Abstract
Background:
The psychotomimetic phencyclidine (PCP) produces behavioral symptoms similar to those observed in schizophrenia, accompanied by increased motor activity. The dopamine and adenosine 3’,5’-cyclic monophosphate-regulated phosphoprotein of 32kDa (DARPP-32) is enriched in the medium spiny neurons (MSNs) of the striatum and has been implicated in the actions of PCP. We examined the effects of deletion of DARPP-32 in distinct populations of striatal MSNs, on the ability of PCP to induce motor activation and memory deficit.
Methods:
The effects of PCP were examined in mice with conditional knockout of DARPP-32 in the MSNs of the direct, or indirect pathway. DARPP-32 phosphorylation was determined by Western blotting. The motor stimulant effects of PCP were determined by measuring locomotion following acute and chronic administration. Memory deficit was evaluated using the passive avoidance test.
Results:
Loss of DARPP-32 in direct MSNs prevents PCP-induced phosphorylation and abolishes the motor stimulation effects of PCP. In contrast, lack of DARPP-32 in indirect MSNs does not affect the ability of PCP to promote DARPP-32 phosphorylation and to increase motor activity. The impairment in passive avoidance induced by PCP is independent of the expression of DARPP-32 in direct or indirect MSNs.
Conclusions:
The increase in DARPP-32 phosphorylation induced by PCP occurs selectively in the MSNs of the direct pathway, which are also specifically involved in the motor stimulant effects of this drug. The memory deficit induced by PCP is not linked to the expression of DARPP-32 in striatal MSNs.
Keywords: basal ganglia, dopamine and cAMP-regulated phosphoprotein of 32 kDa, motor activity, phencyclidine, schizophrenia
Introduction
The non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist phencyclidine (PCP) is a well-known psychotomimetic that produces behavioral changes similar to those observed during acute schizophrenic episodes (Luby et al., 1959). Based on these properties of PCP, it has been suggested that alterations of glutamatergic transmission are implicated in the pathophysiology of schizophrenia (Olney et al., 1999) and glutamatergic antagonists, like PCP and ketamine, have been largely used to generate a pharmacological model of this disorder (Javitt and Zukin, 1991; Abi-Saab et al., 1998; Tamminga, 1998; Jentsch and Roth, 1999; Krystal et al., 1999).
In rodents, acute and chronic administration of PCP results in cognitive deficits (Handelmann et al., 1987; Jentsch et al., 1997; Adams and Moghaddam, 1998; Beraki et al., 2008) and disrupted sensorimotor gating (Mansbach and Geyer, 1989), two major symptoms observed in schizophrenic patients. In addition, PCP increases locomotor activity and repetitive movements, which have been proposed to represent a surrogate marker of the positive symptoms of schizophrenia (Sturgeon et al., 1979; Sams-Dodd, 1996; Bondi et al., 2012).
Studies in humans and in animal models indicate that, besides antagonizing NMDA receptors, PCP also promotes dopamine transmission (Giannini et al., 1984; Ögren and Goldstein, 1994; Seeman and Lasaga, 2005; Seeman and Guan, 2008). In particular, PCP exerts agonistic action at dopamine D2 receptors (D2Rs; Seeman and Lasaga, 2005; Seeman and Guan, 2008) and increases dopamine efflux in several brain regions, including the striatum, a major component of the basal ganglia involved in motor function (Bowyer et al., 1984; Steinpreis and Salamone, 1993; Hertel et al., 1995; Moghaddam and Adams, 1998). In line with these observations, it has been shown that the ability of PCP to induce repetitive movements and impair sensorimotor gating depends on the dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32; Svenningsson et al., 2003), a key component of dopamine signaling (Fienberg et al., 1998).
In the striatum, DARPP-32 is enriched in the GABAergic medium spiny neurons (MSNs; Ouimet et al., 1998), which form the direct and indirect pathways to the output nuclei of the basal ganglia (Albin et al., 1989; Gerfen, 1992). Activation of dopamine D1 receptors (D1Rs), which are selectively expressed in direct MSNs (Gerfen, 1992), leads to phosphorylation of DARPP-32 at Thr34 (Nishi et al., 1997; Svenningsson et al., 1998). This effect is mediated by cAMP-dependent protein kinase (PKA) and converts DARPP-32 into an inhibitor of protein phosphatase-1 (PP-1; Hemmings et al., 1984), thereby reducing dephosphorylation of PKA target proteins and amplifying cAMP-mediated responses (Fienberg et al., 1998; Greengard, 2001). In contrast, activation of D2Rs, which are expressed in indirect MSNs (Gerfen, 1992), leads to inhibition of PKA (Kebabian and Calne, 1979) and reduction of DARPP-32 phosphorylation (Nishi et al., 1997; Svenningsson et al., 2000).
PCP has been shown to increase DARPP-32 phosphorylation at Thr34 in the striatum (Svenningsson et al., 2003; Pozzi et al., 2010). However, the specific localization of this effect in direct or indirect MSNs, as well as the role of these two neuronal populations in the behavioral responses to PCP, remain to be elucidated. In this study, we used transgenic mice in which DARPP-32 was deleted in D1R- or D2R-expressing MSNs (Bateup et al., 2010) to study the role of the direct and indirect MSNs in the motor and cognitive effects of PCP.
Methods
Animals
Mice in which DARPP-32 was conditionally deleted in D1R- or D2R-expressing MSNs (D32 F/F D1RCre +and D32 F/F D2RCre + mice) and control mice (D32 +/+ D1RCre + and
D32 +/+ D2RCre + mice, hereafter referred to as D1RCre and D2RCre mice) were generated as previously described (Bateup et al., 2010). Experiments were carried out in accordance with the guidelines of Research Ethics Committee of Karolinska Institutet, Swedish Animal Welfare Agency, and European Communities Council Directive 86/609/EEC.
Drugs
Phencyclidine hydrochloride (Sigma-Aldrich) was dissolved in saline and injected (3 or 6mg/kg) subcutaneously (s.c.) in the scruff of the neck in a volume of 4ml/kg. Mice were habituated to handling and injection (saline) by the operator for 3 consecutive days before the experiments.
