests that lower MoCA group are nominally healthy individuals who show early signs of impairment in the absence of clinical signs of cognitive decline.
Data analysis
Primary analysis consisted of a targeted analysis of variance to address the five predictions of TP performance outlined in the “Introduction”. Supplementary analyses were also conducted to characterize further TP performance and the effect of unitization in these groups.
Predictions-based analysis for TP conditions
Performance on the TP conditions was evaluated based on performance on the 1-hour delay test to reduce the contribution of extended online maintenance of relations to performance (D’Angelo et al., 2015; Ryan et al., 2013). The five predictions outlined in the “Introduction” were assessed using a targeted mixed-effects analysis of variance (ANOVA) that included Group (higher/lower MoCA) as a between-subject contrast, two within-subject contrasts of Condition: one for Shapes-Standard versus RPS and another for Shapes-Standard versus Shapes-Unitized, and two interactions between Group and Condition, which tested the effect of group on each of the contrasts of Condition. This targeted mixed-effects ANOVA was used to test our predictions directly and to avoid multiple comparisons. In particular we were not interested in comparisons between RPS and Unitization. The targeted ANOVA used here is similar to a group by task repeated-measures ANOVA, but does not include all comparisons between the three TP conditions. Results for the immediate test are reported in the Appendix for comparison.
Our first prediction was that older adults who failed the MoCA would show greater TP impairment than those who passed the MoCA. This prediction was tested based on the effect of Group.
Our second prediction was that older adults would show relational memory impairments for arbitrary relations (i.e., lower performance on the standard version of TP relative to a semantically rich condition). This prediction was tested using the first Condition contrast, which compared performance between the Shapes-Standard and RPS conditions.
Our third prediction was that unitization could ameliorate relational memory impairments in older adults. This prediction was tested using the second Condition contrast, which compared performance between Shapes-Standard and Shapes-Unitized conditions.
Our fourth prediction was that the lower MoCA group would show disproportionate impairments in relational memory for arbitrary relations, which would cause a larger group difference in the Shapes-Standard condition than in the RPS condition, in which prior knowledge can support learning of semantically rich relations (see Moses et al., 2009). This prediction was tested with the interaction between Group and the first Condition contrast (Shapes-Standard vs. RPS). Critically, we predicted a significant interaction with the lower MoCA group showing larger impairments on TP with arbitrary relations (Shapes) than on the semantically rich relations (RPS).
Similarly, our fifth prediction was that the lower MoCA group would show disproportionate impairments in relational memory for arbitrary relations under standard training, which would cause a larger group difference in the Shapes-Standard condition than in the Shapes-Unitized condition. This prediction was tested with the interaction between Group and the second Condition contrast (Shapes-Standard vs. Shapes-Unitized). Critically, we predicted a significant interaction with the lower MoCA group having lower accuracy than the higher MoCA group for arbitrary relations under standard training procedures relative to unitized training.
Supplementary TP analyses
The benefit of unitization was further examined in individuals whose cognitive status was healthy (MoCA ≥ 26) and who were not already performing at ceiling under standard training conditions (Shapes-Standard < 100% correct). Specifically, performance in the Shapes-Standard and Shapes-Unitized conditions was assessed in the healthy group after removing participants who had ceiling performance on the Shapes-Standard delay test. Performance in the remaining participants was analyzed using a repeated -measures ANOVA with condition (Shapes-Standard, Shapes-Unitized) as a within-subject factor.
To examine whether impaired test performance was the result of impaired training, training accuracy was analyzed using an identical ANOVA to what was used in the analysis of 1-hour delay test accuracy. Note that analyses conducted with trials to criterion during training as the dependent measure yielded a similar pattern of results.
Elemental task
To contrast further the two groups on a non-hippocampal dependent task, the accuracy on the 1-hour delay test of the Elemental task was examined as a function of group using a one-way ANOVA with group (higher MoCA/lower MoCA) as a between-subjects factor. Accuracy during training on the Elemental task was also examined as a function of group in an identical analysis.
Results
We first present the results of our prediction-based analyses for the TP conditions, followed by supplementary analyses of the TP conditions and analyses of Elemental task performance.
Predictions-based analysis for TP conditions
Mean accuracy on the TP tests is presented in Figure 2 as a function of group, delay, and condition, along with the elemental threshold (accuracy = 0.67, depicted as a dotted line in Figures 2 and 3). The elemental threshold reflects the maximum score achievable if an elemental learning rule is incorrectly applied to a TP task (e.g., a “winner-takes-all” rule). If an individual fails to use relational learning and instead uses elemental learning, only two-thirds (67%) of the relations can be correctly learned. Therefore, individuals who perform at or below this criterion are considered to have impaired relational learning (D’Angelo et al., 2015; Rickard & Grafman, 1998; Rickard et al., 2006; Ryan et al., 2013).
Figure 2.
