Abstract
Major depression is a serious psychiatric disorder; however, the precise biological basis of depression still remains elusive. A large body of evidence implicates a dysregulated endocrine and inflammatory response system in the pathogenesis of depression. Despite this, given the heterogeneity of depression, not all depressed patients exhibit dysregulation of the inflammatory and endocrine systems. Evidence suggests that inflammation is associated with depression in certain subgroups of patients and that those who have experienced stressful life events such as childhood trauma or bereavement may be at greater risk of developing depression. Consequently, prolonged exposure to stress is thought to be a key trigger for the onset of a depressive episode. This review assesses the relationship between stress and the immune system, with a particular interest in the mechanisms by which stress impacts immune function, and how altered immune functioning, in turn, may lead to a feed forward cascade of multiple systems dysregulation and the subsequent manifestation of depressive symptomology. The identification of stress-related immune markers and potential avenues for advances in therapeutic intervention is vital. Changes in specific biological markers may be used to characterize or differentiate depressive subtypes or specific symptoms and may predict treatment response, in turn facilitating a more effective, targeted, and fast-acting approach to treatment.
Keywords: stress, depression, immune, inflammation
Stress Response System
Stress has long been identified as a risk factor for major depression, while markers of immunological stimulation and inflammation frequently characterize subgroups of severely depressed patients. This review assesses the relationship between stress and the immune system, with a particular interest in the mechanisms by which stress impacts immune function, in an effort to identify stress-related immune markers and potential avenues for advances in therapeutic intervention.
An individual’s capacity to deal with stress is largely controlled by the hypothalamic pituitary adrenal (HPA) axis. A dysregulated stress response system, evidenced by hyperactivity of the HPA-axis, represents a vulnerability factor for major depressive disorders and is one of the most consistent findings in patients with major depression (Carroll et al., 1976a, 1976b; Nemeroff et al., 1984; Pariante and Lightman, 2008), although others suggest that atypical depression may be characterized by HPA axis hypoactivity (Gold and Chrousos, 2002; Parker et al., 2003).
Under normal physiological conditions, the HPA axis is self-regulatory and activity is curtailed via negative feedback inhibition; glucocorticoid receptor (GR) binding in the hypothalamus and the pituitary inhibits the activity of the HPA axis and the subsequent release of glucocorticoids from the adrenal cortex. Additionally, while cytokines such as interleukin-1beta (IL-1β) and IL-6 secreted by immune cells can directly act on the GR in the hypothalamus, activating the HPA axis, glucocorticoids also act to inhibit the synthesis and secretion of inflammatory cytokines, evidenced by the inhibition of endotoxin-induced fever in animals treated with exogenous glucocorticoids (Coelho et al., 1992), inhibition of inflammation, the production of IL-12 by antigen presenting cells, and suppression of proinflammatory cytokine expression (Elenkov and Chrousos, 2002; Pace and Miller, 2009).
The Immune Response
In response to stress, injury or invading pathogens the body’s first line of defence is the activation of the nonspecific innate immune response. The primary task for the host’s innate immune cells is to detect the pathogen and mount an immediate defensive response, resulting in the activation of the inflammatory response, followed by the initiation of a highly diverse, antigen-specific, adaptive immune response and subsequent immunological memory (Hoffmann et al., 1999; Medzhitov, 2001). Upon encountering pathogen associated molecular pathogens or foreign antigen, monocytic innate immune cells respond by producing a variety of cytokines such as IL-1β, IL-6, and IL-12 along with tumor necrosis factor alpha (TNF-α).
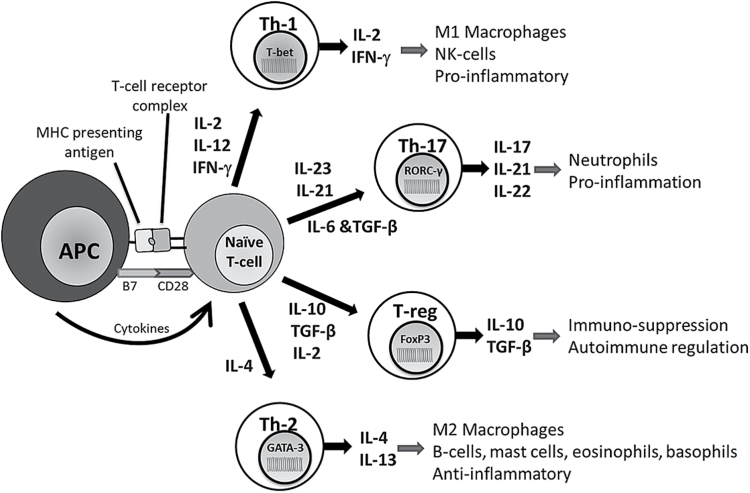
The subsequent process of dendritic cell maturation followed by antigen presentation, cytokine production, and co-stimulation from the innate response system results in the activation of lymphocytes, the key effector cells of the adaptive immune response (Hoebe et al., 2004). Depending on the nature of the signals received, distinct T-cell subsets are activated; proinflammatory T-helper (Th)-1 cells are induced in the presence of IL-2, IL-12, or interferon-gamma (IFN-γ) (Romagnani, 2000), while Il-17, IL-23, and a combination of Il-6 and transforming growth factor beta (TGF-β) are inducers of Th-17 cell activation (Bettelli et al., 2006). The antiinflammatory cytokine IL-4 mediates the differentiation of Th-2 cells (Romagnani, 2000), while the immuno-regulatory mediators IL-10 and TGF-β are potent inducers of T-reg cell differentiation (Bettelli et al., 2006) (Figure 1).