Western Blotting
Mice were treated with a single injection of vehicle or PCP or repeatedly for 8 days with one injection per day and killed by decapitation 30min after acute administration or, in the case of repeated treatment, 30min after the last injection (day 8). The heads of the animals were immediately cooled in liquid nitrogen for 5 seconds and the brains were removed. Striata were dissected out on an ice-cold surface, sonicated in 750 μl of 1% sodium dodecyl sulfate, and boiled for 10min. Aliquots (5 μl) from samples were used for protein quantification using the bicinchoninic acid assay kit (Pierce Europe). Fifteen μg of protein from each sample were loaded onto 10% polyacrylamide gels, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech; Towbin et al., 1979). The membranes were incubated 40min in 5% milk blocking solution and then incubated with antibodies against total DARPP-32, or phospho-Thr34-DARPP-32 (PhosphoSolutions). Blots were then incubated for 2hr in horseradish peroxidase-conjugated secondary antibodies and proteins were visualized by ECL detection (Pierce Europe), followed by quantification with Quantity One software (Bio-Rad). The levels of phosphorylated DARPP-32 were normalized for the amount of the corresponding total protein detected in the sample.
Measurement of Motor Activity
Horizontal motor activity was recorded in motor activity boxes (35 x 25 x 30cm) under dim light (<100 lux on the cage floor). D32 F/F D1RCre + and D32 F/F D2RCre + mice and D1RCre and D2RCre control littermates were first habituated to the novel environment for 60min. At the end of this period, the animals were injected with PCP (3mg/kg) or vehicle and immediately re-introduced in the activity boxes for an additional 60min. The entire 120-min test was video-recorded and the distance covered by each animal was determined using Biobserve GmbH software. Locomotor sensitization was induced by treating the mice with PCP for 7 consecutive days (one injection per day), as described above. At the end of the sensitization procedure (day 7), horizontal motor activity was determined. The mice were then treated for one additional day (day 8) and sacrificed 30min after injection to collect striatal tissue for Western blotting analysis of total and phosphorylated DARPP-32.
Passive Avoidance
Cognitive function was examined in a step-through passive avoidance apparatus (10 x 16 x 18cm; Ugo Basile) composed of two chambers equipped with an electrified floor: a “bright chamber” (white floor and walls, ~1000 1X illumination) was divided from a “dark chamber” (black floor and walls, ~10 1X illumination) by an automatized sliding door (4 x 4cm). The test relies on the natural preference of mice for a dark over a bright environment. D32 F/F D1RCre + and D32 F/F D2RCre + mice and D1RCre and D2RCre controls were examined with a 2-day protocol, as previously described (Madjid et al., 2006). Briefly, on the training day the mice were treated with PCP (6mg/kg) or vehicle and after 30min they were introduced into the bright chamber of the apparatus and allowed to explore it for 60sec before the door to the dark compartment was opened. When the mouse entered the dark chamber with all four paws, the door was closed and a weak electric current (unconditioned stimulus [US]) was delivered (0.35 mA for 2sec). Thirty seconds after the shock the mice were removed from the apparatus and returned to the home cage. On the following day, the retention test was performed drug-free. The mice were reintroduced into the bright chamber for 30sec before the door was opened, allowing them to move between the two chambers for a period of 10min. During the test, the latency to cross the door and enter the dark, shock-associated compartment was recorded.
Statistical Analysis
Data were analyzed using one-, two-, or three- way ANOVA, and comparisons between groups were made using Fisher’s post hoc analysis. When necessary, t-tests with equal variances for groups of two were applied.
Results
Administration of PCP Increases DARPP-32 Phosphorylation in Direct MSNs
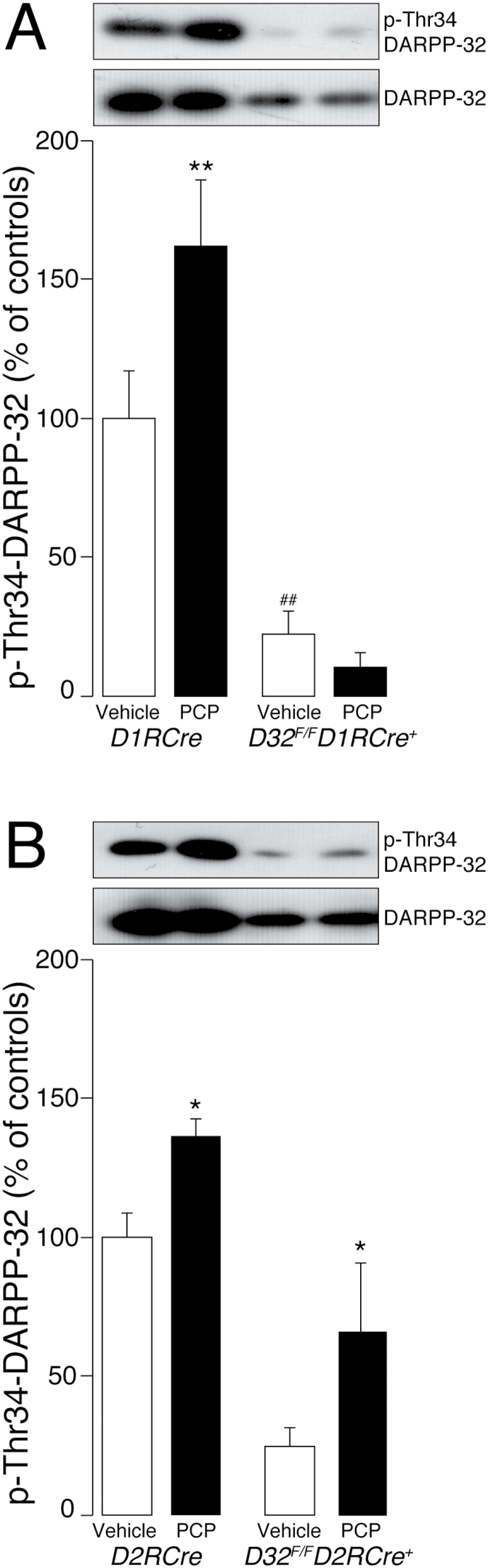
To gain information on the ability of PCP to regulate DARPP-32 in distinct populations of striatal MSNs, we examined its effect in D32 F/F D1RCre + and D32 F/F D2RCre + mice, which lack DARPP-32 in direct and indirect MSNs, respectively (Bateup et al., 2010). As expected, in these mice the levels of DARPP-32 detected in the striatum were reduced in comparison to D1RCre and D2RCre control littermates (Figure 1). Acute administration of 3mg/kg of PCP to D1RCre and D2RCre mice increased the phosphorylation of DARPP-32 at Thr34 to a similar extent as previously reported (Svenningsson et al., 2003; Pozzi et al., 2010; Figure 1). In contrast, we did not observe any increase in DARPP-32 phosphorylation when PCP was administered to D32 F/F D1RCre + mice, which lack DARPP-32 in direct MSNs (statistical comparison indicated significant genotype x treatment interaction: F1,23 = 6.300, p < 0.05; Figure 1A). The ability of PCP to phosphorylate DARPP-32 at Thr34 was instead preserved in D32 F/F D2RCre + mice (statistical comparison indicated significant treatment effect F1,22 = 9.39, p < 0.01; and genotype effect F1,22 = 27.73, p < 0.0001; Figure 1B). These results indicate that acute PCP regulates DARPP-32 phosphorylation preferentially in the D1R-expressing MSNs of the direct pathway.
