Abstract
Background:
Drug-environment associative memory mechanisms and the resulting conditioned behaviors are key contributors in relapse to cocaine dependence. Recently, we reported rat amygdala phospholipase D as a key convergent downstream signaling partner in the expression of cocaine-conditioned behaviors mediated by glutamatergic and dopaminergic pathways. In the present study, 1 of the 2 known upstream serotonergic targets of phospholipase D, the serotonin (5-hydroxytryptamine) 2C receptor, was investigated for its role in recruiting phospholipase D signaling in cocaine-conditioned behaviors altered in the rat amygdala and dorsal hippocampus.
Methods:
Using Western-blot analysis, amygdala phospholipase D phosphorylation and total expression of phospholipase D/5-hydroxytryptamine 2C receptor were observed in early (Day-1) and late (Day-14) withdrawal (cocaine-free) states among male Sprague-Dawley rats subjected to 7-day cocaine-conditioned hyperactivity training. Functional studies were conducted using Chinese Hamster Ovary cells with stably transfected human unedited isoform of 5-hydroxytryptamine 2C receptor.
Results:
Phosphorylation of phospholipase D isoforms was altered in the Day-1 group of cocaine-conditioned animals, while increased amygdala and decreased dorsal hippocampus phospholipase D/5-hydroxytryptamine 2C receptor protein expression were observed in the Day-14 cocaine-conditioned rats. Functional cellular studies established that increased p phospholipase D is a mechanistic response to 5-HT2CR activation and provided the first evidence of a biased agonism by specific 5-hydroxytryptamine 2C receptor agonist, WAY163909 in phospholipase D phosphorylation 2, but not phospholipase D phosphorylation 1 activation.
Conclusions:
Phospholipase D signaling, activated by dopaminergic, glutamatergic, and serotonergic signaling, can be a common downstream element recruited in associative memory mechanisms altered by cocaine, where increased expression in amygdala and decreased expression in dorsal hippocampus may result in altered anxiety states and increased locomotor responses, respectively.
Keywords: amygdala, dorsal hippocampus, phospholipase D, cocaine, conditioned hyperactivity
Introduction
An important hallmark of cocaine addiction is the high propensity of relapse to active use despite extended periods of abstinence (Berke and Hyman, 2000). This relapse can be attributed to a pathological “hijacking” of learning and memory mechanisms in the reward pathway (Kelley, 2004; Hyman, 2005; Hyman et al., 2006). Exposure to cocaine-related cues activates brain areas important in associative memory (Breiter et al., 1997; Kruzich and See, 2001) and the expression of conditioned behaviors.
The involvement of amygdala and hippocampal brain regions has been successfully modeled in preclinical rodents. Reversible inactivation of the basolateral amygdala (BLA) disrupted both formation and expression of the cocaine-conditioned behavior (Kruzich and See, 2001), while electrical stimulation of the BLA was sufficient to reinstate cocaine-seeking behavior in the rat (Hayes et al., 2003). Disruptions in the hippocampus and BLA result in absence of stimulus control in drug-seeking as seen in cocaine-conditioned place preference (Isaac et al., 1989; Hiroi and White, 1991; Brown and Fibiger, 1993; Tzschentke, 2007), cocaine self-administration (Whitelaw et al., 1996), and cocaine reinstatement models (Fuchs et al., 2007).
Certain conditioned responses observed to cocaine-related stimuli in drug addicts (Ehrman et al., 1992) resemble Pavlovian attributes of conditioned behavior to cocaine-associated cues (Pert, 1994). In preclinical rodent models, cocaine-associated environmental response can be investigated as conditioned locomotor behavior in the absence of the drug (Carey and Damianopoulos, 1994; Bardo et al., 1995; Krishnan et al., 2011).
The cocaine-conditioned hyperactivity (CH) model (Barr et al., 1983) differentiates between drug-induced and drug cue-induced increases in locomotor activity in rats (Carey and Damianopoulos, 1994; R. Carey and Gui, 1997; Carey et al., 2008) and mice (Brabant et al., 2003). By repeated cocaine administration prior to placing the animals in a specific environment (eg, locomotor activity monitor), an association of the stimulus environment occurs to the behavioral effects of cocaine, namely hyperactivity, such that later reintroduction into the stimulus environment, without cocaine injection, results in the expression of the hyperactivity. The present study, therefore, employed this behavioral paradigm to investigate the signaling mechanisms recruited by cocaine environment- conditioned responses. Our group recently reported a key role for amygdala phospholipase D (PLD) as a downstream convergent target for dopaminergic and glutamatergic signaling in the expression of cocaine-conditioned response in late (Day-14) withdrawal (drug-free) state (Krishnan et al., 2011). PLD, a lipid-modifying enzyme, catalyzes the conversion of phosphatidyl choline into choline and phosphatidic acid (Cockcroft, 2001; Exton, 2002; Frohman, 2015). But PLD performs other cellular signaling roles, including regulation of exocytosis (Hughes et al., 2004; Huang et al., 2005), endocytosis (Du et al., 2004), and neurotransmitter release (Humeau et al., 2001), all of which are important mechanisms in the neurochemical basis of learning and memory (Bennett and Scheller, 1993). Using CH, the present study addresses the hypothesis that PLD signaling is key to cocaine environment-associated changes in memory mechanism. We also addressed the potential interaction between PLD and serotonergic signaling in the expression of cocaine-conditioned behaviors.
The 5-hydroxytryptamine (5-HT) 2C receptor (5-HT2CR) (Di Giovanni and De Deurwaerdere, 2015) is 1 of 14 identified receptors for serotonin (5-HT) (Meneses, 2015) and is expressed in the amygdala and hippocampus. Interestingly, the complexity of the downstream signaling by this G-protein coupled receptor has been linked to multiple signaling pathways that are G-protein dependent and independent (reviewed in Millan et al., 2008), making it necessary to establish a downstream signaling mechanism to better define the specificity of its action. While the activation of phospholipase C via Gq/11 is most commonly studied (Hoyer et al., 2002), effects of the 5-HT2CR mediated by downstream activation of other phospholipases, including PLD (McGrew et al., 2002, 2004), are largely overlooked.
To test the above, we studied: (1) CH in rats following 7-day repeated cocaine administration in early (Day-1) and late (Day-14) withdrawal (drug-free) states; (2) protein expression levels of 5-HT2CR and the 2 mammalian isoforms of PLD in the amygdala and hippocampus in both drug-free states following cocaine-CH training; (3) functional association; and (4) activation of the PLD by 5-HT2CR as proof of concept in the recruitment of this novel pathway in the expression of conditioned behaviors to cocaine.
Methods
Behavioral Studies
Animals
Forty-eight adult male Sprague-Dawley rats (Harlan Inc., Indianapolis, IN) weighing 225 to 325g were used. Rats acclimated 5 to 7 days in a colony room at a constant temperature (21–23°C) and humidity (45–50%) on a 12-hour-light/-dark cycle (lights on 7:00 am to 12:00 pm). Rats were housed 2 per cage with food and water ad libitum. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Council, 2011) and with the approval of the Institutional Animal Care and Use Committee at University of Texas Medical Branch Galveston.
Drugs
Cocaine HCl, a gift from the National Institute of Drug Abuse (Research Triangle, NC), was dissolved in 0.9% NaCl.
Apparatus
Cocaine-conditioned locomotor activity was monitored and quantified under low light conditions (Valle, 1970).
Experimental Design
Rats were habituated to the locomotor activity monitors for 30 minutes each day for 4 consecutive days after saline (0.9% NaCl) injections. To establish CH, rats were placed in the locomotor activity chamber at the same time once each day for 7 consecutive days. Rats in the control group (n=8) were injected with saline prior to placement in the activity monitors, and the activity was monitored for 30 minutes. At the termination of the session, rats were returned to their home cage and removed 4 hours later and injected with saline and placed back into their home cage. Rats in the unpaired group (n=8) were injected with saline prior to placement in the activity monitors, and activity was monitored for 30 minutes. At the termination of the session, rats were returned to their home cage and received an injection of cocaine (15mg/kg, i.p.) 4 hours later. Rats in the paired group (n=8) were injected with cocaine (15mg/kg, i.p.) immediately prior to placement in the activity monitors, and activity was recorded for 30 minutes. At the termination of the session, rats were returned to their home cage and received an injection of saline 4 hours later. Rats then remained in their home cage until the test; one cohort (n=24, 8 animals per group) was tested after 1-day withdrawal (Day-1 group). After the Day-1 drug-free state, the animals were placed in the locomotor activity chamber after an injection of saline, and locomotor activity was recorded for 30 minutes.