Figure 1.
Simplified overview of T-helper (Th) cell subsets and their functions. The key effector molecules of the inflammatory response system (IRS) are the heterogeneous group of small cell signalling peptides, cytokines (Connor and Leonard, 1998). Released from numerous cell types in the brain and periphery, cytokines act in synergy or antagonistically to direct specific immune responses through the orchestration of immune cell trafficking and the cytokine-induced differentiation of immune cells with roles in innate, cytotoxic, cell-mediated, humoral, and autoimmunity in association with immune suppression (Borish and Steinke, 2003; Commins et al., 2010).
Under normal physiological conditions, proinflammatory Th-1 cells play a key role in protecting the host against intracellular and viral pathogens (Zhu et al., 2010). Alternatively, antiinflammatory Th-2 cells are necessary for the induction of humoral immunity, B cell activation, and the production of Immunoglobulin E antibody and immunoglobulin G neutralizing antibody, in addition to dampening the proinflammatory response (Romagnani, 2000). However, in disease states, it is thought that the balance between pro- and antiinflammatory mediators is lost in favor of a persistent proinflammatory phenotype.
Furthermore, Th-17 cells are thought to be involved in acute inflammatory responses, orchestrating the recruitment of neutrophils to epithelial cells and promoting growth and integrity of epithelial barriers (Wilson et al., 2007). On the other hand, T-reg cells function to maintain immunological homeostasis, protecting against auto-immunity and downregulating T-cell activation and inflammatory cytokine production via NFκB inhibition and/or the depletion or uptake of the lymphocyte-proliferation mediator, IL-2 (Thornton and Shevach, 1998; Bettelli et al., 2005, Popmihajlov and Smith, 2008; Cheng et al., 2011). However, dysregulation of the inflammatory response has detrimental consequences. Th-17 cells are widely implicated in numerous disorders such as irritable bowel syndrome and arthritis (Tesmer et al., 2008), while decreased T-reg cell suppressive function, as a consequence of inflammation, has been reported in rheumatoid arthritis (Nie et al., 2013).
Of particular interest in depressive disorders are the cytokines IL-β, IL-6, and TNF-α. IL-1β is produced by many immune cells, which include mononuclear phagocytes, neutrophils, endothelial cells, and microglia, the resident immune cells in the brain, in response to bacterial endotoxins and other pathogenic agents recognized by pathogen recognition receptors (Dinarello, 1988). The production of IL-1β has a key role to play in the induction and maintenance of the adaptive immune response, promoting increased expression of IL-2 receptors and IL-2 secretion, thereby impacting on T-cell differentiation and B cell activation (Gillis and Mizel, 1981; Kaye et al., 1984).
IL-6 is produced by many cells types such as T and B cells, endothelial cells, and hepatocytes, amongst others; however, the major producers of IL-6 are innate immune mononuclear phagocytes (Commins et al., 2010). IL-6 is a pleiotropic cytokine with both pro- and antiinflammatory effects. IL-6 is the strongest mediator of the acute phase response, stimulating the release of proteins such as C-reactive protein (CRP) and albumin from the liver along with playing a role in immune-mediated HPA axis activation, perhaps via direct interaction with IL-6 receptors in the paraventricular nucleus of the hypothalamus and thereby inducing the stress response (Lenczowski et al., 1999; Horn et al., 2000).
TNF-α is a multi-functional cytokine that plays a central role in mediating host defence against intracellular pathogens and bacterial endotoxin (Pfeffer, 2003). Similar to IL-6 and IL-1β, TNF is produced by many cell types, including neutrophils, lymphocytes, and endothelial cells; however, the major producers of TNF-α are mononuclear phagocytes (Beutler and Cerami, 1989). TNF-α plays a critical role in the inflammatory response, promoting inflammation via the stimulated expression of IL-1β and IL-6 in association with promoting lymphocyte proliferation.
Stress as A Trigger for Activating the Immune System
Further to the findings reviewed by Connor and Leonard (1998), a wealth of evidence has emerged supporting the notion that inflammation may act as a mechanism by which stress can induce depression. A variety of stress paradigms are used preclinically to study the onset and development of depressive-like behaviors (for review, see Stepanichev et al., 2014). Furthermore, it is thought that chronic stressors such as social defeat or chronic mild stress may reflect the clinical condition more closely. More specifically, studies assessing the impact of chronic mild stress in rodents have revealed that prolonged exposure to mild stressors induces anhedonic behaviors, evidenced by a reduced preference for sucrose solutions (Grippo et al., 2005). In accordance with this, naïve mice subjected to a variety of stressors for a 5-week period also displayed depressive-like behavior and decreased sucrose preference in addition to reduced social exploration concomitant with brain inflammation, as evidenced by elevated hippocampal IL-1 levels (Goshen et al., 2008). However, mice with an IL-1 receptor type 1 deletion subjected to a similar chronic mild stress regime did not display the depressive-like behavior observed in naïve mice, suggesting that elevated CNS IL-1 levels, as a consequence of prolonged stress, are responsible for the induction of depressive symptomology via adrenocortical activation and the suppression of neurogenesis (Goshen et al., 2008).