Figure 1.
Effects of acute administration of phencyclidine (PCP) on dopamine and adenosine 3’,5’-cyclic monophosphate activated phosphoprotein of 32 kDa (DARPP-32) phosphorylation in D32 F/F D1RCre + and D32 F/F D2RCre + mice. (A) D32 F/F D1RCre + and (B) D32 F/F D2RCre + conditional knockout mice and (A) D1RCre and (B) D2RCre control mice were treated with one injection of vehicle or PCP (3mg/kg) and killed by decapitation after 30min. DARPP-32 phosphorylation on Thr34 was determined by Western blotting as described in Methods. Upper panels are representative Western blots showing phosphorylated DARPP-32 (top) and total DARPP-32 (bottom). Note the reduction in total DARPP-32 produced by genetic inactivation in (A) D1R- or (B) D2R-expressing MSNs. The increase in phosphorylated DARPP-32 induced by PCP is abolished in (A) D32 F/F D1RCre + mice, but still present in (B) D32 F/F D2RCre + mice. Lower panels are means ± standard error of the mean (n = 5–8/group) of data expressed as percent of (A) D1RCre or (B) D2RCre mice injected with vehicle. *p < 0.05 and **p < 0.01 vs. vehicle-treated mice, same genotype; ##p < 0.01 vs. vehicle-treated mice of respective control genotype (two-way ANOVA, followed by Fisher’s post hoc comparison).
Genetic Inactivation of DARPP-32 in Direct, But Not in Indirect, MSNs Abolishes the Motor Stimulant Properties of PCP
The results of the biochemical experiments led us to examine the effects produced by inactivation of DARPP-32 in direct or indirect MSNs on the motor stimulant properties of PCP. Specifically, we examined PCP-induced motor stimulation, and the motor sensitization produced by repeated drug administration.
D32 F/F D1RCre + and D32 F/F D2RCre + mice and D1RCre and D2RCre control littermates were treated for 7 days with vehicle or PCP (3mg/kg) and their motor activity was measured on day 1 and day 7. Locomotion was first assessed for 60min before drug administration (habituation) and then for an additional 60min immediately after administration of vehicle or PCP.
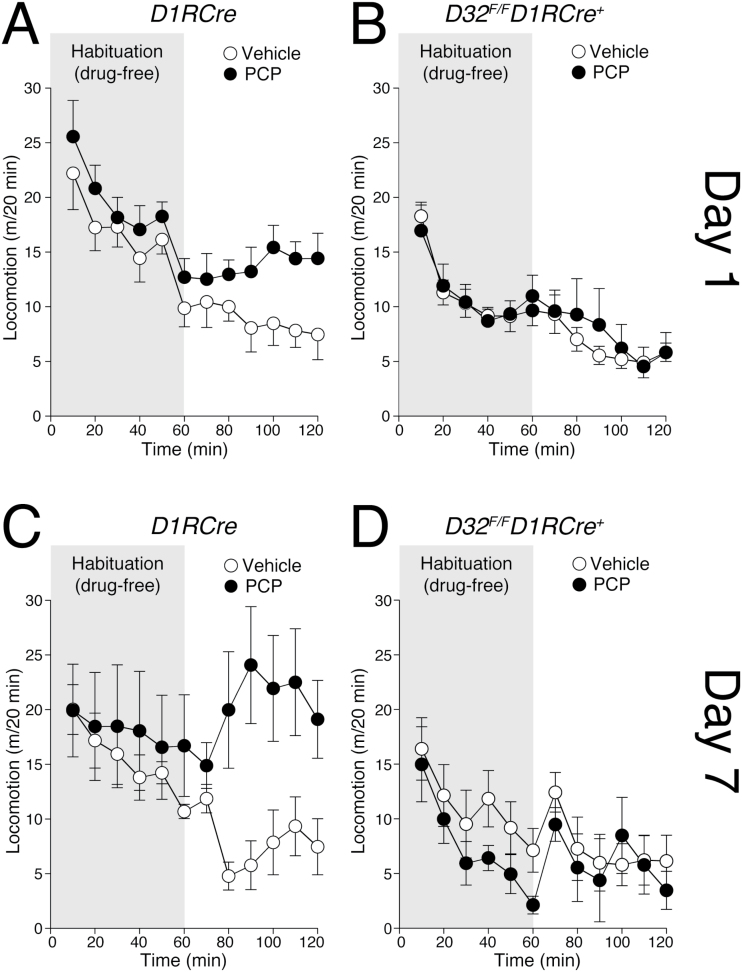
Analysis of motor activity during the first 60min of day 1 indicated that all genotypes habituated to the novel home cage (two-way ANOVA with repeated measures indicated a significant effect of time: F5,16 = 13.502, p < 0.0001 for D32 F/F D1RCre + and D1RCre mice, and F5,21 = 10.578, p < 0.0001 for D32 F/F D2RCre + and D2RCre mice; Figures 2A and B and 3A and B grey shadow). During habituation, D32 F/F D1RCre + mice showed reduced locomotion, whereas D32 F/F D2RCre + mice showed elevated locomotion, in comparison to their respective controls (A and B panels in Figures 2 and 3). Statistical analysis of the distance covered during the habituation phase indicated a significant effect of the genotype for D32 F/F D1RCre + versus D1RCre mice (student’s t-test, p < 0.01) and D32 F/F D2RCre + versus D2RCre mice (student’s t-test, p < 0.01). Analysis at day 1 showed that PCP increased motor activity in D1RCre control mice, but not in D32 F/F D1RCre + mice (two-way ANOVA indicated genotype effect: F1,18 = 7.095, p < 0.05, and treatment effect: F1,18 = 3.30, p < 0.05; Figure 2A and B).