Another cohort of 24 rats (n=8 each for control, unpaired and paired) underwent the same habituation and training as mentioned above, but were kept for 14 days following their last dose of cocaine in their home cage with food and water ad libitum before testing (Day-14 group).
Biochemical Assay
Immediately after the termination of the CH test (for both Day-1 and Day-14 groups), the animals were anesthetized using chloral hydrate solution (400mg/kg). Subsequent decapitation and microdissection of the brain to obtain amygdala and dorsal hippocampus (dHC) were performed using the rat brain stereotaxic coordinates (Paxinos and Watson, 2013). The brain regions were isolated into microcentrifuge tubes, immersed in liquid nitrogen to prevent degradation, and stored at -80°C till further use. A crude synaptosomal prep was made from these brain tissues, and subsequent Western blotting was performed using standard procedures. A uniform concentration (10–20 µg) of protein was diluted in Laemmli sample buffer (6X) and heated for 20 minutes at 70°C (Chinese hamster ovary [CHO] cell extracts were denatured for 5 minutes to prevent aggregation that occurred on longer duration of heating).
Antibodies (45 µg/reaction) were covalently crosslinked onto protein A/G resin according to the manufacturer’s instructions (Pierce Crosslink Immunoprecipitation Kit, Pierce Biotechnology, Rockford, IL), and immunoprecipitation studies were conducted using standard protocols.
Antibodies
Primary antibodies include: 5-HT2CR detected by mouse monoclonal D-12 (sc-17797, Santa Cruz; 1:100); 5-HT2CR detected by goat polyclonal N-19 (sc-15081, Santa Cruz; 1:250); PLD1 detected by rabbit polyclonal H-160 (sc-25512, Santa Cruz; 1:100); PLD2 detected by rabbit polyclonal H-133 (sc-25513, Santa Cruz; 1:100); monoclonal mouse anti-b-actin (MAB1501, Chemicon International, Temecula, CA, 1:5000); monoclonal mouse anti pan-cadherin (C-19, Abcam, Cambridge, MA, 1:5000); Phospho-PLD1 (Thr147, pPLD1T147) (#3831, Cell Signaling Technology, Danvers, MA, 1:1000); Phospho-PLD1 (Ser561, pPLD1S561) (#3834, Cell Signaling Technology, Danvers, MA, 1:1000); Phospho-PLD2 (Phospho-Tyr169, pPLD2Y169) (A8400, Assay Biotech, Sunnyvale, CA, 1:1000). Secondary antibodies included infrared-labeled goat anti-mouse (IRDye 680; 926–32220, LI-COR Biosciences, Lincoln, NE); goat anti-rabbit (IRDye 800; 827–08365, LI-COR Biosciences); donkey anti-goat (IRDye 800CW; 605-731-125, Rockland Immunochemicals, Inc., Gilbertsville, PA) and sheep anti-mouse (IRDye 680, Rockland Immunochemicals).
Data Analysis
Data from the activity monitors was organized into mean total counts (± SEM) for the dependent measure of total horizontal activity (ambulation and fine movements) recorded during the test session. A 1-way ANOVA (GraphPad InStat software for Windows V.3.01, GraphPad Software Inc., La Jolla, CA) followed by Tukey’s posthoc analysis was used to analyze CH measures at both drug-free states. Time course data were broken down into 3 separate 10-minute time points. The criterion for statistical significance was set at P<.05.
Membranes were imaged using the Odyssey Infrared Imaging System (LI COR Biosciences) at 700 and/or 800nm at 169 μm resolution. The integrated intensity of each band was analyzed with the Odyssey Infrared Imaging System Application version 2.1 Software. The ratio of 5-HT2CR or PLD band intensity to pan-cadherin band intensity was determined for each sample for normalization. Further analysis to study the time-dependent changes between Day-1 and Day-14 was conducted as follows. For each antibody, normalized control values described above were averaged. Unpaired and paired values were divided by the respective average control value for each respective antibody and converted to percent increase or decrease and further assessed for changes.
Cellular Studies
CHO K1 cell lines, stably transfected with nonedited human 5-HT2CR (5-HT2CR-CHO) cells in the p198–DHFR–Hygro vector containing a hygromycin resistance gene, were generous gifts from Drs. K. Berg and W. Clarke from the University of Texas Health Science Center at San Antonio (San Antonio, TX). Two lines of 5-HT2CR-CHO cells were employed in the present experiment: the “low”-expressing CHO-1C19 cells express ~200fmol/mg of the 5-HT2CR protein, while the “high”-expressing CHO-1C7 cells express 5 to 10 pmol/mg of the 5-HT2CR protein (Berg et al., 1994, 1999). Protein expression in 1C19 cells was assessed at 200fmol/mg protein, which approximates physiological levels in the brain (Berg et al., 2001). Cells were grown at 37°C, 5% CO2 and 85% relative humidity in GlutaMaxα-MEM (Invitrogen, Carlsbad CA), 5% fetal bovine serum (Atlanta Biologicals, Atlanta GA), and 100 µg/mL hygromycin (Mediatech, Manassas VA) and were passaged when they reached 80% confluence in 150-cm2 tissue culture plates before extracting protein.
Drugs
Serotonin (5-HT; Acros Organics, Thermo Fisher Scientific, Rockford, IL) was dissolved in 1X Dulbecco’s Phosphate Buffered Saline (DPBS; Cellgro, Thermo Fisher Scientific, Rockford, IL). WAY163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-(b)(1,4)diazepino (6,7,1hi)indole], a gift from Pfizer (New York, NY), was dissolved in 0.9% NaCl. SB242084 [6-chloro-5-methyl-1-((2-(2-methylpyrid-3-yloxy) pyrid-5-yl)carbamoyl)indoline dihydrochloride, Tocris Bioscience, Ellisville MO] was dissolved in 1X DPBS containing 10 mmol/L citric acid (Sigma, St. Louis, MO) and 8% 2-hydroxypropyl-β-cyclodextrin (Trappsol Hydroxypropyl Beta Cyclodextrin, pharmaceutical grade; Cyclodextrin Technologies Development, Alachua, FL) with the final pH adjusted to 5.6. SB216641 [N-[3-[3-(Dimethylamino)ethoxy]-4-me thoxyphenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol- 3-yl)-[1,1′-biphenyl]-4-carboxamide hydrochloride, Tocris Bioscience, Ellisville MO] was dissolved in 1X DPBS.
Confluent cells in the 150-cm2 plates were starved with serum/antibiotic free medium overnight at 37ºC. The next day, the media was aspirated and replaced with Hank’s Balanced Saline Solution (HBSS; Mediatech, Manassas VA). Drugs were diluted in HBSS at the specified concentrations and applied to the cells for the requisite time as specified in Results. At the end of the treatment, cells were rinsed with HBSS and scraped in cellular protein buffer (pH 7.4) containing 50mM Tris-HCl, 10mM MgCl2, 0.1mM EDTA, plus protease inhibitor cocktail, and phosphatase inhibitor 1 and 2 cocktails (10 µL/mL Sigma-Aldrich) and 1 µM dithiothreitol (Sigma-Aldrich). Centrifugation was performed at 2000 g at 4ºC for 10 minutes to pellet the cells. Following a second wash, a second centrifugation at 20,000 g for 30 minutes was performed. The pellet obtained was resuspended in the above recipe of cellular protein buffer plus 1% Nonidet P-40 (Sigma-Aldrich) detergent. Protein concentration was estimated using the BCA assay. Western-blot analysis was performed as described in the earlier sections, except that actin was the loading control used for coimmunoprecipitation studies, since cadherin was not detected in cellular preparations.