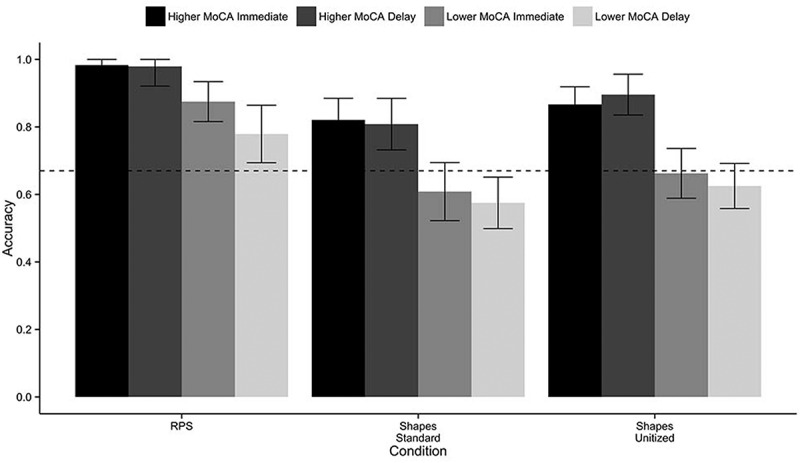
Mean accuracy in the test phases of the TP tasks as a function of group, delay, and condition. For each condition, we plot performance in the higher MoCA and lower MoCA groups for the immediate and 1-hour delay tests. Error bars represent the 95% confidence interval of the mean corrected for between-subject variability (Morey, 2008). The dotted line represents the elemental threshold (0.67—see text for details).
Figure 3.
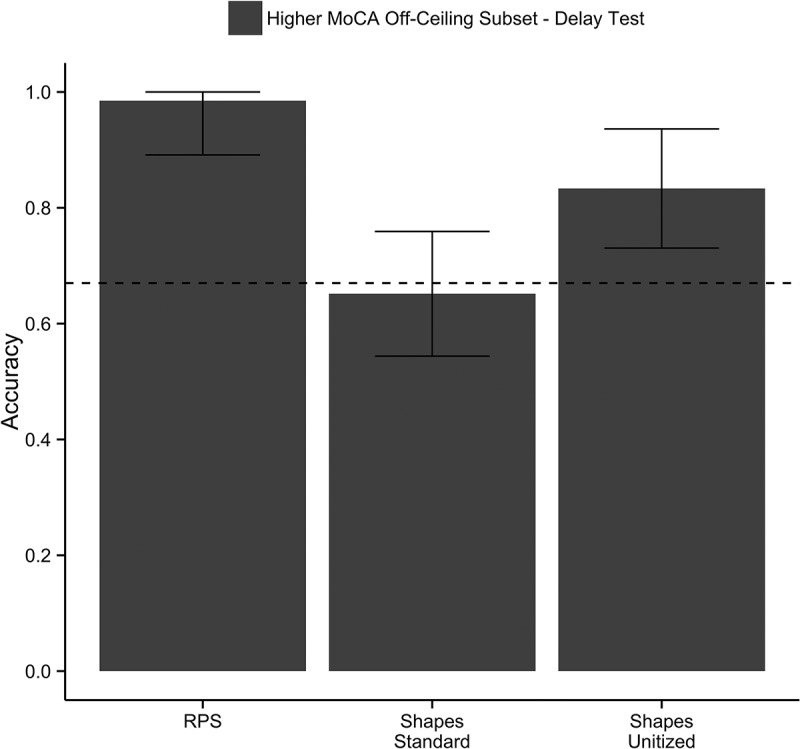
Mean accuracy on the hour delay test as a function of condition for higher MoCA older adults who were not at ceiling on the immediate test. Error bars represent the 95% confidence interval of the mean corrected for between-subject variability (Morey, 2008). The dotted line represents the elemental threshold (accuracy = 0.67).
Below we report analyses on accuracy from the 1-hour delay tests (analyses on accuracy from the immediate tests showed the same pattern and are presented in the Appendix). Table 2 presents the results of the overall ANOVA with the contrasts specified above.
Our first prediction that older adults in the lower MoCA group would show greater impairments than those in the higher MoCA group was supported. Participants in the lower MoCA group had lower overall accuracy (M = 0.66, SE = 0.05) than did those in the higher MoCA group (M = 0.89, SE = 0.04; F(1, 38) = 26.3, p < .001, η2 p = 0.41).
Our second prediction that older adults would show greater impairments on TP with arbitrary relations than with semantically rich relations was supported. Overall, accuracy was lower in the Shapes-Standard condition (M = 0.69, SE = 0.04) than in the RPS condition (M = 0.88, SE = 0.03; F(1, 76) = 24.9, p < .001, η2 p = 0.25).
Our third prediction that unitization would support relational memory impairments for arbitrary relations in older adults was marginally supported. Although overall accuracy was numerically higher in the Shapes-Unitized condition (M = 0.76, SE = 0.03) than in the Shapes-Standard condition (M = 0.69, SE = 0.04), the effect of condition was statistically indeterminate (F(1, 76) = 3.8, p = .056, η2 p = 0.05).
Our fourth prediction was that the lower MoCA group would show disproportionate impairments in relational memory for arbitrary relations, which would cause a larger group difference in the Shapes-Standard condition than in the RPS condition, in which prior knowledge can support learning of semantically rich relations. Our fourth prediction was not supported (F(1, 76) = 0.72, p = .399, η2 p = 0.01). Failure to find a significant interaction indicates that the difference in the group effect between the Shapes-Standard condition and the RPS condition was not sufficiently large. This negative result was not driven by equivalent performance in the Shapes-Standard condition for the two groups (lower MoCA: M = 0.58, SE = 0.05; higher MoCA: M = 0.81, SE = 0.05), but was due to large differences between groups in both conditions (RPS performance—lower MoCA: M = 0.87, SE = 0.03; higher MoCA: M = 0.98, SE = 0.01), as was found in the results from Prediction 1.