Early-life stress also has a crucial impact upon the development of neurobiological systems implicated in stress and mood responses, thereby increasing one’s vulnerability to stress and hence the risk of developing depression later in life, especially in response to secondary stress (Hammen et al., 2000; Mazure et al., 2000; Chapman et al., 2004; Nemeroff, 2004; Heim and Binder, 2012; Cattaneo et al., 2015). Preclinical studies assessing the effects of maternal separation in adult offspring have reported an altered stress response system in association with a proinflammatory phenotype (O’Mahony et al., 2009). Additionally, recent findings by Slusarczyk et al. (2015) highlight the detrimental effect of prenatal stress on microglial activity in adult offspring, evidenced by an elevated presence of inflammatory and microglial activation markers in the hippocampus and frontal cortex, in association with increased anhedonic and depressive-like behaviors. Similarly, clinical studies reveal a causal relationship between prenatal maternal stress and a dysregulated inflammatory profile in adolescent children (Veru et al., 2015). Furthermore, Frodl et al. (2012) report a positive association between childhood maltreatment and the general inflammatory marker and acute phase protein, CRP, in a depressed cohort. In accordance with this, depressed patients with a history of childhood trauma are often characterized by an elevated inflammatory signature in association with glucocorticoid resistance (GR) and altered brain derived neurotrophic factor (BDNF) concentrations, both thought to be as a consequence of the persistent, chronic, low-grade inflammatory phenotype (Heim et al., 2008; Mondelli et al., 2011).
Preclinical studies indicate that psychological stress increases gut permeability, thereby enabling gut flora to access the systemic system (Bowe and Logan, 2011). In line with this observation, Maes et al. (2008) have reported the presence of antibodies against endotoxin from a number of commensal bacteria in plasma from depressed patients. Consequently, it is possible that the potent innate immune stimulus and bacterial endotoxin, lipopolysaccharide (LPS), found on the outer cell wall of gram negative bacteria could stimulate a systemic, low-grade inflammatory response in depressed patients, although this remains to be fully elucidated.
The mechanistic driving force underpinning stress-induced immune activation involves the synergistic effects of the HPA axis, the sympatho-adrenal-medullary (SAM) axis, and the parasympathetic nervous system (PNS) (for review, see Miller et al., 2009). Under stress, in vitro and in vivo studies have shown that adrenergic receptor activation by noradrenaline culminates in the activation of inflammatory signalling pathways and the production of inflammatory cytokines (Miller et al., 2009). Catecholamines acting through alpha and beta adrenoceptors have been shown to have immunoregulating properties (Johnson et al., 2005). Stimulation of beta2 adrenoceptors on immune cells leads to the production of cAMP, which promotes the production of cytokines that can suppress cell-mediated immune responses (Suberville et al., 1996; Platzer et al., 2000). For instance, activated monocytes and dendritic cells exposed to noradrenaline have reduced IL-12 concentrations in parallel with increased production of the antiinflammatory cytokine IL-10 (Elenkov et al., 1996). Sustained activation of the SAM axis with chronic stress results in substantial increases in circulating catecholamine concentrations, and this can lead to adaptive changes in the expression of beta2-adrenoceptors. For example, it has been demonstrated that continuous exposure to catecholamines leads to a reduced receptor response by a process of receptor phosphorylation, internalization, and downregulation (Bawa-Khalfe et al., 2007). Therefore, in a similar fashion to GRs, stress-induced reduction of beta2 adrenoceptors could be involved in a diminished negative feedback response to catecholamines on immune cells. Conversely, others have reported an increase in catecholamine reactivity in mice subjected to chronic mild stress (Edgar et al., 2002, 2003). Lymphocytes from mice subjected to chronic mild stress have an increased response to catecholamine-mediated inhibition or enhancement of proliferation in T and B cells coupled with an increase in beta2 adrenoceptor density and responsivity. In addition, the PNS may also play a role in the regulation of inflammation. Activation of efferent vagus nerve fibers, acetylcholine release, and subsequent alpha7 nicotinic receptor activation on immune cells can inhibit cytokine responses to endotoxin in laboratory animals (Pavlov and Tracey, 2005; for review, see Miller et al., 2009). Increased muscarinic receptor expression has been reported in T and B cells isolated from mice exposed to chronic stress (Edgar et al., 2002). Thus, long-term adaptive responses are also evident in the PNS in response to stress, which may also impact on immune function.