Figure 2.
Effects of phencyclidine (PCP) on motor activity and motor sensitization in D32 F/F D1RCre + mice. (A, C) D1RCre mice and (B, D) D32 F/F D1RCre + conditional knockout mice were injected with vehicle or PCP (3mg/kg) for 7 consecutive days. Motor activity (measured as distance covered in cm) was measured on (A, B) Day 1 and (C, D) Day 7, during two consecutive sessions of 60min, immediately preceding (habituation, grey) and following injection of vehicle (open circles) or PCP (filled circles). Note the absence of acute motor response (Day 1; B) and motor sensitization (Day 7; D) to PCP in D32 F/F D1RCre + mice in comparison to D1RCre control mice (A, C). Data are expressed as mean ± standard error of the mean (n = 5–8/group).
Repeated treatment (7 days) enhanced the motor stimulant effect of PCP in control mice. Thus, in D1RCre mice, the increase in locomotion produced by PCP at day 7 was significantly larger in comparison to day 1 (Figure 2A and C). In contrast, in D32 F/F D1RCre + mice, repeated administration of PCP did not produce any sensitization (three-way ANOVA with repeated measures indicated an overall interaction genotype x treatment x time F1,18 = 6.095, p < 0.05; Figure 2D).
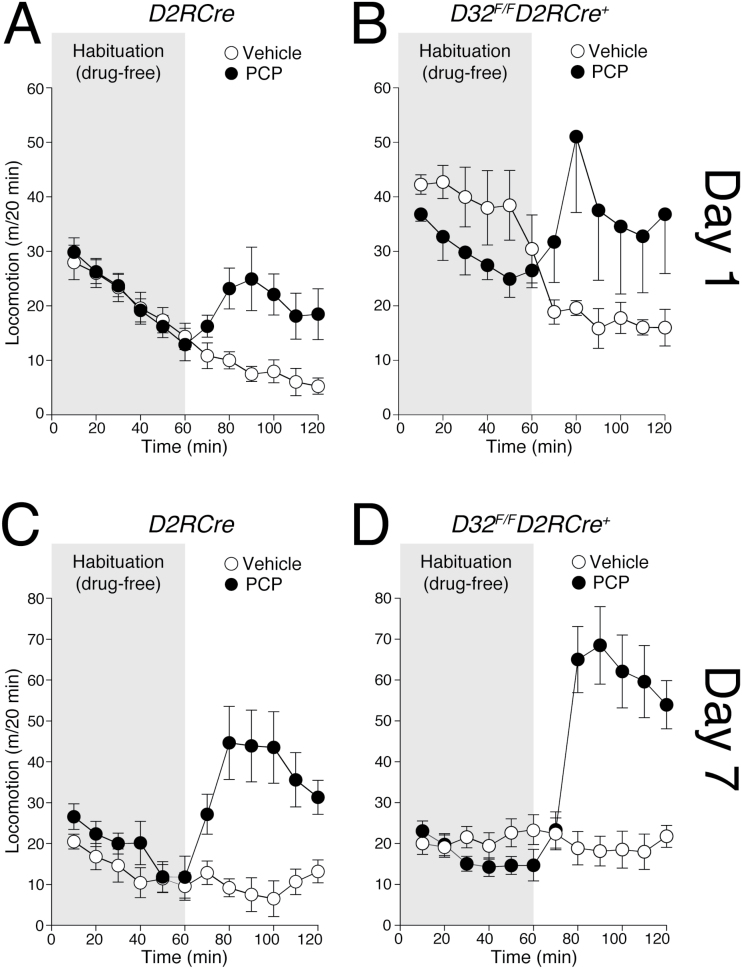
In parallel experiments, the effect of PCP was examined in D32 F/F D2RCre + mice and D2RCre control littermates (Figure 3). Both strains responded to the first injection of PCP (day 1) with a significant increase in motor activity (two-way ANOVA genotype x treatment revealed treatment effect: F1,20 = 9.356, p < 0.01; Figure 3A and B). Moreover, repeated administration resulted in a robust sensitization of the motor stimulant response to PCP in both D2RCre and D32 F/F D2RCre + mice (three-way ANOVA with repeated measures indicated a time x treatment interaction F1,16 = 9.727, p < 0.01; Figure 3C and D).
Figure 3.
Effects of phencyclidine (PCP) on motor activity and motor sensitization in D32 F/F D2RCre + mice. (A, C) D2RCre mice and (B, D) D32 F/F D2RCre + conditional knockout mice were injected with vehicle or PCP (3mg/kg) for 7 consecutive days. Motor activity (measured as distance covered in cm) was measured on (A, B) Day 1 and (C, D) Day 7, during two consecutive sessions of 60min, immediately preceding (habituation, grey) and following injection of vehicle (open circles) or PCP (filled circles). Data are expressed as mean ± standard error of the mean (n = 5–8/group).