Our fifth prediction was that the lower MoCA group would show disproportionate impairments in relational memory for arbitrary relations, which would cause a larger group difference in the Shapes-Standard condition than in the Shapes-Unitized condition. Our fifth prediction was not supported (F(1, 76) = 0.28, p = .598, η2 p = 0.003). Once again, failure to find a significant interaction was driven by worse performance in the lower MoCA group in both conditions, consistent with the results from Prediction 1. Note that even with unitized training, mean accuracy in the Shapes-Unitized condition in the lower MoCA group was below the elemental threshold and the confidence interval around their performance included the elemental threshold (M = 0.62; 95% CI = 0.54–0.71).
Table 2.
Results of overall ANOVA on delay test performance with specified contrasts (Df, degrees of freedom; SS, sum of squares; MS, mean squared values).
| Df | SS | MS | F | p | η2p | |
|---|---|---|---|---|---|---|
| Group | 1 | 1.653 | 1.653 | 26.3 | <.001 | 0.41 |
| Error (Group) | 38 | 2.391 | 0.063 | |||
| Condition Contrast 1: Shapes-Standard vs. RPS | 1 | 0.625 | 0.625 | 24.9 | <.001 | 0.25 |
| Condition Contrast 2: Shapes-Standard vs. Shapes-Unitized | 1 | 0.095 | 0.095 | 3.77 | .056 | 0.05 |
| Group by Contrast 1 (Shapes-Standard vs. RPS) | 1 | 0.018 | 0.018 | 0.72 | .399 | 0.01 |
| Group by Contrast 2 (Shapes-Standard vs. Shapes-Unitized) | 1 | 0.007 | 0.007 | 0.28 | .598 | 0.003 |
| Error (condition) | 76 | 1.908 | 0.025 |
The results from our tests of predictions four and five suggest that individuals in the lower MoCA group likely have broader cognitive impairments than previously thought. 2 The nature of their impairments is discussed in the “General discussion” section.
Supplementary TP analyses
As is evident from Figure 2, performance in the higher MoCA group was above the elemental threshold in the Shapes-Standard condition. Nine of the 20 higher MoCA participants had 100% accuracy on the Shapes-Standard condition, even after the hour delay. Anecdotally, some of these participants spontaneously self-reported using a unitization-like strategy to remember the relations (e.g., imagining the items interacting together). As a more stringent test of how unitization can support relational memory impairments in aging, performance was re-examined in the higher MoCA group after excluding the participants who were at ceiling on the Shapes-Standard delay test. Mean accuracy from the remaining 11 participants was assessed using a repeated measures ANOVA with condition (Shapes/Shapes-Unitized) as the within-subjects factor. Accuracy for this subgroup is shown in Figure 3 as a function of condition. This subgroup had impaired performance (i.e., at or below the elemental threshold) in the Shapes-Standard condition (M = 0.65, SE = 0.06, 95% CI = 0.52–0.79), which improved to the above-threshold performance with unitization training (M = 0.83, SE = 0.06, 95% CI = 0.69–0.98; F(1, 10) = 6.92, p = .025, η2 p = 0.41). Importantly, these individuals did not show impairment on RPS (M = 0.99, SE = 0.01), and thus unlike what was observed in the lower MoCA group, this subgroup has a selective relational memory deficit in TP with arbitrary relations that can be ameliorated through unitization.
TP training data
The analyses presented above revealed consistent effects of group, showing that the lower MoCA group had lower accuracy than the higher MoCA group across conditions. These analyses also showed that performance on RPS was greater than in the Shapes-Standard condition, that performance on the Shapes-Unitized condition was numerically greater than in the Shapes-Standard condition, while also suggesting that the lower MoCA group may not simply have impairments selective to relational binding for arbitrary relations. To examine whether these findings found at test were due to differences that were present during training, we examined whether the differences between the two groups were similar across the RPS and Shapes-Standard conditions during training (as they were at test), as well as whether the differences between the two groups were similar across the Shapes-Standard and Shapes-Unitized conditions during training (as they were at test) using an identical ANOVA to the one described for the analysis of accuracy on the 1-hour delay test. Mean accuracy and mean number of trials presented in the training phase are presented in Table 3 as a function of group and condition.
Table 3.
Mean accuracy and mean number of trials (95% confidence interval) presented in the training phase as a function of group and condition.
| TP task |
|||||
|---|---|---|---|---|---|
| RPS | Shapes- Standard |
Shapes-Unitized | Elemental task |
||
| Higher MoCA | Accuracy | 0.96 | 0.81 | 0.97 | 0.96 |
| (0.95–0.97) | (0.74–0.88) | (0.92–1.02) | (0.95–0.97) | ||
| Number of trials | 207 | 217 | 207 | 207 | |
| (207–207) | (204–231) | (207–207) | (207–208) | ||
| Lower MoCA | Accuracy | 0.83 | 0.61 | 0.89 | 0.87 |
| (0.77–0.90) | (0.54–0.68) | (0.83–0.96) | (0.79–0.94) | ||
| Number of trials | 211 | 245 | 212 | 217 | |
| (207–215) | (220–269) | (207–216) | (207–226) | ||
In the training phase, participants in the lower MoCA group had lower overall accuracy (M = 0.78, SE = 0.04) than did those in the higher MoCA group (M = 0.91, SE = 0.03; F(1, 38) = 19.7, p < .001, η2 p = 0.34). As in the test phase, accuracy across both groups in the training phase was lower in the Shapes-Standard condition (M = 0.71, SE = 0.03) than in the RPS condition (M = 0.90, SE = 0.02; F(1, 76) = 13.4, p < .001, η2 p = 0.15). In the training phase, accuracy across both groups was significantly higher in the Shapes-Unitized condition (M = 0.93, SE = 0.02) than in the Shapes-Standard condition (M = 0.71, SE = 0.03; F(1, 76) = 86.8, p < .001, η2 p = 0.53).