Immunological Activation Induces “Stress-Like” Behaviors
Further to and in corroboration with the above-mentioned findings, numerous studies investigating the impact of immunological insult in naïve rodents indicate that immunological activation induces stress-like behaviors. IL-1β has a critical role to play in the manifestation of sickness behavior, a strategic physiological response induced by the immune system in an effort to conserve energy in order to fight infection (Hart, 1988; Dantzer, 2006). Symptoms of sickness behavior include fever, malaise, loss of appetite, and fatigue in association with an activated stress response system (Konsman et al., 2002). Preclinical evidence suggests that the actions of IL-1β in the brain have a pivotal role to play in the sickness response. This is supported by the manifestation of symptomology and sickness behavior following an immune challenge with IL-1β or the bacterial endotoxin LPS, which abates in the presence of the IL-1 receptor antagonist (IL-1Ra) (for review, see Dantzer, 2006). However, prolonged activation of IL-1β in association with IL-6 and TNF-α appears to result in a maladaptive sickness response and the subsequent manifestation of depressive symptoms (Dantzer et al., 2008).
As reviewed by Gadek-Michalska et al. (2013), chronic inflammation also has a negative impact on central HPA axis function. Preclinical evidence suggests that exposure to endotoxin in early life has detrimental consequences on normal HPA axis functioning, evidenced by increased corticosterone concentrations in combination with stress-mediated alterations in lymphocyte proliferative abilities (Shanks et al., 2000). Further to this, recent findings indicate that early-life inflammatory challenges in naive mice are associated with depressive-like behavior in adulthood in association with decreased prefrontal cortex GR phosphorylation in the absence of altered corticosterone levels (Dinel et al., 2014). Moreover, early-life exposure to inflammatory stimuli appears to be a vulnerability factor, as reexposure to endotoxin later in life resulted in memory impairment and altered neurogenesis (Dinel et al., 2014).
Furthermore, others have shown that IL-1β and to a lesser extent IL-6, TNF-α, and IL-2 have a role to play in the modulation of neurotransmitters (Dunn, 1992; Palazzolo and Quadri, 1992; Song et al., 1999; Zhu et al., 2006). Under stress, dysregulation of serotonergic neurotransmission in particular may be, in part, responsible for the alterations in mood, emotion, and cognitive processing that are major characteristics of a depressive episode. Reichenberg et al. (2001) have shown that healthy male volunteers treated with low-dose endotoxin display increased circulating concentrations of IL-1Ra, IL-6, and TNF-α, which are positively correlated with emotional and cognitive disturbances evidenced by depressed mood, memory impairments, and anxiety.
Acute activation of the inflammatory response system (IRS) with an adaptive component is an essential host defence mechanism against stress and infection. Under normal physiological conditions this process is self-limiting with a distinct termination. However, failure of the IRS to resolve results in the subsequent development of a maladaptive, chronic, low-grade inflammatory process with detrimental consequences. Evidence of a low-grade inflammatory phenotype is apparent in numerous disorders such as cardiovascular diseases, obesity, type 2 diabetes, asthma, and psychiatric disorders such as major depression. It is not clear what instigates this maladaptive process, although Medzhitov (2008) suggests that the emergence of chronic inflammation may be a consequence of impaired homeostasis and the dysfunction of physiological systems not necessarily associated with host defence or tissue regeneration, which are commonly the principal initiators of an inflammatory response.
Evidence for Activation of the Immune System In Major Depression
The monocyte-T-lymphocyte theory of depression, devised by Smith and Maes (Smith, 1991; Maes et al., 1995b) in the early 1990s, proposes that increased proinflammatory cytokine secretion in the form of IL-1β, TNF-α, and IFN-γ is responsible for the initiation and maintenance of a depressive episode. While the earliest studies by Kronfol et al. (1983) and Schleifer et al. (1984), investigating alterations in immune function in stressed and depressed individuals, reported a decreased T-cell proliferative response upon mitogen stimulation, much of the research to date has largely focused on the involvement of the innate immune response with numerous reports highlighting an association between major depression and activation of the innate immune response (Dantzer et al., 2008; Miller et al., 2009; Anisman, 2011; Leonard and Maes, 2012). In particular, evidence suggests that depression is associated with increased circulating concentrations of proinflammatory cytokines such as IL-1β, IL-6, TNF-α, and IFN-γ in association with elevations in chemokines and the acute phase protein, CRP (Maes et al., 1995a; Lanquillon et al., 2000; Cizza et al., 2008; Simon et al., 2008; Diniz et al., 2010; Hughes et al., 2012). Additionally, in the case of IL-6, IL-1, TNF-α, the soluble IL-2 receptor (sIL-2R), and CRP, these results have been supported by meta-analyses (Dowlati et al., 2010; Haroon et al., 2012; Liu et al., 2012; Haapakoski et al., 2015).