PCP-Induced DARPP-32 Phosphorylation is Maintained During Repeated Administration
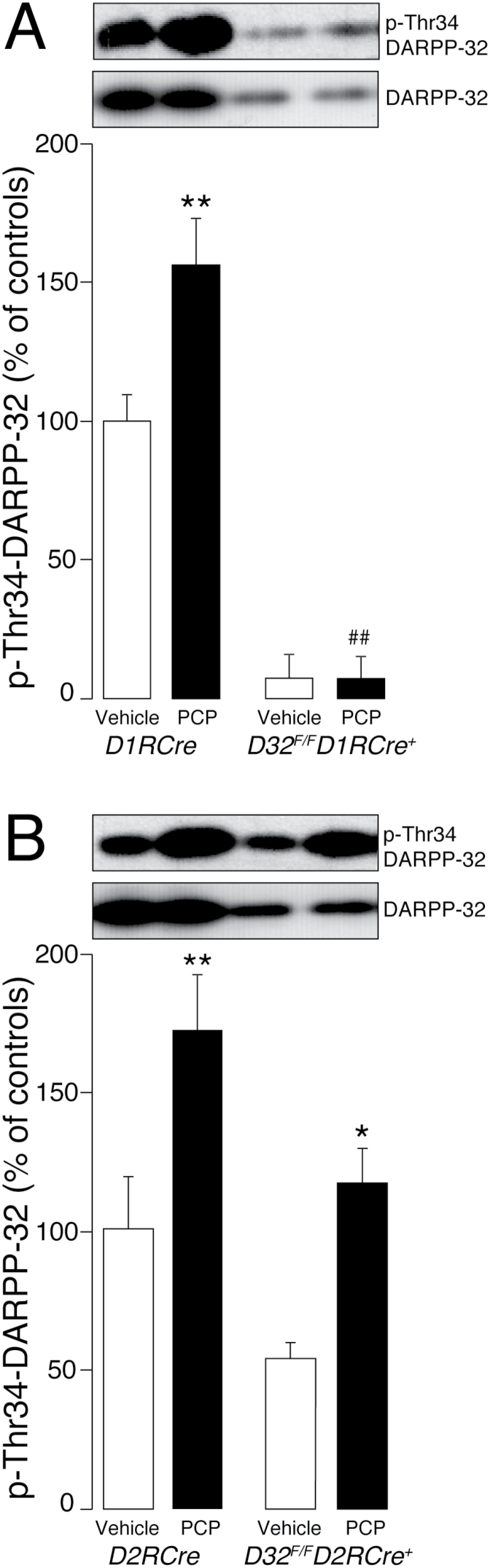
To further substantiate the idea of an involvement of DARPP-32 in PCP-induced motor stimulation, we examined whether activation of DARPP-32 was maintained even after repeated drug administration. D32 F/F D1RCre + and D32 F/F D2RCre + mice and D1RCre and D2RCre control littermates previously subjected to the behavioral sensitization procedure, were treated one additional day (day 8) with PCP and the levels of phosphorylated DARPP-32 were determined by Western blotting 30min after drug administration. We found that the ability of PCP (3mg/kg) to increase DARPP-32 phosphorylation in D1RCre and D2RCre control mice (Figure 4), as well as in D32 F/F D2RCre + mice (Figure 4B), persisted even after repeated administration. Indeed, in these mice the effect produced 30min after the last administration of PCP was similar to that observed 30min after a single injection. In line with the results obtained after acute PCP administration (Figure 1A), we also confirmed the lack of PCP-induced DARPP-32 phosphorylation in D32 F/F D1RCre + mice after 8 days of drug treatment (Figure 4A; two-way ANOVA, genotype x treatment interaction F1, 21 = 6.02, p < 0.05 for D32 F/F D1RCre + and D1RCre control mice, Figure 3A; only-treatment effect F1, 20 = 15.10, p < 0.01 and genotype effect F1, 20 = 8.62, p < 0.01 for D32 F/F D2RCre + and D2RCre control mice).
Figure 4.
Effects of repeated administration of phencyclidine (PCP) on dopamine and adenosine 3’,5’-cyclic monophosphate activated phosphoprotein of 32 kDa (DARPP-32) phosphorylation in D32 F/F D1RCre + and D32 F/F D2RCre + mice. (A) D32 F/F D1RCre + and (B) D32 F/F D2RCre + conditional knockout mice and (A) D1RCre and (B) D2RCre control mice were treated for 8 days (one injection/day) with vehicle or PCP (3mg/kg) and killed by decapitation 30min after the last injection. DARPP-32 phosphorylation on Thr34 was determined by Western blotting as described in Methods. Upper panels are representative Western blots showing phosphorylated DARPP-32 (top) and total DARPP-32 (bottom). Note the reduction in total DARPP-32 produced by genetic inactivation in (A) D1R- or (B) D2R-expressing MSNs. The increase in phosphorylated DARPP-32 induced by PCP is abolished in (A) D32 F/F D1RCre + mice, but still present in (B) D32 F/F D2RCre + mice. Lower panels are mean ± standard error of the mean (n = 5–8/group) of data expressed as percent of (A) D1RCre or (B) D2RCre mice injected with vehicle. *p < 0.05 and **p < 0.01 vs. vehicle-treated mice, same genotype; ##p < 0.01 vs. vehicle-treated mice of respective control genotype (two-way ANOVA, followed by Fisher’s post hoc comparison).