In the analysis of delay test accuracy, the contrast for our fourth prediction revealed that performance across groups did not differ between the Shapes-Standard and RPS conditions. The analysis of accuracy during the training showed similar results, as the interaction between group and condition was not significant (F(1, 76) = 0.13, p = .720, η2 p = 0.01). These results indicate that the effect of group did not differ between the Shapes-Standard and RPS conditions during the training phase, similar to what was found in the test phase. This finding indicates that the effects observed at test between RPS and Shapes-Standard for the two groups could be due to differences that were measured during the training phase.
In the delay test, the examination of our fifth prediction revealed that the effect of group did not differ between the Shapes-Standard and Shapes-Unitized conditions. The contrast of accuracy during the training phase in the Shapes-Standard and Shapes-Unitized conditions for the two groups revealed that the group difference was significantly larger for the Shapes-Standard condition (difference between groups = 0.20) than the Shapes-Unitized condition (difference between groups = 0.07; F(1, 76) = 8.03, p = .006, η2 p = 0.10). This result shows that the differences between groups observed in the delay test were not driven by differences during training—specifically, that the low performance observed in the lower MoCA group in the Shapes-Unitized condition at test was not due to poor performance in this condition in the training phase.
Elemental task
Accuracy on the Elemental tests is summarized in Table 4. The analysis of performance on the 1-hour delay test revealed a main effect of group, with better performance in the higher MoCA compared to the lower MoCA group (F(1, 38) = 7.05, p = .011, η2 p = .16). Accuracy and the number of trials to criterion in the training phase for the Elemental task are summarized in Table 3 as a function of group and task condition. Similar to the analysis on accuracy on the delay test, the ANOVA on the training accuracy revealed better performance for the higher MoCA compared to the lower MoCA group (F(1, 38) = 6.67, p = .014, η2 p = 0.15).
Table 4.
Mean accuracy (95% confidence interval) on the immediate and one-hour delay Elemental tests as a function of group.
| Immediate | One-hour delay | |
|---|---|---|
| Higher MoCA | 0.99 | 0.99 |
| (0.97–1.00) | (0.98–1.00) | |
| Lower MoCA | 0.87 | 0.88 |
| (0.78–0.96) | (0.78–0.97) |
Accuracy in the lower MoCA group for training and subsequent delay test was significantly lower than that of the higher MoCA group, suggesting a relative impairment. However, the lower MoCA group showed learning and retention of the elemental discriminations, as evidenced by delay test performance (M = 0.88, SE = 0.04) that was significantly above chance performance of 0.50 (t(20) = 9.0, p < .001).
General discussion
Although older adults have traditionally shown deficits in relational memory tasks (Naveh-Benjamin et al., 2004), such as TP (Driscoll et al., 2003), the present work demonstrates that unitization is a viable strategy to reduce these deficits. Findings presented here extend prior work (Newsome et al., 2012, 2013; Yeung et al., 2013) by showing that individuals who fail the MoCA have impairments on TP relative to those who pass the MoCA. Our findings also directly replicate previous work that showed deficits in older adults for learning arbitrary relations in the TP task (Shapes-Standard condition), but intact performance when the stimuli and their relations were known prior to the experimental session and were grounded in semantically rich knowledge (RPS condition) (Ostreicher et al., 2010). Recently, we demonstrated that a unitization strategy can circumvent TP impairments in some amnesic cases (D’Angelo et al., 2015; Ryan et al., 2013). Here, we extended this recent work and showed that a unitization strategy can lead to better memory for the relations among distinct objects. Critically, in healthy older adults who showed selective impairments on standard TP, unitization considerably improved performance. Therefore, unitization can support healthy older adult performance in what is traditionally considered to be a relational memory task.
The hypothesis that individuals who fail the MoCA have greater impairments selective to MTL-dependent processes, such as relational memory, was not supported in this work. The present set of findings suggests that those who fail the MoCA may have more widespread impairments, as evidenced by their lower accuracy on both the RPS and Elemental conditions. Furthermore, we did not find evidence that unitization can support the large relational memory impairment observed in the lower MoCA group. Although individuals in the lower MoCA group had high performance on the Shapes-Unitized condition during training, their test performance was not significantly greater than that exhibited by the higher MoCA group, and was not greater than the elemental threshold.
In our previous work, we proposed that by using a unitization strategy, amnesic cases, D.A. and N.C., were able to shift reliance away from hippocampal-dependent relational binding and toward an alternative cognitive function (D’Angelo et al., 2015; Ryan et al., 2013). The engagement of unitization results in the formation and use of fused representations to represent arbitrary relations. We have proposed that these fused or unitized representations are created by incorporating arbitrary information with information in semantic memory through the use of visual imagery and action representations, and are strengthened by maintaining these unitized representations online in working memory (Ryan et al., 2013). Upon retrieval of a unitized representation, the relation between the two items may be derived online by interpreting the actions that are contained therein.