Further to this, it has also been shown that cytokine immunotherapy in the form of IL-2 and IFN-α for the treatment of hepatitis C (Hep C) and certain types of cancer, such as malignant melanoma, can induce depression in 30% to 50% of these patients who are otherwise psychiatrically normal (Capuron et al., 2000; Bonaccorso et al., 2001; Capuron et al., 2001). Additionally, increased concentrations of monocytic cytokines such as IL-6, in association with indicators of cell-mediated immune activation and T-cell subset cytokine production, have been highly associated with the onset of depressive symptoms following IFN-α treatment (Bonaccorso et al., 2001; Wichers et al., 2007). Significant symptom overlap between idiopathic and cytokine-induced depression has been observed (Capuron et al., 2009). Moreover, the development and progression of the cytokine-induced depressive symptoms can be inhibited with antidepressant treatment, suggesting that the therapeutic efficacy of antidepressants may be related to their immunomodulatory properties (Musselman et al., 2001; Raison et al., 2005).
In accordance with this, numerous studies have reported on the antiinflammatory properties of antidepressants (for review, see Kenis and Maes, 2002). Specifically, Kubera et al. (2001) suggest that antidepressants may exert their effects via immunoregulatory mechanisms evidenced by elevated IL-10 concentrations and a suppressed IFN-γ/IL-10 production ratio in stimulated whole blood from severely depressed patients treated with antidepressants in vitro. Others have shown reduced whole blood TNF-α concentrations in patients who responded to a 6-week course of amitriptyline (Lanquillon et al., 2000), while Seidel et al. (1995) reported a normalization of the elevated IFN-γ and sIL-2R concentrations in a patient cohort following antidepressant treatment. Increased TNF-α plasma concentrations were reduced following a course of electroconvulsive therapy (Hestad et al., 2003), while others have shown that treatment with selective serotonin reuptake inhibitors can reduce circulating CRP concentrations in the absence of therapeutic efficacy (O’Brien et al., 2006). Additionally, antiinflammatory agents have been shown to enhance the efficacy of antidepressant treatment with findings by Muller et al. (2006) showing an enhanced antidepressant efficacy of the noradrenaline reuptake inhibitor reboxetine when given in combination with the cyclooxygenase-2 inhibitor, celecoxib, a known inhibitor of prostaglandin E2 and proinflammatory cytokines, while Raison et al. (2013) have shown that depressed patients with higher baseline inflammatory markers respond to treatment with the TNF-α antagonist, infliximab. It is noteworthy however, that the meta-analysis by Hannestad et al. (2011), which assesses the effect of antidepressant treatment on serum cytokines levels of IL-1, IL-6, and TNF-α in 22 independent studies, report that while antidepressant treatment appears to reduce levels of IL-1 and possibly IL-6, TNF-α levels were not reduced in accordance with reduced depressive symptomology.
An increased prevalence of depression has also been observed in association with autoimmune disorders with up to 50% of multiple sclerosis patients developing clinical depression (Feinstein, 2011). Additionally, the chronic inflammatory disorder rheumatoid arthritis is also highly associated with clinical depression symptoms with up to 42% of patients reporting comorbid depression (Bruce, 2008). Therefore, the presence of an autoimmune disease appears to put people at a risk 3 times greater than that posed to the general population.
Elevated T-cell-stimulated inflammatory cytokine production prior to experiencing a stressful life event such as military deployment has recently been shown to be a risk factor for the development of depression (van Zuiden et al., 2011). This finding represents the emergence of a new body of literature, once again addressing the involvement of the adaptive immune response in the pathogenesis of depression.
Adaptive Immune System Activation in Depression
The innate and adaptive immune response collaborate in a bidirectional manner to maintain a homeostatic balance (Reiner, 2007). Increased IL-2 receptor expression and secretion upon T-cell activation promotes T-cell proliferation and differentiation (Medzhitov and Janeway, 1997). Additionally, the production of either proinflammatory (IFN-γ and IL-12) or antiinflammatory (IL-4 and IL-10) cytokines also has a key role to play in T-cell polarization (Medzhitov and Janeway, 1997). The complex adaptive cellular immune network and differentiation of T-cells into proinflammatory (Th-1 and Th-17), antiinflammatory (Th-2), or regulatory (T-reg) results in direct and specific immune responses to various pathogens as well as offering protection against potentially harmful effector responses and autoimmunity.
Furthermore, T-cell activation results in the further production of various cytokines. More specifically, and in relation to the discussion in the following paragraph, the production of IFN-γ from Th-1 cells promotes further activation of monocytes and macrophages, resulting in the additional release of monocytic cytokines such as IL-1, macrophage inflammatory mediators such as neopterin, and interferon-inducible genes, thereby sustaining a proinflammatory phenotype (Zhou et al., 2009; Maes, 2011). The interaction between T-cells and macrophages via the production of a range of inflammatory cytokines is known as cell-mediated immune activation and functions to stimulate and recruit new macrophages, kill infected macrophages, and maintain the inflammatory response (Maes, 2011).