PCP-Induced Impairment of Passive Avoidance is Independent of DARPP-32 Expression in Striatal MSNs
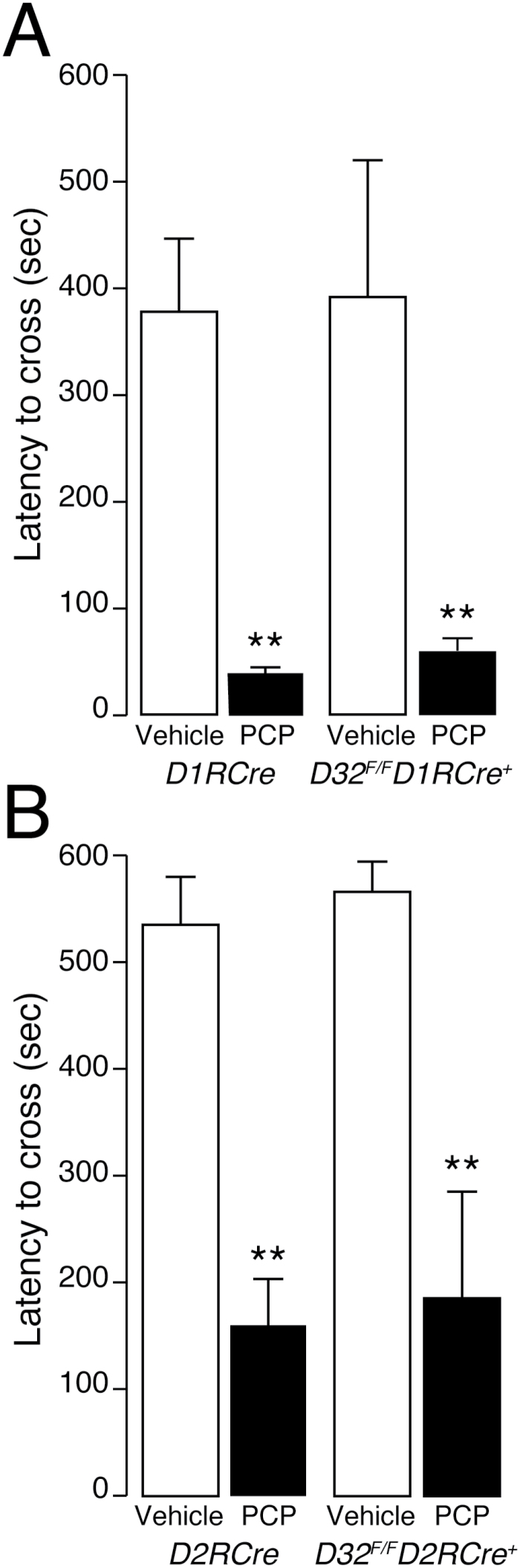
Finally, D32 F/F D1RCre + and D32 F/F D2RCre + mice and D1RCre and D2RCre control littermates were examined in the passive avoidance test. PCP (6mg/kg) reduced the latency of the animals to enter the dark box during the retention test, which was performed 24hr after drug administration (Figure 5). Statistical analysis revealed a significant treatment effect independent of the genotype (two-way ANOVA, only-treatment effect: for D32 F/F D1RCre + F1, 21 = 27.38, p < 0.0001; for D32 F/F D2RCre + F1, 18 = 33.75, p < 0.0001).
Figure 5.
Effect of phencyclidine (PCP) on passive avoidance in D32 F/F D1RCre + and D32 F/F D2RCre + mice. (A) D32 F/F D1RCre +, (B) D32 F/F D2RCre + conditional knockout mice and (A) D1RCre and (B) D2RCre control mice were injected with vehicle (open bars) or PCP (6mg/kg, i.p.; filled bars) and after 30min they were introduced into the bright chamber of the passive avoidance apparatus for the training phase. The following day, drug-free animals were reintroduced in the apparatus and the latency (sec) to cross the door and enter the dark, shock-associated compartment was recorded (see Methods). Data are expressed as mean ± standard error of the mean (n = 5–7/group). *p < 0.01 vs vehicle-treated mice, same genotype (two-way ANOVA, followed by Fisher′s post hoc comparison).
Discussion
This study shows that the motor activating properties of PCP, which are regarded as a surrogate marker of the positive symptoms in experimental models of schizophrenia, are associated with increased phosphorylation of DARPP-32 at Thr34 in the striatal MSNs of the direct pathway. Moreover, we show that conditional knockout of DARPP-32 in direct, but not in indirect, MSNs prevents both the acute and repeated motor stimulant effects of PCP.
PCP has a complex pharmacological profile, since it acts both as NMDA receptor antagonist and by promoting dopamine transmission. This latter effect has been proposed to occur via inhibition of dopamine reuptake (Garey and Heath, 1976; Smith et al., 1977) or by increasing dopamine release (Vickroy and Johnson, 1982), which may lead to activation of dopamine receptors and phosphorylation of DARPP-32. For instance, stimulation of D1Rs, which activates cAMP signaling, could be responsible for the increase in phosphorylation produced by PCP on Thr34 (Svenningsson et al., 2003; Pozzi et al., 2010), a site regulated by PKA (Nishi et al., 1997; Svenningsson et al., 1998). However, this possibility is questioned by the observation that depletion of dopamine or genetic inactivation of D1Rs does not prevent the ability of PCP to increase DARPP-32 phosphorylation at Thr34 (Svenningsson et al., 2003).
An alternative mechanism by which PCP could regulate DARPP-32 is through its antagonistic action at NMDA receptors. In this case, a blockade of NMDA receptors would enhance the levels of phosphorylated DARPP-32 by reducing dephosphorylation at Thr34, which is mediated by the Ca2+/calmodulin-dependent protein phosphatase, calcineurin (King et al., 1984; Halpain et al., 1990; Nishi et al., 2002). In line with this idea, it has been shown that incubation of striatal slices with NMDA receptor antagonists, such as ketamine and MK-801, produces an increase in DARPP-32 phosphorylation at Thr34 similar to that elicited by PCP (Svenningsson et al., 2003).
The impact of PCP on the regulation of DARPP-32 is particularly prominent in the D1R-expressing MSNs of the direct pathway. The lack of effect of PCP in indirect MSNs may be explained by the agonistic action played by this drug at D2Rs (Seeman and Lasaga, 2005; Seeman and Guan, 2008), which would inhibit cAMP signaling (Kebabian and Calne, 1979), thereby suppressing basal PKA activity and Thr34 phosphorylation. This, in turn, would counteract the increase in DARPP-32 phosphorylation induced by PCP through the blockade of NMDA receptors and reduction of calcineurin-mediated dephosphorylation.