Our present findings from the higher MoCA group are consistent with our prior work showing that unitization can support learning of arbitrary relations in TP in individuals with amnesia (D’Angelo et al., 2015; Ryan et al., 2013). In Ryan et al. (2013), we report on D.A., an amnesic individual who used a self-generated strategy to learn relations in TP. To achieve successful performance on TP, D.A. would spontaneously imagine the interaction and fusion of pairs of items in a manner that allowed him to determine which item would be the winner (e.g., one object covers another). This unitization strategy was the basis of the experimenter-given strategy used in the present study. When provided with the unitization strategy in a separate condition, D.A. was able to learn arbitrary relations that he had previously failed to learn, and retained them over considerable delays. In this same study, two other amnesic individuals, K.C. and R.F.R., showed no improvements with unitization. It is noteworthy that both K.C. and R.F.R. had more diffuse patterns of damage. D.A. has bilateral damage to the MTL, affecting his hippocampus, perirhinal cortex, and parahippocampal cortex, with additional damage to his entorhinal cortex on the right side, as well as damage to his right anterior temporal lobe. Given the differences in affected regions across these cases, we previously proposed that K.C. and R.F.R. may not have benefited from unitization because their damage extended to, or affected, areas necessary for the underlying processing mechanisms that support unitization.
More recently, we have shown that unitization can support learning of arbitrary relations in another amnesic case, N.C. (D’Angelo et al., 2015). N.C. is a case with developmental amnesia, who has more circumscribed damage than the previously tested individuals. Although N.C. has no apparent reductions to his hippocampus, he has damage to the extended hippocampal system (Aggleton, 2014; Aggleton & Brown, 1999), including bilateral damage to the mediodorsal nuclei of the thalamus, reductions in the left and right mammillary bodies, and in the right fornix. N.C.’s amnesia is consistent with integrated views of temporal lobe and diencephalic amnesia (Aggleton, 2008). N.C.’s specific impairment in standard TP conditions is consistent with a previous report of impaired TP learning in a case with bilateral thalamic damage (Rickard et al., 2006). Overall, despite their differences in etiology, D.A. and N.C. both show impaired learning under standard training that is mitigated with unitization, similar to the findings here with healthy older adults who pass the MoCA.
Unitization incorporates information from semantic memory with visual imagery and action representation, and as a result may depend on a network of neural regions outside of the hippocampus and its extended network. Based on the neural regions spared in the amnesic cases who did, versus did not, benefit from unitization (D’Angelo et al., 2015; Ryan et al., 2013), we have suggested that the following neural structures underlie the cognitive processes that are required for unitization. The anterior temporal lobes in interactions with the ventrolateral frontal cortex may mediate the retrieval of existing representations from semantic memory (Moses et al., 2009; Noppeney et al., 2007). Subsequently, the incorporation of currently presented information with semantic knowledge through visual imagery may be supported by posterior visual cortices (Staresina & Davachi, 2010) and the precuneus (Cavanna & Trimble, 2006). These fused representations may then be maintained and manipulated online through frontal regions (Badre, Kayser, & D’Esposito, 2010; Moscovitch & Winocur, 2002). The function of these systems may be inferred to be intact in the higher MoCA group of older adults, given their ability to benefit from unitization. However, additional research is necessary to further examine the role of these structures in supporting performance that would ordinarily require relational memory through unitization.
The present findings are consistent with the recent work examining paired-associate learning, in which improved memory for relations has been found in older adults when they are given strategies that encourage the fusion of items (Bastin et al., 2013) or when given materials that promote unitization (Ahmad, Fernandes, & Hockley, 2015). Improved memory for paired-associates with unitization strategies has also been demonstrated in amnesic cases, but only in cases with damage limited to the hippocampus and intact familiarity-based recognition (Quamme, Yonelinas, & Norman, 2007). Within the paired-associates learning literature, improvements in relational memory with unitization strategies have been related to differential patterns of activation in areas along the ventral visual pathway (Staresina & Davachi, 2010) and in perirhinal cortex (O’Neil, Barkley, & Köhler, 2013). This prior work in paired-associates learning has shown that unitization helps individuals remember which items were studied together, potentially through familiarity-based recognition (Diana, Yonelinas, & Ranganath, 2008; Parks & Yonelinas, 2015). Knowledge of which items were previously presented together is insufficient to support performance in the TP task, as each item occurs in the context of each of the other two items an equal number of times. Our findings of improved TP learning with unitization suggest that information regarding the directionality of the relations between items is encapsulated into the representations formed using a unitization strategy. In order to encapsulate directional information, the unitization strategy presented here may require properties that go above and beyond fusion of two items. In particular, we hypothesize that in the present case, unitization critically involves linking items through actions that are within semantic knowledge. To our knowledge, this is the first demonstration that unitization can support learning of this nature in older adults.