As reviewed by Miller (2010), during the last 10 years the research focus has shifted with a reemergence of reports in corroboration with early theories by Smith and Maes (Maes et al., 1995b) suggesting the involvement of the adaptive immune response and cell-mediated immune activation in the pathophysiology of major depression. In the early 1990s elevated macrophage-secreted neopterin levels, which represent increased IFN-γ-mediated macrophage activation, in association with increased circulating concentrations of the cell-mediated immune activation markers sIL-2R were observed in depressed and melancholic patients, suggesting an elevated presence of cell-mediated immune activation (Caruso et al., 1993; Maes, 1995). In support of this, findings by Celik et al. (2010) also suggest increased cell-mediated immune activity evidenced by elevated neopterin concentrations that are positively correlated with recurrent depression and an increased number of depressive episodes. Additionally, some reports suggest an imbalance in T-cell subset cytokine production with elevated levels of prototypical Th-1 cytokines, such as IFN-γ, while antiinflammatory IL-4 and TGF-β produced by Th-2 and Th-3 cells, respectively, have been found to be significantly lower in depressed cohorts (Myint et al., 2005; Sutcigil et al., 2007). However, it is important to note that the literature is varied in this regard (Pavon et al., 2006; Kim et al., 2007). In addition, others have reported elevations in both pro- and antiinflammatory cytokines (IFN-γ, IL-6, IL-7, IL-8, IL-10, IL-1Ra, and G-CSF) in depressed cohorts relative to controls that were then normalized following therapeutic intervention (Dahl et al., 2014) (Table 1). It is also noteworthy and apparent in Table 1 that some of the discrepancies in the literature arise as a consequence of a number of highly variable and influential factors. These include the wide variety of depressive subtypes investigated (endogenous, reactive, melancholic, or atypical), first episode vs recurrent or treatment-resistant depression, depression severity, comorbid disorders, the presentation of a diverse range of symptoms (affective or somatic), medical history, medication status, the immunological profile under assessment, the number of participants included, and the methods employed in the study.
Table 1.
Summary of the Dysregulation of Th-1/Th-2 Cytokines in Depressive Disorders
| Participants | Sample Size | Diagnostic Test Employed | Study Design | Medication Status | Inflammatory Mediators Investigated | Source | Study Methods Employed | Baseline Inflammatory Profile | Inflammatory Profile Post Treatment | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| MDD and healthy controls | -30 depressed patients (9 first episode, 12 second episode and 9 third episode) -26 controls |
SCID-1 and HAM-D (17 item) |
Controls vs depressed | Patients were free of psychoactive medication for 6 weeks prior to the study | Neopterin | Serum | -HPLC | -Increased neopterin levels in patients with recurrent depression relative to controls and first onset patients -Neopterin levels increased in accordance with the number of previous episodes |
N/A | (Celik et al., 2010) |
| Mixed depressive subtypes and nondepressed controls | -238 cases with depressive symptoms (Inc. major, minor depression and dysthymia) -357 non- depressed controls |
CES-D | Control vs depressed | Neopterin | Plasma | -HPLC | -No difference in neopterin levels between depressed subjects and controls -No association between neopterin levels and depressive symptoms |
N/A | (Tiemeier et al., 2006) | |
| MDD and healthy controls | -40 depressed patients (22 completed the 8 week study) -80 controls |
HAM-D | -Controls vs depressed -Pre- and post- antidepressant treatment |
Patients were medication naïve (first onset) or medication free for 4 months prior to the study | IFN-γ, IL4 and TGFβ1 |
Plasma | -8 weeks course of antidepressants -ELISA |
-Increased IFN-γ/IL-4 and IFNγ/TGF-β1 ratio relative to controls -Negative correlation between TGF-β1 and HAM-D scores |
-Decreased IFN-γ / IL-4 ratio post- treatment relative to pre-treatment -Increased TGF-β1 post-treatment relative to pre- treatment levels |
(Myint et al., 2005) |
| MDD and healthy controls | -23 depressed patients (first episode) -25 controls |
HAM-D | -Controls vs depressed -Pre- and post- antidepressant treatment |
Patients were medication free for 6 weeks prior to the study | IL-2, IL-4, IL-12, TNF-α, TGF-β1 and MCP-1 |
Serum | -8 week course of sertraline treatment -ELISA |
-Increased levels of IL-2, IL-12, TNF-α and MCP-1 relative to controls -Lower levels of IL-4 and TGF-β relative to controls |
-IL-12 levels decreased and IL-4 and TGF-β levels increased post treatment relative to pre-treatment levels | (Sutcigil et al., 2007) |
| MDD and healthy controls | -32 depressed patients -63 controls |
HAM-D | Controls vs depressed -Pre- and post- antidepressant treatment |
IL-6, TNF-α, IFN-γ, IL-2, IL-4, TGF-β1 |
Whole blood | -6 weeks course of antidepressants -In-vitro stimulation with phyto- hemagglutinin (4μg/ ml) and LPS (20μg/ ml) for 48hrs -ELISA |
-Increased production of IL-6, TNF-α, TGF-β1 and IFN-γ/IL-4 ratio relative to controls -Decreased production of IFN-γ, IL-4 and IL-2 relative to controls |