Results
Robust CH Is Observed in Both Day-1 and Day-14 Drug-Free States
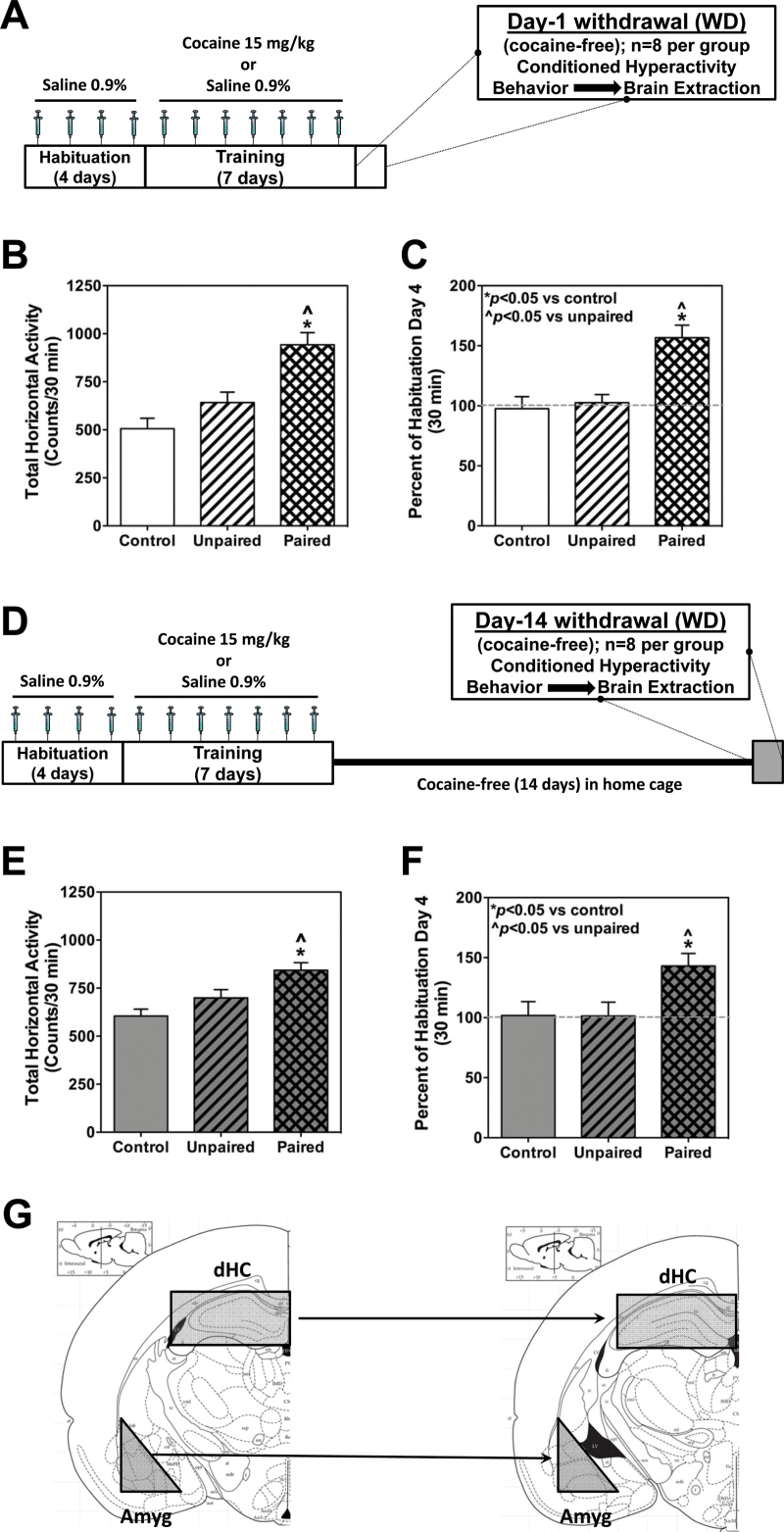
We tested the hypothesis that CH provides a robust measure of cocaine-induced conditioned responses at Day-1 (Figure 1B-C) and Day-14 (Figure 1E-F) drug-free states. Total horizontal activity over the entire 30-minute interval (the sum of all ambulatory and fine movements – motions that interrupted the infrared beams) was significantly increased in the paired group of animals (942.1±63.2) compared with the unpaired (642.1±53.7) or control (505.9±54.1) group in Day-1 drug-free state (Figure 1B). The apparent increased locomotor activity trend in the unpaired group of animals compared with control was accounted by the variability between animals (Figure 1C). Increase in locomotor activity as a percent of the last habituation day in the paired group of animals registered ~50% increase (156.7±10.4) compared with either unpaired (102.6±6.8) or control (97.6±9.9) groups in the Day-1 cohort.
Figure 1.
Cocaine-induced conditioned hyperactivity is observed in both (B-C) Day-1 (clear background) and (E-F) Day-14 (dark background) drug-free states. Panels A and D provide a schematic of the training and testing regimen used in the conditioned hyperactivity paradigm. Data represent the mean total horizontal activity counts (±SEM) summed over the 30-minute test session in rats conditioned to 15mg/kg of cocaine in (B) Day-1 drug-free state (cohort of 24 animals, n=8/group) or (E) Day-14 drug-free state (another cohort of 24 animals, n=8/group). While increase in conditioned hyperactivity in the paired group of animals in Day-1 (C) and Day-14 (F) drug-free states, as a percent of the total horizontal activity on habituation day 4, remains the same, the analysis also demonstrates an absence of hyperactivity in the unpaired group of animals compared with control, thus validating the use of this paradigm to delineate the signaling mechanisms affected by cocaine pharmacology in contrast to cocaine-conditioned memory. Following Day-1 or Day-14 test, the animals were immediately sacrificed and the brain removed and sectioned to isolate specific brain regions. (G) A schematic of the coronal section from -2.5mm (left) to -3.5mm (right) bregma (modified from Paxinos and Watson, 2013). After isolating this section, the shaded rectangular and triangular cuts were performed with razor blades to isolate the dorsal hippocampus (dHC) and amygdala (Amyg), respectively, for further biochemical analysis. *P < .05 vs control; ^ P < .05 vs unpaired.
In the Day-1 drug-free state, the paired group of animals demonstrated an increase of ~50% in their horizontal locomotor activity (156.7±10.4) compared with either unpaired (102.6±6.8) or control (97.7±10.0) groups. The increase in locomotor activity was significant for each 10-minute epoch (P<.05) compared with both unpaired and control groups (data not shown).
In the Day-14 drug-free state, the CH in the paired group (843.0±39.0, n=8) was significantly different compared with the unpaired (698.8±43.4, n=8) or the control (604.3±36.06, n=8) group (Figure 1E). Increase in locomotor activity as a percent of the last habituation day in the paired group of animals still registered ~50% increase (143.1±10.5) compared with either unpaired (101.3±11.6) or control (101.9±11.5) groups (Figure 1F) in the Day-14 cohort and was not different from the increased CH levels in the Day-1 drug-free state. These results suggest that cocaine-environment association elicits a robust conditioned response that is quantified at the same levels between Day-1 and Day-14 cocaine-free days.
Thus, the CH paradigm uniquely provided an opportunity to address whether PLD and 5-HT2CR signaling occurs in response to pharmacological effects of cocaine or the conditioned response to the cocaine-associated environment or both. Studying the effect on PLD and 5-HT2CR expressions at 2 distinct time points addressed the next step of verifying whether these key therapeutic targets were associated with cocaine-conditioned responses.