Our findings also inform whether all individuals benefit from errorless learning training procedures. Although not the focus of the present study, the unitization training approximated errorless learning procedures (Glisky, Schacter, & Tulving, 1986); by providing a cue to the answer on each trial, fewer errors were observed during training for both groups. Relative to trial-and-error learning, errorless learning conditions have generally been found to improve memory performance in amnesic cases (Glisky et al., 1986) and in healthy older adults (Da Silva & Sunderland, 2010; but see Cyr & Anderson, 2012; 2015). Both groups showed near-errorless learning conditions during training in the Shapes-Unitized condition. However, the lower MoCA group’s performance on Shapes-Unitized dropped at test, even in the immediate test phase. The qualitative difference observed between the two groups during the test phases suggests that errorless learning conditions may not mitigate memory impairments in older adults who show early signs of cognitive decline. More work is needed to test this possibility.
Our failure to find evidence that the unitization strategy supports relational memory performance in older adults who fail the MoCA suggests that these older adults have deficits that go beyond relational binding as mediated by the hippocampus and its extended system. The lower MoCA group’s impaired performance in the Shapes-Unitized condition, coupled with their relatively lower accuracy in the RPS condition, likely reflects early deficits in semantic memory, which presumably would otherwise be used in RPS and in support of unitization. However, our neuropsychological test battery did not include traditional tests of semantic memory (e.g., Boston naming test, category fluency test), and more work is needed to bolster our interpretation that impaired RPS performance reflects impaired semantic memory. We also acknowledge that participants in the current study did not receive MRI scans and thus conclusions regarding brain–behavior relationships must be tentative.
Overall, the present work demonstrates that relational memory impairments in healthy older adults can be offset through the use of a unitization strategy. This strategy may support memory by shifting reliance away from relational representations as mediated by the binding functions of the hippocampus and instead toward fused or unitized cortical representations that are formed by integrating relations along with existing information in semantic memory into a coherent unit through visual imagery. Whereas healthy older adults benefit from a unitization strategy, we did not find evidence of such a benefit in older adults who show early signs of cognitive decline. This latter finding suggests that unitization may require a certain threshold of cognitive and neural integrity that is particularly compromised in individuals who fail the MoCA—including those undiagnosed, community-dwelling participants who volunteer their time in psychology labs.
Appendix
Table A1.
Results of overall ANOVA on immediate test performance (Df = degrees of freedom, SS = sum of squares, MS = mean squared values).
| Df | SS | MS | F | p | η2p | |
|---|---|---|---|---|---|---|
| Group | 1 | 0.919 | 0.919 | 22.0 | <.001 | 0.37 |
| Error (Group) | 38 | 1.585 | 0.042 | |||
| Condition Contrast 1: Shapes-Standard vs. RPS | 1 | 0.958 | 0.958 | 45.1 | <.001 | 0.37 |
| Condition Contrast 2: Shapes-Standard vs. Shapes-Unitized | 1 | 0.050 | 0.050 | 2.35 | .129 | 0.03 |
| Group by Contrast 1 (Shapes-Standard vs. RPS) | 1 | 0.067 | 0.067 | 3.14 | .081 | 0.04 |
| Group by Contrast 2 (Shapes-Standard vs. Shapes-Unitized) | 1 | 0.000 | 0.000 | 0.02 | .899 | 0.00 |
| Error (Condition) | 76 | 1.614 | 0.021 |
Results from the analysis of accuracy on the immediate test are summarized in Table A1. The analysis of accuracy on the immediate test generally replicated the analysis of accuracy on the delay test. Older adults in the lower MoCA group showed greater impairments than those in the higher MoCA group even on the immediate test. Participants in the lower MoCA group had lower overall accuracy (M = 0.71, SE = 0.05) than did those in the higher MoCA group (M = 0.89, SE = 0.04; F(1, 38) = 22.0, p < .001, η2 p = 0.37). As in the delay test, older adults showed greater impairments on TP with arbitrary relations than when TP was supported by semantic relations. Overall, accuracy was lower in the Shapes-Standard condition (M = 0.71, SE = 0.04) than in the RPS condition (M = 0.93, SE = 0.02; F(1, 76) = 45.1, p < .001, η2 p = 0.37). In contrast to the results from the analysis on delay test performance, the overall accuracy was not significantly higher in the Shapes-Unitized condition (M = 0.76, SE = 0.03) than in the Shapes-Standard condition (M = 0.72, SE = 0.04), the effect of condition was only marginally significant (F(1, 76) = 2.35, p = .129, η2 p = 0.03).
The group difference was numerically larger in the Shapes-Standard condition (difference = 0.21) than in the RPS condition (difference = 0.11), although this effect was not significant (F(1, 76) = 3.14, p = .081, η2 p = 0.04). Lastly, group differences did not differ in the Shapes-Standard (difference = 0.21) and Shapes-Unitized (difference = 0.21) conditions (F(1, 76) = 0.02, p = .899, η2 p = 0.00). Surprisingly, even on the immediate test, the lower MoCA group scored below the elemental threshold (M = 0.66, SE = 0.04, 95% CI = 0.58–0.74), while the higher MoCA group scored above the threshold (M = 0.87, SE = 0.04, 95% CI = 0.80–0.94).
Funding Statement
This research was supported in part by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR) awarded to M.C.D., as well as support from the Ontario Ministry of Health and Long-Term Care through the Ontario Research Coalition of Research Institutes/Centres on Health & Aging (ORC) to M.C.D. This work was also supported by CIHR Operating Grants awarded to J.D.R. [grant number MOP-126003] and M.D.B. [grant number MOP-115148], and by Tier II Canada Research Chair Awards awarded to J.D.R. and M.D.B.