The results of the biochemical studies are consistent with those of the behavioral experiments, which indicate that the acute motor stimulant effect of PCP requires the expression of DARPP-32 in the D1R-enriched MSNs of the direct pathway, but not in the D2R-enriched MSNs of the indirect pathway. PCP-induced motor sensitization was also abolished by selective knock-out of DARPP-32 in D1R-expressing MSNs. However, in control D1R-Cre mice, repeated administration did not enhance the ability of PCP to induce DARPP-32 phosphorylation, which remained similar to that observed after acute treatment. Therefore, the absence of motor sensitization observed in D32 F/F D1RCre + mice should be regarded as a consequence of the lack of motor response to PCP, caused by deletion of DARPP-32 in direct MSNs.
The involvement of direct MSNs in the motor response to PCP is in line with the observation that the increase in spontaneous activity produced by this drug is reduced by the selective D1R antagonist SCH23390, but not by the D2Rs antagonist sulpiride (Tsutsumi et al., 1995). Previous work also showed that the preferential D2R antagonists haloperidol and raclopride, injected at non-cataleptogenic doses, failed to block the increase in locomotion produced by PCP in rats (Ögren and Goldstein, 1994). However, higher doses of D2R antagonists have been reported to counteract the motor stimulant effect of PCP (Sturgeon et al., 1981; Jackson et al., 1994; Kitaichi et al., 1994). Interestingly, these ataxia-producing doses increase DARPP-32 phosphorylation preferentially in the MSNs of the indirect pathway (Bateup et al., 2008). Therefore, it is possible that the motor stimulant effect of PCP, which activates DARPP-32 in direct MSNs, is counterbalanced by stimuli able to produce a concomitant activation in indirect MSNs.
The abolishment of the locomotor stimulant effects of PCP observed in D32 F/F D1RCre + mice is reminiscent of the reduction of the locomotor response to acute cocaine, a drug which acts by increasing dopamine efflux, previously described in the same strain of mice (Bateup et al., 2010). Altogether, these findings indicate that increased Thr34 phosphorylation of DARPP-32 in the striatal MSNs of the direct pathway is an important step in mediating the motor stimulant properties of distinct classes of psychoactive drugs.
Genetic inactivation of DARPP-32 in indirect MSNs correlates with an exacerbation of the motor stimulant effects of PCP. These results are in line with the hyperlocomotor phenotype previously reported in D32 F/F D2RCre + mice (Bateup et al., 2010) and confirmed in the present study, showing that deletion of DARPP-32 in indirect MSNs enhances spontaneous locomotion, whereas deletion of DARPP-32 in direct MSNs results in an opposite effect.
PCP produces a broad range of behavioral responses, which have been proposed to reproduce various aspects of the symptomatology of schizophrenia. Therefore, we examined the potential involvement of DARPP-32 in the cognitive deficit produced by acute PCP, which models a cardinal feature of the disease. To this purpose we used the passive avoidance test, a model of cognitive symptoms of schizophrenia induced by NMDA receptor antagonists (Danysz et al., 1988). Genetic inactivation of DARPP-32 in D1R- and D2R-expressing MSNs did not affect the impairment of passive avoidance produced by PCP. This suggests that the contribution of DARPP-32 to the behavioral responses elicited by PCP is particularly important with regard to the stimulant effect on motor activity, rather than to the cognitive deficits associated with the administration of this drug. In agreement with this finding, it has been shown that the ability of PCP to impair performance of rats in the five-choice serial reaction time task, a cognitive test of attention and executive function, is independent of increased Thr34 phosphorylation of DARPP-32 (Pozzi et al., 2010). These observations are consistent with earlier studies indicating that the cognitive deficits caused by PCP involve actions related to serotonin and not dopamine (Madjid et al., 2006).
It has been shown that D1Rs, which play an important role in the activation of DARPP-32, are required for spatial learning and memory (El-Ghundi et al., 1999). Interestingly, the present study shows that the deficit in passive avoidance produced by PCP is accompanied by increased DARPP-32 phosphorylation in direct MSNs. These findings indicate that the disruption of performance in the passive avoidance test caused by PCP involves multiple mechanisms, which altogether overcome the potentially pro-cognitive effect of DARPP-32 activation.
In conclusion, this study shows the involvement in the motor stimulant properties of PCP of a distinct population of striatal projection neurons, corresponding to the D1R-expressing MSNs of the direct pathway. It also indicates that PCP regulates PKA-dependent phosphorylation of DARPP-32 selectively in these neurons, without producing any effect in the MSNs of the indirect pathway. Moreover, the present data suggest that the cognitive deficit caused by administration of PCP is independent of DARPP-32 expression in striatal MSNs. Thus, activation of DARPP-32 in direct MSNs is linked to a specific behavioral output produced by PCP and regarded as a surrogate marker of the positive symptoms of schizophrenia.
Statement of Interest
None.
Acknowledgments
This work was supported by the Swedish Research Council (grant number 13482 to Dr Fisone), StratNeuro at Karolinska Institutet (Dr Fisone), the foundation Blanceflor Boncompagni-Ludovisi née Bildt (Dr Bonito-Oliva), and Åhlén-stiftelsen (Dr Bonito-Oliva)
The authors thank Dr Paul Greengard for generously providing the DARPP-32 F/F, D1Rcre +, and D2RCre + transgenic mice used in this study.