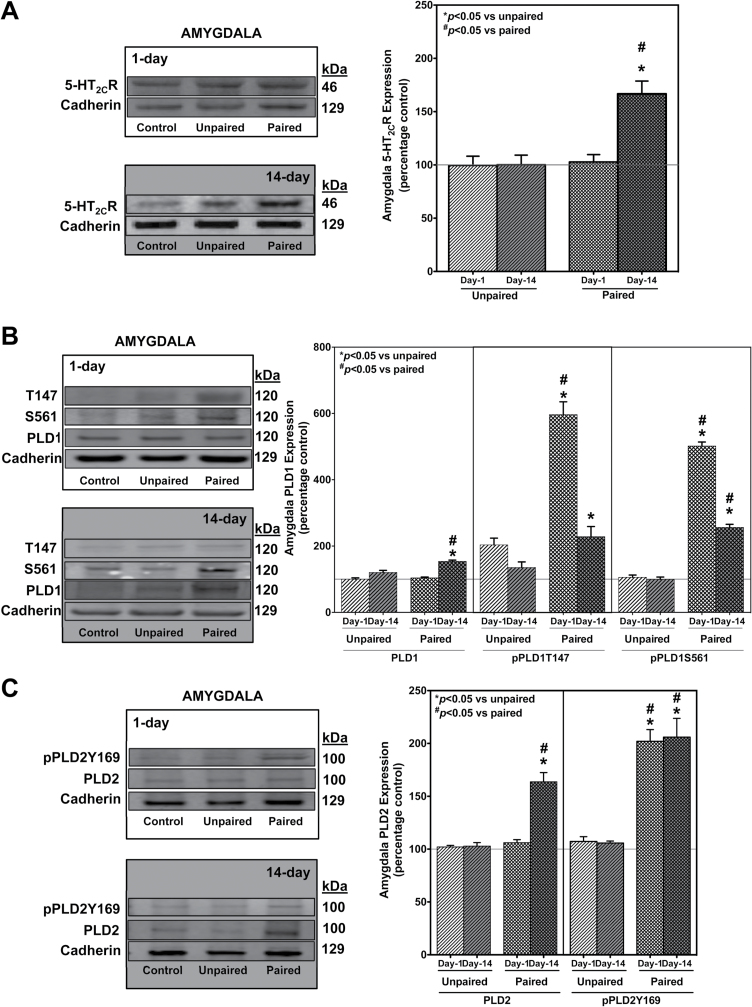
CH Is Associated with Elevated Day-14, but Not Day-1, Expression of Amygdala PLD and 5-HT2CR While Increased Phosphorylation of PLD Isoforms Are Observed in Both Drug-Free States in the Paired Group of Animals
Changes in phosphorylation states (pPLD) were used, in addition to protein expression profiles, as a measure of PLD signaling (Kim et al., 1999; Watanabe and Kanaho, 2000; Z. Xie et al., 2000a, 2000b; Hu and Exton, 2003, 2005). For both Day-1 (clear background) and Day-14 (dark background) drug-free states, increased levels of phosphorylation were observed in the paired group of animals (cross-hatch bars) compared with unpaired (slanted bars) at the threonine 147 (pPLD1T147, Figure 2B, middle), serine 561 (pPLD1S561, Figure 2B, last) in PLD1 isoform, and tyrosine 169 (pPLD2Y169, Figure 2C, last) in the PLD2 isoform. Interestingly, crude synaptosomal levels for both PLD1 (Fig 2B, first) and PLD2 (Fig 2C, first) were increased by 50% in Day-14 (dark background) but not Day-1 (clear background) drug-free states in the paired (hatched bars) compared with the unpaired (slanted bars) group. 5-HT2CR expression in the paired group (Fig 2A, hatched bars) is elevated by 50% only in the Day-14 (dark background), not in the Day-1 (clear background), drug-free state compared with the unpaired group (slanted bars).
Figure 2.
Amygdala crude synaptosomal membrane phospholipase D (PLD)1/PLD2 isoforms show elevated phosphorylation states in the Day-1, while both phosphorylation and expression levels are increased in the Day-14 drug-free states in the paired group following conditioned hyperactivity (CH). 5-HT2CR expression remains unchanged in Day-1 and increases in the Day-14 drug-free state in the paired group following CH. Results represent the percent change in expression of the unpaired and paired compared to control group (±SEM). For both Day-1 (clear background) and Day-14 (dark background) drug-free states, increased levels of phosphorylation were observed in the paired group of animals (cross-hatch bars) compared to unpaired (slanted bars) at the [B] pPLD1T147 (middle panel), pPLD1S561 [last panel] in PLD1 isoform and [C] pPLD2Y169 (last panel) in the PLD2 isoform. Interestingly, crude synaptosomal levels for both PLD1 (B, first panel) and PLD2 (C, first panel) were increased by 50% in Day-14 (dark background), but not Day-1 (clear background) drug-free states in the paired (hatched bars) compared to the unpaired (slanted bars) group. 5-Hydroxytryptamine (5-HT) 2C receptor (5-HT2CR) expression in the paired group (A, hatched bars) is elevated only in the Day-14 (dark background), not in the Day-1 (clear background), drug-free state compared with the unpaired group (slanted bars). # P<.05 vs paired; *P<.05 vs unpaired; n=8 animals/group.
Absolute levels of crude synaptosomal 5-HT2CR/PLD fractions in control/unpaired amygdala were indistinguishable from naïve animals (not exposed to the environment, injections, or handling; data not shown), verifying that the neurochemical changes observed are a result of conditioned responses to cocaine. 5-HT2CR-PLD levels in the dHC (Fuchs et al., 2007; X. Xie et al., 2010) were assessed next.
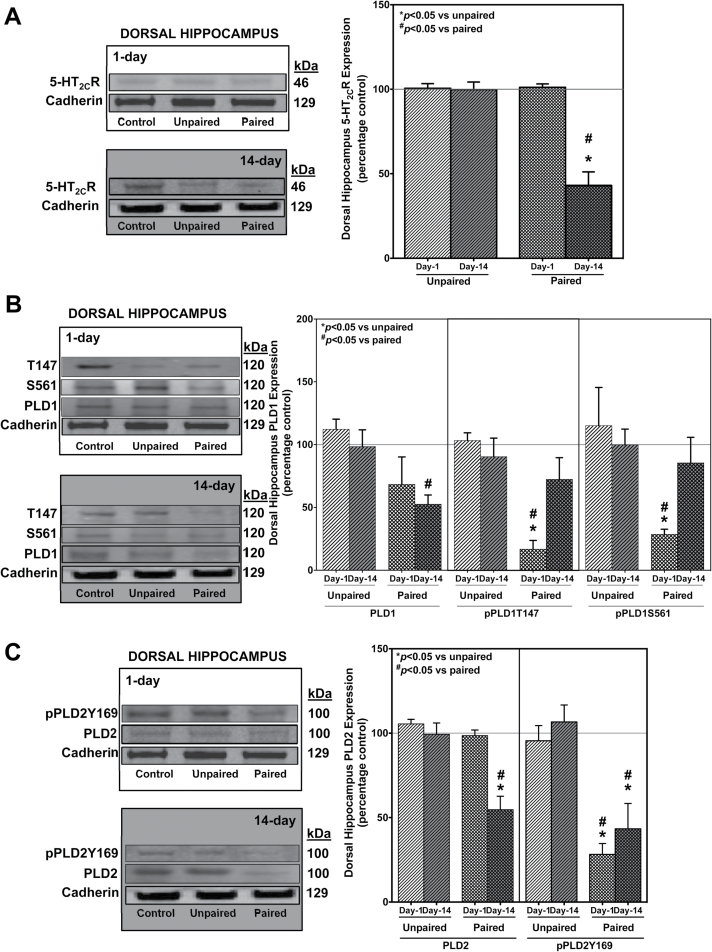
CH Is Associated with Diminished Day-14, but Not Day-1, Expression of dHC PLD and 5-HT2CR with Differential Changes in the Phosphorylation States of the PLD Isoforms in the Paired Group of Animals
Interestingly, crude synaptosomal expression levels of 5-HT2CR, PLD1, and PLD2 in the dHC demonstrated a contrasting profile of phosphorylation as well as expression states compared with the amygdala (Figure 3). Contrary to the increased amygdala profile, 5-HT2CR expression in the paired group (Figure 3A, hatched bars) is diminished by 50% only in the Day-14 (dark background), not in the Day-1 (clear background), drug-free state compared with the unpaired group (slanted bars). Again, contrasting to the increased amygdala profile, decreased phosphorylation states in the dHC were observed in the paired group of animals (cross-hatch bars) compared with unpaired (slanted bars) at the threonine 147 (pPLD1T147, Figure 3B, middle), serine 561 (pPLD1S561, Figure 3B last) in PLD1 isoform and tyrosine 169 (pPLD2Y169, Figure 3C, last) in the PLD2 isoform Day-1 (clear background) drug-free state.
Figure 3.