Notes
Prior knowledge of RPS rules was included as an additional between-subjects factor in all analyses, and did not affect performance unless otherwise noted.
Group assignment based on neuropsychological test performance yielded similar results as assignment based on MoCA status. Results of these analyses are available upon request.
Disclosure statement
The authors state that they do not have any conflicts of interest to declare.
References
- Aggleton J. P. EPS Mid-Career Award 2006. Understanding anterograde amnesia: Disconnections and hidden lesions. The Quarterly Journal of Experimental Psychology. 2008;61:1441–1471. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- Aggleton J. P. Looking beyond the hippocampus: Old and new neurological targets for understanding memory disorders. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140565–20140565. doi: 10.1098/rspb.2014.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J. P., Brown M. W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–444. doi: 10.1017/S0140525X99002034. discussion 444–89. [DOI] [PubMed] [Google Scholar]
- Ahmad F. N., Fernandes M., Hockley W. E. Improving associative memory in older adults with unitization. Aging, Neuropsychology, and Cognition. 2015;22:452–472. doi: 10.1080/13825585.2014.980216. [DOI] [PubMed] [Google Scholar]
- Alvarado M. C., Bachevalier J. Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus. 2005;15:118–131. doi: 10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- Alvarado M. C., Rudy J. W. Rats with damage to the hippocampal-formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behavioral Neuroscience. 1995;109:204–211. doi: 10.1037/0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- Badre D., Kayser A. S., D’Esposito M. Frontal cortex and the discovery of abstract action rules. Neuron. 2010;66:315–326. doi: 10.1016/j.neuron.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense M. D., Groen I. I. A., Lee A. C. H., Yeung L.-K., Brady S. M., Gregori M. Intact memory for irrelevant information impairs perception in amnesia. Neuron. 2012;75:157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C., Diana R. A., Simon J., Collette F., Yonelinas A. P., Salmon E. Associative memory in aging: The effect of unitization on source memory. Psychology and Aging. 2013;28:275–283. doi: 10.1037/a0031566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey T. J., Warburton E., Aggleton J. P., Muir J. L. Fornix lesions can facilitate acquisition of the transverse patterning task: A challenge for “configural” theories of hippocampal function. The Journal of Neuroscience. 1998;18:1622–1631. doi: 10.1523/JNEUROSCI.18-04-01622.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A. E., Trimble M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cyr -A.-A., Anderson N. D. Trial-and-error learning improves source memory among young and older adults. Psychology and Aging. 2012;27:429–439. doi: 10.1037/a0025115. [DOI] [PubMed] [Google Scholar]
- Cyr -A.-A., Anderson N. D. Mistakes as stepping stones: Effects of errors on episodic memory among younger and older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015;41:841–850. doi: 10.1037/xlm0000073. [DOI] [PubMed] [Google Scholar]
- D’Angelo M. C., Kacollja A., Rabin J. S., Rosenbaum R. S., Ryan J. D. Unitization supports lasting performance and generalization on a relational memory task: Evidence from a previously undocumented developmental amnesic case. Neuropsychologia. 2015;77:185–200. doi: 10.1016/j.neuropsychologia.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Da Silva L., Sunderland A. Effects of immediate feedback and errorless learning on recognition memory processing in young and older adults. Neuropsychological Rehabilitation. 2010;20:42–58. doi: 10.1080/09602010903036731. [DOI] [PubMed] [Google Scholar]
- Diana R. A., Yonelinas A. P., Ranganath C. The effects of unitization on familiarity-based source memory: Testing a behavioral prediction derived from neuroimaging data. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:730–740. doi: 10.1037/0278-7393.34.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I., Hamilton D. A., Petropoulos H., Yeo R. A., Brooks W. M., Baumgartner R. N., Sutherland R. J. The aging hippocampus: Cognitive, biochemical and structural findings. Cerebral Cortex (New York, N.Y.: 1991) 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Driscoll I., Howard S. R., Prusky G. T., Rudy J. W., Sutherland R. J. Seahorse wins all races: Hippocampus participates in both linear and non-linear visual discrimination learning. Behavioural Brain Research. 2005;164:29–35. doi: 10.1016/j.bbr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Otto T., Cohen N. J. Two functional components of the hippocampal memory system. Behavioral and Brain Sciences. 1994;17:449–471. doi: 10.1017/S0140525X00035391. [DOI] [Google Scholar]
- Giovanello K. S., Kensinger E. A., Wong A. T., Schacter D. L. Age-related neural changes during memory conjunction errors. Journal of Cognitive Neuroscience. 2010;22:1348–1361. doi: 10.1162/jocn.2009.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky E. L., Schacter D. L., Tulving E. Learning and retention of computer-related vocabulary in memory-impaired patients: Method of vanishing cues. Journal of Clinical and Experimental Neuropsychology. 1986;8:292–312. doi: 10.1080/01688638608401320. [DOI] [PubMed] [Google Scholar]
- Graf P., Schacter D. L. Unitization and grouping mediate dissociations in memory for new associations. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:930. doi: 10.1037//0278-7393.15.1.3. [DOI] [PubMed] [Google Scholar]