-IL-6 and TGF- β1 levels were decreased post treatment relative to pre-treatment levels | (Kim et al., 2007) | |
| MDD (with comorbid anxiety in some cases) and healthy controls | -33 depressed patients (80% first episode, 20% recurrent depression) -33 controls |
HAM-D (17 item) | Control vs depressed | Patients were medication free for 3 weeks prior to the study | TNF-α, IL-6, IL-1β, IL-2, IFN-γ, IL-4 and IL-13 |
Serum | -ELISA | -Increased levels of TNF-α, IL-4 and IL-13 relative to controls -Decreased levels of IL-2 and IFN-γ |
N/A | (Pavon et al., 2006) |
| MDD patients and healthy controls | -50 depressed patients (76% had melancholic depression at baseline; 43 completed the follow-up assessment) -34 controls |
IDS scale | Controls vs depressed -Pre- and post- antidepressant treatment |
Patients were medication free for 3 weeks prior to the study | IL-1β, IL-1Ra, IL-5, IL-6, IL- 7, IL-8, IL-10 and G-CSF |
Plasma | -12 weeks course of antidepressants (treatment as usual) - Bio-Plex Pro Human Cytokine Group I with the Luminex 100 |
-Increased levels of IL-1, IL-1Ra, IFN-γ, IL-5, IL-6, IL-7, IL-8, IL-10 and G-CSF relative to controls |
-Decreased levels of IL-1Ra, IFN-γ, IL-6, IL-7, IL-8, IL-10 and G-CSF post- treatment relative to pre-treatment levels and did not differ relative to controls -Normalization of cytokine levels only observed in treatment responders |
(Dahl et al., 2014) |
Abbreviations: CES-D, The Centre for Epidemiologic Studies Depression Scale; ELISA, enzyme-linked immunosorbent assay; G-CSF, granulocyte-colony stimulating factor; HAM-D, The Hamilton Rating Scale for Depression; HPLC, High Performance Liquid Chromatography; IDS, Inventory of Depressive Symptomology scale; IFN, interferon; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MDD, Major Depressive Disorder; SCID-1, The Structured Clinical Interview for Depression; TGF, transforming growth factor; TNF, tumor necrosis factor.
There are many inconsistencies in the literature largely owing to the fact the major depression is a highly heterogeneous disorder and therefore discrepancies in the immunological profile arise as a consequence of a number of highly variable and influential factors. ‘These include the wide variety of depressive subtypes investigated (endogenous, reactive, melancholic, or atypical), first episode vs recurrent or treatment-resistant depression, depression severity, co-morbid disorders, the presentation of a diverse range of symptoms (affective or somatic), medical history, medication status, the immunological profile under assessment, the number of participants included, and the methods employed in the study’. These factors are highlighted in the text and are further illustrated in the table which summarizes the variable nature of studies undertaken and the results obtained, particularly in relation to the adaptive immune response and the imbalance/dysregulation of T-cell subset cytokines in depression.
It has also been suggested that the balance between inflammatory Th-17 cells and immunoregulatory T-reg cells may be disturbed in depressed patients in favor of an increased proinflammatory Th-17 cell profile (Haroon et al., 2012). While there is little direct evidence to support or refute this, it may be conceivable in light of recent preclinical findings by Beurel et al. (2013) that directly implicate stress-induced elevations of brain Th-17 cells in the promotion of depressive-like behaviors in mice, which are then attenuated following the targeted inhibition of Th-17 cell production and function. In addition, Th-17 cells are highly implicated in the pathogenesis of inflammatory disorders such as rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease, often presenting comorbid with depression (Wilson et al., 2007; Tesmer et al., 2008). On the other hand, with regard to T-reg cells and in further support of this theory, Li et al. (2010) reported a decreased expression of CD4+CD25+ T-reg cells in unmedicated, first-episode, melancholic major depressed patients relative to healthy age- and sex-matched control subjects. Moreover, Himmerich et al. (2010) reported that, in accordance with a decreased expression of IL-1 and IL-6 during antidepressant therapy, expression of T-reg cells was increased in patients who had been suffering from a mild depressive episode, while a significantly larger increase in CD4CD25hi cells posttreatment was evident in the more severely depressed patients. Seemingly contrary to the above-mentioned findings, recent studies investigating T-cell and monocytic inflammatory systems in bipolar and schizophrenia patients reported an elevated presence of T-reg cells in association with a monocytic inflammatory signature (Drexhage et al., 2011a, 2011b). Additionally, Ronaldson et al. (2015) also reported an elevated T-reg cell presence in association with blunted cortisol and increased IL-6 levels in a healthy cohort of volunteers following acute stress. The elevated T-reg cell presence was also found to be correlated with worse physical and mental health status and higher levels of depressive symptomology (Ronaldson et al., 2015). Conversely, Drexhage et al. (2011a, 2001b) suggest that a higher T-reg cell presence in psychiatric patients at admission was associated with better clinical outcome at discharge.