Dorsal hippocampus (dHC) crude synaptosomal membrane phospholipase D (PLD)1/PLD2 isoforms show reduced phosphorylation states in the Day-1, while either decreased (PLD2) or unchanged (PLD1) phosphorylation and decreased PLD1/PLD2 expression in the Day-14 drug-free states in the paired group following conditioned hyperactivity (CH). 5-Hydroxytryptamine (5-HT) 2C receptor (5-HT2CR) expression remains unchanged in Day-1 and decreases in the Day-14 drug-free state in the paired group following CH. Results represent the percent change in expression of the unpaired and paired compared to control group (±SEM). 5-HT2CR expression in the paired group (A, hatched bars) is diminished by 50%, only in the Day-14 (dark background), not in the Day-1 (clear background) drug-free state compared with the unpaired group (slanted bars). Decreased phosphorylation states were observed in the paired group of animals (cross-hatch bars) compared with unpaired (slanted bars) at (B) pPLD1T147 (middle panel), pPLD1S561 (last panel) in PLD1 isoform, and (C) pPLD2Y169 (last panel) in the PLD2 isoform Day-1 (clear background) drug-free state. Phosphorylation states of the PLD1 isoform (B, middle and last panel) did not show any significant increase or decrease in paired group (cross hatched bars) in the Day-14 (dark background) drug-free state. However, the overall dHC crude synaptosomal expression for PLD1 (B, first panel) and PLD2 (C, first panel) levels were decreased by 50% in the paired (cross-hatched bars) compared with the unpaired (slanted bars) animals in the Day-14 (dark background) drug-free state. No changes in the paired (cross hatched bars) compared with unpaired (slanted bars) groups were observed in either PLD1 (B, first panel) or PLD2 (C, first panel) in the Day-1 (clear background) drug-free state. Diminished phosphorylation state of the PLD2 isoform Y169 (C, last panel) was observed in both the Day-1 (clear background) and Day-14 (dark background) drug-free states. # P<.05 vs paired; *P<.05 vs unpaired; n=8 animals/group.
In contrast to the changed amygdala profile, phosphorylation states in the dHC for the PLD1 isoform (Figure 3B, middle and last) did not show any significant increase or decrease in the paired group (cross hatched bars) in the Day-14 (dark background) drug-free state. However, the overall dHC crude synaptosomal expression for PLD1 (Figure 3B, first) and PLD2 (Figure 3C, first) levels were decreased by 50% in the paired (cross-hatched bars) compared with the unpaired (slanted bars) animals in the Day-14 (dark background) drug-free state. Similar to the amygdala profile, there were no changes in the paired (cross hatched bars) compared with unpaired (slanted bars) groups observed in either PLD1 (Figure 3B, first) or PLD2 (Figure 3C, first) in the Day-1 (clear background) drug-free state. Thus, it is possible that the lack of a diminished phosphorylation profile in Day-14 drug-free state could be attributed to floor-level reduction in expression of the PLD1 isoform. Intriguingly, the diminished phosphorylation state of the PLD2 isoform Y169 (Figure 3C, last) was observed in both the Day-1 (clear background) and Day-14 (dark background) drug-free states. One possible explanation is that PLD2 isoform, being the constitutively expressed isoform with high basal activity (Slaaby et al., 2000), has sufficient basal activity despite the decrease for observing a change in the phosphorylation state. Next, we tested the hypothesis that 5-HT2CR can functionally activate PLD using 2 approaches: coimmunoprecipitation studies and cell culture experiments.
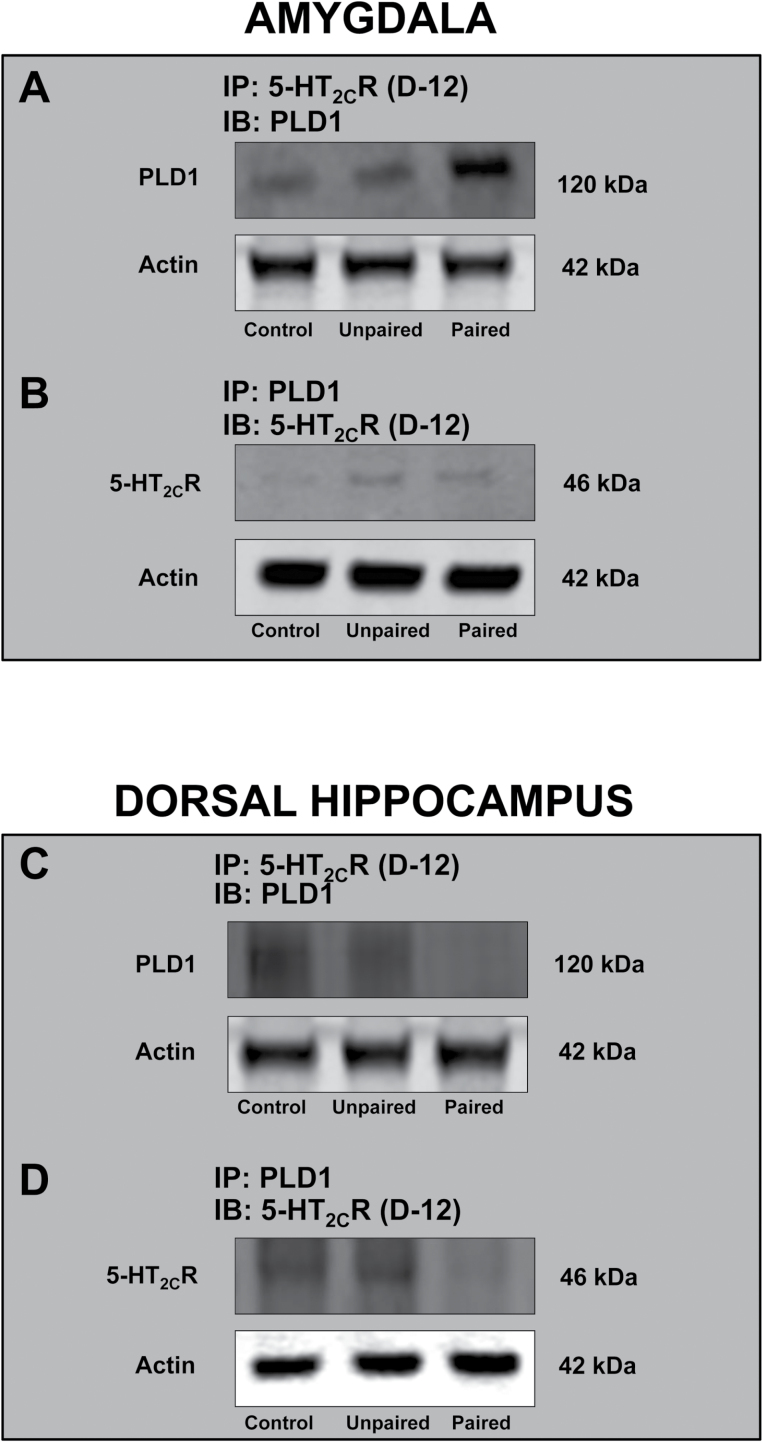
CH Is Characterized by Increased Amygdala and Decreased Hippocampal 5-HT2CR-PLD1 Association
Qualitative coimmunoprecipitation studies established a functional association between 5-HT2CR and PLD (Figure 4). Increased association of PLD1 (Figure 4A) was observed in the 5-HT2CR pulldowns from amygdala crude synaptosomal fractions of paired compared with control and unpaired groups. A similar increased association was demonstrated in the reverse immunoprecipitation scheme using PLD1 antibody for pulldowns (Figure 4B). These results mirrored the increased expression profile observed in the Day-14 paired group. On the other hand, decreased expression in dHC corresponds with decreased 5-HT2CR-PLD1 association in the paired group (Figure 4C-D).
Figure 4.
Functional (immunoprecipitation) studies demonstrate that phospholipase D (PLD)1 expression tracks 5-hydroxytryptamine (5-HT) 2C receptor (5-HT2CR) expression in crude synaptosomal fractions of the amygdala and hippocampus in the paired group of animals in Day-14 drug-free state. (A, C) Synaptosomal fractions were immunoprecipitated (IP) with 5-HT2CR antibody and immunoblotted (IB) with PLD1, and the converse was also performed (C-D) in both the amygdala (A-B) and the hippocampus (C-D). Uniform loading was verified by measuring β-actin. Each experiment was repeated twice. These studies establish that increased association in the amygdala and decreased association in the hippocampus of 5-HT2CR-PLD at the crude synaptosomal levels are involved with the expression of conditioned hyperactivity.
While reciprocal coimmunoprecipitations were attempted with PLD2 antibody, 5-HT2CR expression was not observed. Interestingly, PLD2 expression (data not shown) in 5-HT2CR pulldowns mirrored the expression of PLD1 in the amygdala and dHC. Perhaps the epitope recognized by PLD2 antibody was limiting the ability of the present study to detect 5-HT2CR expression in the PLD2 pulldowns.