- Miller S. L., Celone K., DePeau K., Diamond E., Dickerson B. C., Rentz D. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. D. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorials in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Moscovitch M., Winocur G. The frontal cortex and working with memory. Principles of Frontal Lobe Function. 2002 doi: 10.1093/acprof:oso/9780195134971.003.0012. [DOI] [Google Scholar]
- Moses S. N., Ostreicher M. L., Rosenbaum R. S., Ryan J. D. Successful transverse patterning in amnesia using semantic knowledge. Hippocampus. 2008;18:121–124. doi: 10.1002/hipo.20378. [DOI] [PubMed] [Google Scholar]
- Moses S. N., Ryan J. D. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. 2006;16:43–65. doi: 10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- Moses S. N., Ryan J. D., Bardouille T., Kovacevic N., Hanlon F. M., McIntosh A. R. Semantic information alters neural activation during transverse patterning performance. NeuroImage. 2009;46:863–873. doi: 10.1016/j.neuroimage.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1170–1187. doi: 10.1037/0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M., Guez J., Kilb A., Reedy S. The associative memory deficit of older adults: Further support using face-name associations. Psychology and Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Newsome R. N., Duarte A., Barense M. D. Reducing perceptual interference improves visual discrimination in mild cognitive impairment: Implications for a model of perirhinal cortex function. Hippocampus. 2012;22:1990–1999. doi: 10.1002/hipo.22071. [DOI] [PubMed] [Google Scholar]
- Newsome R. N., Pun C., Smith V. M., Ferber S., Barense M. D. Neural correlates of cognitive decline in older adults at-risk for developing MCI: Evidence from the CDA and P300. Cognitive Neuroscience. 2013;4:152–162. doi: 10.1080/17588928.2013.853658. [DOI] [PubMed] [Google Scholar]
- Noppeney U., Patterson K., Tyler L. K., Moss H., Stamatakis E. A., Bright P. Temporal lobe lesions and semantic impairment: A comparison of herpes simplex virus encephalitis and semantic dementia. Brain: A Journal of Neurology. 2007;130:1138–1147. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- O’Neil E. B., Barkley V. A., Köhler S.2013Representational demands modulate involvement of perirhinal cortex in face processing Hippocampus23592–605. 10.1002/hipo.22117 [DOI] [PubMed] [Google Scholar]
- Old S. R., Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23:104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. A.1944Le test de copie d“une figure complexe; contribution à l”étude de la perception et de la mémoire Archives De Psychologie 30 [Google Scholar]
- Ostreicher M. L., Moses S. N., Rosenbaum R. S., Ryan J. D. Prior experience supports new learning of relations in aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2010;65B:32–41. doi: 10.1093/geronb/gbp081. [DOI] [PubMed] [Google Scholar]
- Parks C. M., Yonelinas A. P. The importance of unitization for familiarity-based learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015;41:881–903. doi: 10.1037/xlm0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme J. R., Yonelinas A. P., Norman K. A. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Reitan R. M., Wolfson D. The Halstead-Reitan neuropsychological test battery. Reitan Neuropsychology; 1985. [Google Scholar]
- Rickard T. C., Grafman J. Losing their configural mind: Amnesic patients fail on transverse patterning. Journal of Cognitive Neuroscience. 1998;10:509–524. doi: 10.1162/089892998562915. [DOI] [PubMed] [Google Scholar]
- Rickard T. C., Verfaellie M., Grafman J. Transverse patterning and human amnesia. Journal of Cognitive Neuroscience. 2006;18:1723–1733. doi: 10.1162/jocn.2006.18.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina R., Olsen R. K., McQuiggan D., Fatima Z., Li L., Oziel E. Age-related changes to oscillatory dynamics in hippocampal and neocortical networks. Neurobiology of Learning and Memory. 2015 doi: 10.1016/j.nlm.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Ryan J. D., Cohen N. J. Evaluating the neuropsychological dissociation evidence for multiple memory systems. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:168–185. doi: 10.3758/CABN.3.3.168. [DOI] [PubMed] [Google Scholar]
- Ryan J. D., Moses S. N., Barense M., Rosenbaum R. S. Intact learning of new relations in amnesia as achieved through unitization. Journal of Neuroscience. 2013;33:9601–9613. doi: 10.1523/JNEUROSCI.0169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida L. M., Bussey T. J., Buckmaster C. A., Murray E. A. Impairment and facilitation of transverse patterning after lesions of the perirhinal cortex and hippocampus, respectively. Cerebral Cortex. 2006;17:108–115. doi: 10.1093/cercor/bhj128. [DOI] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Annals of the New York Academy of Sciences. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Staresina B. P., Davachi L.2010Object unitization and associative memory formation are supported by distinct brain regions The Journal of Neuroscience309890–9897. 10.1523/JNEUROSCI.0826-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E. K., James M. The visual object and space perception battery: VOSP. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. WMS-IV: Wechsler memory scale 4th edition. New York, NY: The Psychological Corporation; 2009. [Google Scholar]
- Wechsler, D. 2008WAIS-IV: Wechsler adult intelligence scale [Google Scholar]
- Yeung L.-K., Ryan J. D., Cowell R. A., Barense M. D. Recognition memory impairments caused by false recognition of novel objects. Journal of Experimental Psychology: General. 2013;142:1384–1397. doi: 10.1037/a0034021. [DOI] [PubMed] [Google Scholar]