References
- Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH. (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31(Supp 2):104–109. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18:5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. (2008) Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. (2010) Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA 107:14845–14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraki S, Kuzmin A, Tai F, Ogren SO. (2008) Repeated low dose of phencyclidine administration impairs spatial learning in mice: blockade by clozapine but not by haloperidol. Eur Neuropsychopharmacol 18:486–497. [DOI] [PubMed] [Google Scholar]
- Bondi C, Matthews M, Moghaddam B. (2012) Glutamatergic animal models of schizophrenia. Curr Pharm Des 18:1593–1604. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Spuhler KP, Weiner N. (1984) Effects of phencyclidine, amphetamine and related compounds on dopamine release from and uptake into striatal synaptosomes. J Pharm Exp Ther 229:671–680. [PubMed] [Google Scholar]
- Danysz W, Wroblewski JT, Costa E. (1988) Learning impairment in rats by N-methyl-D-aspartate receptor antagonists. Neuropharmacology 27:653–656. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O’Dowd BF, George SR. (1999) Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol 383:95–106. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, et al. (1998) DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science 281:838–842. [DOI] [PubMed] [Google Scholar]
- Garey RE, Heath RG. (1976) The effects of phencyclidine on the uptake of 3H-catecholamines by rat striatal and hypothalamic synaptosomes. Life Sci 18:1105–1110. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. (1992) The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci 15:285–320. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Nageotte C, Loiselle RH, Malone DA, Price WA. (1984) Comparison of chlorpromazine, haloperidol and pimozide in the treatment of phencyclidine psychosis: DA-2 receptor specificity. J Toxicol Clin Toxicol 22:573–579. [DOI] [PubMed] [Google Scholar]
- Greengard P. (2001) The neurobiology of slow synaptic transmission. Science 294:1024–1030. [DOI] [PubMed] [Google Scholar]
- Halpain S, Girault JA, Greengard P. (1990) Activation of NMDA receptors induces dephosphorylation of DARPP-32 in rat striatal slices. Nature 343:369–372. [DOI] [PubMed] [Google Scholar]
- Handelmann GE, Contreras PC, O’Donohue TL. (1987) Selective memory impairment by phencyclidine in rats. Eur J Pharmacol 140:69–73. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr., Greengard P, Tung HY, Cohen P. (1984) DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature 310:503–505. [DOI] [PubMed] [Google Scholar]
- Hertel P, Mathe JM, Nomikos GG, Iurlo M, Mathe AA, Svensson TH. (1995) Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav Brain Res 72:103–114. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Johansson C, Lindgren LM, Bengtsson A. (1994) Dopamine receptor antagonists block amphetamine and phencyclidine-induced motor stimulation in rats. Pharmacol Biochem Behav 48:465–471. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psych 148:1301–1308. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. (1997) Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology 17:92–99. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. (1979) Multiple receptors for dopamine. Nature 277:93–96. [DOI] [PubMed] [Google Scholar]
- King MM, Huang CY, Chock PB, Nairn AC, Hemmings HC, Jr., Chan KF, Greengard P. (1984) Mammalian brain phosphoproteins as substrates for calcineurin. J Biol Chem 259:8080–8083. [PubMed] [Google Scholar]
- Kitaichi K, Yamada K, Hasegawa T, Furukawa H, Nabeshima T. (1994) Effects of risperidone on phencyclidine-induced behaviors: comparison with haloperidol and ritanserin. Jpn J Pharmacol 66:181–189. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S. (1999) NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 7:125–143. [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. (1959) Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 81:363–369. [DOI] [PubMed] [Google Scholar]
- Madjid N, Tottie EE, Luttgen M, Meister B, Sandin J, Kuzmin A, Stiedl O, Ögren SO. (2006) 5-Hydroxytryptamine 1A receptor blockade facilitates aversive learning in mice: interactions with cholinergic and glutamatergic mechanisms. J Pharm Exp Ther 316:581–591. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. (1989) Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology 2:299–308. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. (1997) Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci 17:8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. (2002) Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. J Neurochem 81:832–841. [DOI] [PubMed] [Google Scholar]
- Ögren SO, Goldstein M. (1994) Phencyclidine- and dizocilpine-induced hyperlocomotion are differentially mediated. Neuropsychopharmacology 11:167–177. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. (1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Langley-Gullion KC, Greengard P. (1998) Quantitative immunocytochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res 808:8–12. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Greco B, Sacchetti G, Leoni G, Invernizzi RW, Carli M. (2010) Blockade of serotonin 2A receptors prevents PCP-induced attentional performance deficit and CREB phosphorylation in the dorsal striatum of DBA/2 mice. Psychopharmacology (Berl) 208:387–399. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. (1996) Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol 7:3–23. [PubMed] [Google Scholar]
- Seeman P, Lasaga M. (2005) Dopamine agonist action of phencyclidine. Synapse 58:275–277. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC. (2008) Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse 62:819–828. [DOI] [PubMed] [Google Scholar]
- Smith RC, Meltzer HY, Arora RC, Davis JM. (1977) Effects of phencyclidine on [3H]catecholamine and [3H]serotonin uptake in synaptosomal preparations from rat brain. Biochem Pharmacol 26:1435–1439. [DOI] [PubMed] [Google Scholar]
- Steinpreis RE, Salamone JD. (1993) The role of nucleus accumbens dopamine in the neurochemical and behavioral effects of phencyclidine: a microdialysis and behavioral study. Brain Res 612:263–270. [DOI] [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, Meltzer HY. (1979) Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol 59:169–179. [DOI] [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, London SF, Meltzer HY. (1981) A comparison of the effects of neuroleptics on phencyclidine-induced behaviors in the rat. Eur J Pharmacol 76:37–53. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. (1998) Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience 84:223–228. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, Fisone G. (2000) Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci USA 97:1856–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. (2003) Diverse psychotomimetics act through a common signaling pathway. Science 302:1412–1415. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. (1998) Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 12:21–36. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi T, Hirano M, Matsumoto T, Nakamura K, Hashimoto K, Hondo H, Yonezawa Y, Tsukashima A, Nakane H, Uchimura H, et al. (1995) Involvement of dopamine D1 receptors in phencyclidine-induced behavioral stimulation in rats. Clin Neuropharmacol 18:64–71. [DOI] [PubMed] [Google Scholar]
- Vickroy TW, Johnson KM. (1982) Similar dopamine-releasing effects of phencyclidine and nonamphetamine stimulants in striatal slices. J Pharm Exp Ther 223:669–674. [PubMed] [Google Scholar]