Activation of Human 5-HT2CR Results in Increased PLD Phosphorylation States in Vitro
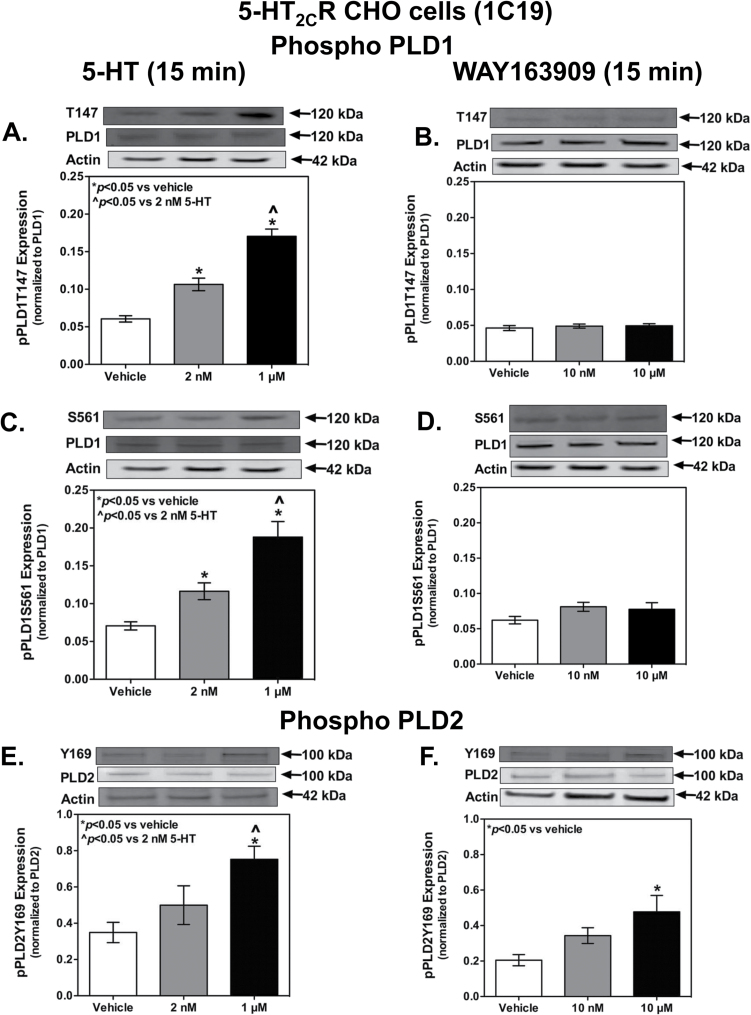
Functional 5-HT2CR-PLD signaling was assessed by the effect of acute activation of human 5-HT2CR (5-HT2CR-CHO cells) on pPLD expression. After reaching 80% confluence, cells were serum-starved overnight at 37°C to minimize the downregulation of the 5-HT2CR (Berg et al., 2001). Cells were treated with endogenous ligand, 5-HT (2nM or 1 µm), or the selective 5-HT2CR agonist WAY163909 (10nM or 10 µM) in vehicle (1X DPBS) for 5 minutes in the presence or absence of the selective 5-HT2CR antagonist SB242084 (300nM) or the 5-HT1BR antagonist SB216641 (1 µM), applied 10 minutes prior to agonist application. 5-HT2CR-CHO cells are reported to express 5-HT1BR (Berg et al., 1994). No significant decrease in pPLD1 states (data not shown) was observed in the presence of the 5-HT1BR antagonist. Overall expression of PLD1 or PLD2 remained unchanged (Figure 5). Increasing phosphorylation levels of PLD1 isoform were observed at T147 (Figure 5A), S561 (Figure 5C), and PLD2 at Y169 (Figure 5E) with increasing concentrations of 5-HT. Interestingly, application of WAY163909 resulted in increased phosphorylation only at the 10-μM concentration at the Y169 site on PLD2 (Figure 5F) but did not affect the phosphorylation states of T147 (Figure 5B) and S561 (Figure 5D) at any concentration. We addressed the hypothesis that increased receptor levels using the “high”-expressing CHO-1C7 may increase the signal-noise ratio and demonstrate robust levels of elevations in phosphorylation states, especially by WAY163909. However, the results mirrored the expression in the “low”-expressing CHO-1C19 cells, suggesting that perhaps WAY163909 may be demonstrating biased agonism by selectively activating PLD2, not PLD1, isoform downstream to human 5-HT2CR.
Figure 5.
Endogenous (5-hydroxytryptamine [5-HT]-) or selective agonist (WAY163909-) mediated activation (15 minutes) of stable but “low-expressing” (~250fmol/mg) unedited human isoform of the 5-hydroxytryptamine (5-HT) 2C receptor (5-HT2CR) in Chinese hamster ovary (CHO)-K1 cells increases phosphorylation states of the 2 different phospholipase D (PLD) isoforms in a dose-dependent manner. Whole cell homogenates were analyzed by Western blot (n=3–6 samples/each). Densitometric analyses were conducted on the (A-B) pPLD1T147 (120kDa), (C-D) pPLD1S561 (120kDa), (E-F) pPLD2Y169 (100kDa), (A-D) PLD1 (120kDa), and (E-F) PLD2 bands. Interestingly, 5-HT activation results in increased phosphorylation states for all 3 phosphorylation sites (A, C, E) tested, however, WAY163909 activation results in a significant increase only in the Y169 phosphorylated state at the highest concentration (F). Results represent the mean density (±SEM) of the respective IR band of each phosphorylated form normalized to the total protein levels. Actin was used as a loading control. *P<.05 vs vehicle; ^P<.05 vs 2nM 5-HT.
Discussion
The present study demonstrated: (1) there is a ~50% increase in the conditioned locomotor response in both Day-1 and Day-14 groups following 7-day CH; (2) this involves increased phosphorylation states (Day-1, Day-14) and 50% increase in the expression (Day-14) of PLD in amygdala crude synaptosomal fractions; (3) decreased PLD phosphorylation states (Day-1) and (50%) 5-HT2CR/PLD expression (Day-14) in the dHC; (4) increased (amygdala) and decreased (dHC) crude synaptosomal 5-HT2CR/PLD association in Day-14 paired group; and (5) biased agonism profile for the 2 PLD isoforms via activation by endogenous (5-HT) and a specific (WAY163909) activator of human 5-HT2CR stably expressed in CHO cells.
Robust CH Is Observed Irrespective of the Withdrawal Period
CH in the present study was chosen for its ability to quantitatively differentiate between pharmacological and conditioned responses to cocaine. Conditioned locomotor activity provides a direct measure of cue-attributed salience without the confounding variable of goal-seeking behavior and motivation state (Olmstead et al., 2001). Habituation sessions, prior to cocaine conditioning, were important for discounting rat locomotor activity that occurs when placed in novel environments (Alex and Pehek, 2007). This allowed us to utilize the 4th habituation day to eliminate intervariability among the rats and demonstrate that locomotor activity (Figure 1C, F) is increased significantly (~50%) in the Day-1 and Day-14 drug-free states exclusively in the paired group. An incubation effect (increase in cue-induced activity with increasing withdrawal time) that is reported in self-administration paradigms (Tran-Nguyen et al., 1998; Neisewander et al., 2000; Lu et al., 2004) was not observed in this noncontingent drug administration study.
CH Increases Amygdala PLD/5-HT2CR Signaling
The present study is the first to show significant changes in the 5-HT2CR-PLD expression profiles after a short (Day-1) and longer (Day-14) period of drug-free state. Increased phosphorylation at pPLD1T147 can occur via PKCα (Kim et al., 1999) and is present in the pleckstrin homology domain that increases PLD enzymatic (lipolytic) activity (Lee et al., 2005) and the rate of interaction with other proteins (Sung et al., 1999). Additionally, simultaneous activation at pPLD1T147 and pPLD1S561 (located in the negative regulatory loop exclusive to the PLD1 isoform) increases endogenous PLD1 activity (Kim et al., 1999).
Direct phosphorylation at the pPLD2Y169 by PKCδ (Han et al., 2002) at the pleckstrin homology domain of PLD2 can bind to SH2/SH3 containing tyrosine kinases (Ahn et al., 2003; Choi et al., 2004), resulting in increased PLD2 activity. Thus, the increased phosphorylation states of pPLD1T147 and pPLD1S561 levels as well as increased expression of PLD1/PLD2 and 5-HT2CR (Figure 2) could trigger PKC/PLD-dependent downstream events in response to CH, some of which have been discussed below.
CH Decreases dHC PLD/5-HT2CR Signaling
The hippocampus is well established for its role in associative memory of context-specific information (Burgess et al., 2001; Davachi and DuBrow, 2015). It is also implicated in the associative memory mechanisms underlying addiction (White, 1996; Crombag et al., 2008; Marchant et al., 2014). The present study investigated the dHC (also referred to as septal hippocampus; posterior hippocampus in primates) because of its preferential role in spatial learning and memory (Moser and Moser, 1998; Bannerman et al., 2004) vs the ventral hippocampus (or temporal hippocampus; anterior hippocampus in primates), which is implicated in anxiety (Bannerman et al., 2004; Engin and Treit, 2007; Koob and Volkow, 2010; Allsop et al., 2014; Strange et al., 2014) and is the subject of our future investigations of effects on anxiety-like behaviors due to cocaine conditioning.
CH reduces both phosphorylation and expression of PLD1 isoforms (Figure 3), suggesting that downstream signaling is attenuated in this brain region during conditioned responses. Since the overall PLD1 isoform expression was decreased by 50%, it is very possible that any changes in the phosphorylation states may be occluded from our analyses. A decrease noted at both withdrawal states in the phosphorylation state of PLD2 (Y169, Figure 3C) supports the above possibility, since basal PLD2 isoform expression is greater than PLD1 (Slaaby et al., 2000).
5-HT2CR/PLD Brain Region-Specific Association Tracks with the Protein Expression Profiles
Reciprocal coimmunoprecipitation studies (Figure 4) established that crude synaptosomal fractions of PLD protein expression tracks that of 5-HT2CR in the amygdala and dHC for the paired group of animals in the Day-14 drug-free state. Based on this study, we investigated some of the known downstream signaling targets of PLD, such as G-protein-independent RhoA, that could underlie 5-HT2CR activation (McGrew et al., 2004). Since we failed to see a band for RhoA in any of the lanes (data not shown), the interaction may be G-protein coupled. Next, we tested for G12/13 implicated in 5-HT2CR-mediated PLD activation (McGrew et al., 2002, 2004) and observed that only G13 expression was observed in the paired group of animals in the amygdala (data not shown), suggesting that a specific G-protein-coupled signaling is recruited. Since mTOR (mammalian target of rapamycin), a serine and threonine kinase physically associates with PLD (Sun and Chen, 2008; Foster et al., 2014) where it is central to neuroadaptation signaling mechanisms mediated by drugs of abuse (Neasta et al., 2014), we investigated the pulldowns for mTOR expression. We did not observe mTOR (data not shown), presumably because it is not membrane associated. Future studies of downstream signaling targets using cytoplasmic and nuclear fractions will be key to establish signaling downstream to PLD.
PLD Phosphorylation Levels Increase in Response to Increasing Concentrations of Human 5-HT2CR Agonists in CHO Cells
Using human 5-HT2CR in CHO cells, we demonstrated a functional activation of PLD where we observe specificity in the phosphorylation states for the individual isoforms (Figure 5). While PLD2, the constitutively expressed basal isoform, showed increased phosphorylation to WAY163909, there was no activation of PLD1, thus indicating a possible biased agonism in 5-HT2CR-PLD-mediated signaling.
A Model for Amygdala/dHC PLD Signaling in CH
Increase in amygdala 5HT2CR/PLD expression/association may increase anxiogenic responses (Vicente and Zangrossi, 2012, 2014). BLA infusions of the nonselective 5-HT2A/2B/2CR antagonist, ritanserin, prevents the anxiogenic response associated with systemic MK212 (5-HT2A/2CR agonist) administration (de Mello Cruz et al., 2005), while mCPP (5-HT2B/2CR agonist) infusion into the BLA/CeA complex (but not dHC/vHC) enhances elevated plus maze associated anxiogenic behavior (Cornelio and Nunes-de-Souza, 2007), suggesting that amygdala 5-HT2R receptors play a positive modulatory role in expression of anxiogenic behaviors. Importantly, the anxiogenic responses are completely blocked by preinfusion of SDZ SER 082 (at doses that preferentially blocked 5-HT2CR), further localizing the effect of the anxiogenic responses to 5-HT2CR action specifically in the amygdala (Cornelio and Nunes-de-Souza, 2007). Most importantly, a recent study provides direct evidence for our observations, where administration of a 5-HT2CR agonist in the BLA increased anxiety-like behavior in cocaine-conditioned rats (Pockros-Burgess et al., 2014). However, increased anxiety is routinely associated with reduced locomotor activity and/or exploration in paradigms such as elevated plus maze and bright open field. Such cessation was not observed in CH, because the animals were placed back into a familiar (conditioned) rather than novel environment typically used in studies of anxiety. Such a hypothesis is supported by another study where overexpression of 5-HT2CR in the forebrain, with highest levels in the dHC, results in decreased wheel running activity as well as open field activity (Kimura et al., 2009). Thus, the opposite scenario of a decreased 5-HT2CR expression that we observe in the dHC could contribute towards cocaine-conditioned locomotor activity in CH.
Future studies that address a direct causal effect between CH-associated specific conditioned behaviors of anxiety and increased locomotor responses to cocaine-cue-induced memory will be important in addressing whether the 5-HT2CR-PLD signaling pathway will be amenable to therapeutic intervention against cocaine addiction.
Statement of Interest
None.
Acknowledgements
I thank the Center for Addiction Research (CAR) and the Mitchell Center for Neurodegenerative Diseases at University of Texas Medical Branch (UTMB) for providing necessary laboratory space and major equipment support. But most of all, I would like to acknowledge the confidence, support, and relentless intellectual input of Anusha Srinivasan that was instrumental in seeing this manuscript to its present status.
This work was supported by National Institute on Drug Abuse Grant R03 DA033428 to B.K. Additional support was provided by the CAR Pilot Grant, and the Institute for Translational Sciences at the UTMB, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health.
References
- Ahn SJ, Yoon MS, Hyuk S, Han W, Yoon YD, Han JS, Noh DY. (2003) Phospholipase C-protein kinase C mediated phospholipase D activation pathway is involved in tamoxifen induced apoptosis. J Cell Biochem 89:520–528. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharm & Ther 113:296–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM. (2014) Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Fron Behav Neurosci 8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. (2004) Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev 28:273–283. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. (1995) Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19:39–51. [DOI] [PubMed] [Google Scholar]
- Barr GA, Sharpless NS, Cooper S, Schiff SR, Paredes W, Bridger WH. (1983) Classical conditioning, decay and extinction of cocaine-induced hyperactivity and stereotypy. Life Sci 33:1341–1351. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. (1993) The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A 90:2559–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. (1994) Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharm 46:477–484. [PubMed] [Google Scholar]
- Berg KA, Stout BD, Maayani S, Clarke WP. (2001) Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J Pharm Exp Ther 299:593–602. [PubMed] [Google Scholar]
- Berke JD, Hyman SE. (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532. [DOI] [PubMed] [Google Scholar]
- Brabant C, Tambour S, Tirelli E. (2003) Quasi-asymptotic development of conditioned hyperactivity induced by intermittent injections of cocaine in C57BL/6J mice. Pharm Biochem Beh 75:273–280. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. (1993) Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharm 113:123–130. [DOI] [PubMed] [Google Scholar]
- Burgess N, Becker S, King JA, O’Keefe J. (2001) Memory for events and their spatial context: models and experiments. Phil Trans Royal Soc, Biol Sci 356:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R, Gui J. (1997) A simple and reliable method for the positive identification of pavlovian conditioned cocaine effects in open-field behavior. J Neurosci Mthds 73:1–8. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN. (1994) Conditioned cocaine induced hyperactivity: an association with increased medial prefrontal cortex serotonin. Behav Brain Res 62:177–185. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN, Shanahan AB. (2008) Cocaine conditioned behavior: a cocaine memory trace or an anti-habituation effect. Pharm Biochem Beh 90:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Hiragun T, Lee JH, Kim YM, Kim HP, Chahdi A, Her E, Han JW, Beaven MA. (2004) Activation of RBL-2H3 mast cells is dependent on tyrosine phosphorylation of phospholipase D2 by Fyn and Fgr. Mol Cell Biol 24:6980–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S. (2001) Signalling roles of mammalian phospholipase D1 and D2. Cell Mol Life Sci 58:1674–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelio AM, Nunes-de-Souza RL. (2007) Anxiogenic-like effects of mCPP microinfusions into the amygdala (but not dorsal or ventral hippocampus) in mice exposed to elevated plus-maze. Behav Brain Res 178:82–89. [DOI] [PubMed] [Google Scholar]
- Council NR (2011) Guide for the care and use of laboratory animals. 8th ed. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. (2008) Review. Context-induced relapse to drug seeking: a review. Phil Trans Royal Soc, Biol Sci 363:3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, DuBrow S. (2015) How the hippocampus preserves order: the role of prediction and context. Trends in Cog Sci 19:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello Cruz AP, Pinheiro G, Alves SH, Ferreira G, Mendes M, Faria L, Macedo CE, Motta V, Landeira-Fernandez J. (2005) Behavioral effects of systemically administered MK-212 are prevented by ritanserin microinfusion into the basolateral amygdala of rats exposed to the elevated plus-maze. Psychopharm 182:345–354. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdere P. (2015) New therapeutic opportunities for 5-HT receptor ligands in neuropsychiatric disorders. Pharmacol & Ther 15:222–223. [DOI] [PubMed] [Google Scholar]
- Du G, Huang P, Liang BT, Frohman MA. (2004) Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell 15:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharm 107:523–529. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. (2007) The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol 18:365–374. [DOI] [PubMed] [Google Scholar]
- Exton JH. (2002) Regulation of phospholipase D. FEBS Lett 531:58–61. [DOI] [PubMed] [Google Scholar]
- Foster DA, Salloum D, Menon D, Frias MA. (2014) Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J Biol Chem 289:22583–22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman MA. (2015) The phospholipase D superfamily as therapeutic targets. Trends in Pharm Sci 36:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498. [DOI] [PubMed] [Google Scholar]
- Han JM, Kim JH, Lee BD, Lee SD, Kim Y, Jung YW, Lee S, Cho W, Ohba M, Kuroki T, Suh PG, Ryu SH. (2002) Phosphorylation-dependent regulation of phospholipase D2 by protein kinase C delta in rat Pheochromocytoma PC12 cells. J Biol Chem 277:8290–8297. [DOI] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. (2003) Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharm 168:75–83. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. (1991) The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci 11:2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharm Biochemistry Behav 71:533–554. [DOI] [PubMed] [Google Scholar]
- Hu T, Exton JH. (2003) Mechanisms of regulation of phospholipase D1 by protein kinase Calpha. J Biol Chem 278:2348–2355. [DOI] [PubMed] [Google Scholar]
- Hu T, Exton JH. (2005) 1-Butanol interferes with phospholipase D1 and protein kinase Calpha association and inhibits phospholipase D1 basal activity. Biochem Bipophys Res Comm 327:1047–1051. [DOI] [PubMed] [Google Scholar]
- Huang P, Altshuller YM, Hou JC, Pessin JE, Frohman MA. (2005) Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol Biol Cell 16:2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WE, Elgundi Z, Huang P, Frohman MA, Biden TJ. (2004) Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic beta-cells. J Biol Chem 279:27534–27541. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Vitale N, Chasserot-Golaz S, Dupont JL, Du G, Frohman MA, Bader MF, Poulain B. (2001) A role for phospholipase D1 in neurotransmitter release. Proc Natl Acad Sci U S A 98:15300–15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. (2005) Addiction: a disease of learning and memory. Am J Psych 162:1414–1422. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT. (1989) Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci 103:345–355. [DOI] [PubMed] [Google Scholar]
- Kelley AE. (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–179. [DOI] [PubMed] [Google Scholar]
- Kim JH, Han JM, Lee S, Kim Y, Lee TG, Park JB, Lee SD, Suh PG, Ryu SH. (1999) Phospholipase D1 in caveolae: regulation by protein kinase Calpha and caveolin-1. Biochem 38:3763–3769. [DOI] [PubMed] [Google Scholar]
- Kimura A, Stevenson PL, Carter RN, Maccoll G, French KL, Simons JP, Al-Shawi R, Kelly V, Chapman KE, Holmes MC. (2009) Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur J Neurosci 30:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Genzer KM, Pollandt SW, Liu J, Gallagher JP, Shinnick-Gallagher P. (2011) Dopamine-induced plasticity, phospholipase D (PLD) activity and cocaine-cue behavior depend on PLD-linked metabotropic glutamate receptors in amygdala. PLoS One 6:e25639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. (2001) Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci 21:Rc155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim JH, Jang IH, Kim HS, Han JM, Kazlauskas A, Yagisawa H, Suh PG, Ryu SH. (2005) Phosphatidylinositol (3,4,5)-trisphosphate specifically interacts with the phox homology domain of phospholipase D1 and stimulates its activity. J Cell Sci 118:4405–4413. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharm 47 Suppl 1:214–226. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. (2014) Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res 14:1191–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L, Chang MS, Sanders-Bush E. (2002) Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol 62:1339–1343. [DOI] [PubMed] [Google Scholar]
- McGrew L, Price RD, Hackler E, Chang MS, Sanders-Bush E. (2004) RNA editing of the human serotonin 5-HT2C receptor disrupts transactivation of the small G-protein RhoA. Mol Pharmacol 65:252–256. [DOI] [PubMed] [Google Scholar]
- Meneses A. (2015) Serotonin, neural markers, and memory. Fron Pharm 6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. (2008) Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends in Pharm Sci 29:454–464. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. (1998) Functional differentiation in the hippocampus. Hippocampus 8:608–619. [DOI] [PubMed] [Google Scholar]
- Neasta J, Barak S, Hamida SB, Ron D. (2014) mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem 130:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Lafond MV, Everitt BJ, Dickinson A. (2001) Cocaine seeking by rats is a goal-directed action. Behav Neurosci 115:394–402. [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2013) The rat brain in stereotaxic coordinates. 7th ed. Academic Press, Cambridge, MA. [Google Scholar]
- Pert A. (1994) Neurobiological mechanisms underlying the acquisition and expression of incentive motivation by cocaine-associated stimuli: relationship to craving. NIDA Res Monograph 145:163–190. [PubMed] [Google Scholar]
- Pockros-Burgess LA, Pentkowski NS, Der-Ghazarian T, Neisewander JL. (2014) Effects of the 5-HT2C receptor agonist CP809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int J Neuropsychopharm 17:1751–1762. [DOI] [PubMed] [Google Scholar]
- Slaaby R, Du G, Altshuller YM, Frohman MA, Seedorf K. (2000) Insulin-induced phospholipase D1 and phospholipase D2 activity in human embryonic kidney-293 cells mediated by the phospholipase C gamma and protein kinase C alpha signalling cascade. Biochem J 351 Pt 3:613–619. [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. (2014) Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15:655–669. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen J. (2008) mTOR signaling: PLD takes center stage. Cell Cycle 7:3118–3123. [DOI] [PubMed] [Google Scholar]
- Sung TC, Zhang Y, Morris AJ, Frohman MA. (1999) Structural analysis of human phospholipase D1. J Biol Chem 274:3659–3666. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. (1998) Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharm 19:48–59. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biol 12:227–462. [DOI] [PubMed] [Google Scholar]
- Valle FP. (1970) Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psych 83:103–111. [PubMed] [Google Scholar]
- Vicente MA, Zangrossi H. (2012) Serotonin-2C receptors in the basolateral nucleus of the amygdala mediate the anxiogenic effect of acute imipramine and fluoxetine administration. Int J Neuropsychopharm 15:389–400. [DOI] [PubMed] [Google Scholar]
- Vicente MA, Zangrossi H., Jr (2014) Involvement of 5-HT2C and 5-HT1A receptors of the basolateral nucleus of the amygdala in the anxiolytic effect of chronic antidepressant treatment. Neuropharm 79:127–135. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kanaho Y. (2000) Inhibition of phosphatidylinositol 4,5-bisphosphate-stimulated phospholipase D2 activity by Ser/Thr phosphorylation. BBA 1495:121–124. [DOI] [PubMed] [Google Scholar]
- White NM. (1996) Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91:921–949; Discussion 951–965. [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. (1996) Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharm 127:213–224. [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. (2010) Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharm 208:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Ho WT, Exton JH. (2000a) Conserved amino acids at the C-terminus of rat phospholipase D1 are essential for enzymatic activity. Eur J Biochem / FEBS 267:7138–7146. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ho WT, Exton JH. (2000b) Association of the N- and C-terminal domains of phospholipase D. Contribution of the conserved HKD motifs to the interaction and the requirement of the association for Ser/Thr phosphorylation of the enzyme. J Biol Chem 275:24962–24969. [DOI] [PubMed] [Google Scholar]