This is of interest in the context of stress and depression, with alterations in immune proliferative responses frequently observed in depressed and stressed cohorts. Furthermore, regulatory T-cells can also be induced under circumstances of low tryptophan availability as a consequence of increased indoleamine 2,3 dioxygenase (IDO) activity (Fallarino et al., 2006), which is thought to be key in linking inflammation and kynurenine pathway activation in depression as discussed below. Consequently, the elevated or prolonged presence of T-reg cells may represent a vulnerability factor or early marker for major depression.
It may seem, however, that the earliest reports investigating the association between inflammation and depression are in contrast to a more active T-cell role, with the suggestion that stress and severe depression negatively affect T-cell function with reduced responses upon mitogen stimulation (Zorrilla et al., 2001; Irwin and Miller, 2007; Miller, 2010). While an immunosuppressed T-cell response in the face of immune challenge may appear in contrast with the proinflammatory signature that severely depressed patients are often characterized by, it is possible that inflammation itself (as maybe the case with T-reg cells) and a dysregulated stress response may also have a part to play in the diminished T-cell responses in major depression (Miller, 2010). This too is reasonable given the findings from numerous studies investigating the disruptive impact of chronic TNF-α exposure on T-cell proliferation, cytokine production, and apoptosis and its disruptive effects on T-cell receptor signalling and NFκB activation, culminating in nonresponsive T-cells (Cope et al., 1994, 1997; Lee et al., 2008; Nie et al., 2013). Furthermore, chronic inflammation is also thought to impair GR expression and function in addition to promoting a steroid refractory phenotype via the expression of the inactive beta isoform of the GR (Goleva et al., 2002; Wang et al., 2004; Schewitz et al., 2009). As a consequence, T-cells may no longer be capable of responding to vital neuroendocrine trafficking signals and the mobilization of T-cells to the brain, where, in times of stress, they impart neuroprotective effects (Miller, 2010). As such, alterations in T-cell function, trafficking, and immune regulation as a consequence of persistent inflammation and GR may have detrimental consequences on CNS responses (Miller, 2010).
Biological Mechanisms Implicated in Depression Associated With Inflammatory Markers
The Kynurenine Pathway
In recent years, theories linking the serotonergic and cytokine hypotheses of depression have emerged (Maes et al., 2011; Leonard and Maes, 2012). In support of this, preclinical reports studying animal models of depression have highlighted a possible role for kynurenine pathway activation (Figure 3) in the biological basis of depression (O’Connor et al., 2009a, 2009b). Specifically, O’Connor et al. (2009b) report that blockade of IDO activation in naïve mice following peripheral administration of the bacterial endotoxin LPS prevents the development of depressive-like behavior. Additionally, kynurenine administration to mice dose-dependently induces depressive-like behavior as assessed using the forced swim and tail suspension tests (O’Connor et al., 2009b). Moreover, while Bacille Calmette-Guérin induces inflammation, IDO activity and subsequent depressive-like behavior in wild-type mice, IDO-knockout mice inoculated with Bacille Calmette-Guérin display an increased inflammatory profile in the absence of IDO activity and depressive-like behavior, thereby suggesting that IDO has a central role to play in the onset of depressive symptomology (O’Connor et al., 2009a).
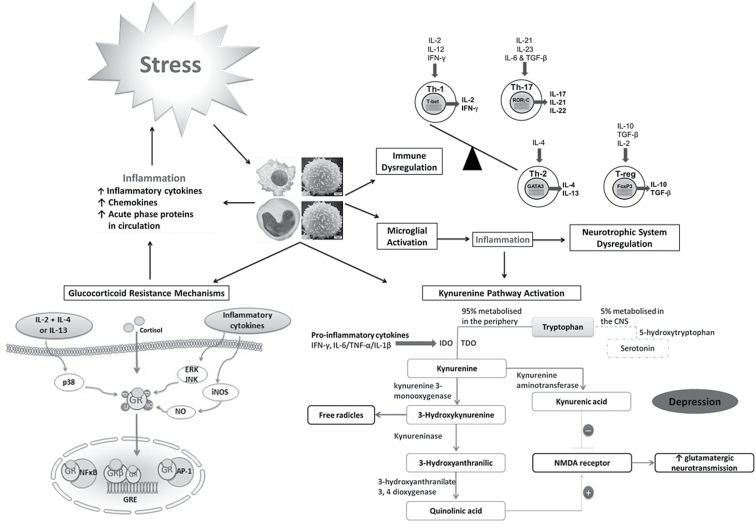
Figure 3.
Summary of the mechanisms by which stress-related immune alterations may precipitate depression. Stress, psychological, physical, or combinational, is thought to be a major risk factor for depression. Chronic stress is thought to impact negatively on the inflammatory response system (IRS), potentially culminating in the manifestation of depressive symptoms. Specifically, a chronic inflammatory state, as a consequence of stress, may lead to activation and further dysregulation of both the innate and adaptive immune response, further promoting an inflammatory environment. The synthesis of serotonin in the CNS is dependent upon the availability of the essential dietary amino acid tryptophan (Russo et al., 2009). In this regard, the kynurenine pathway is the major metabolic pathway for tryptophan in the body, resulting in the production of kynurenine and many downstream metabolites. Indoleamine 2,3 dioxygenase (IDO) is the rate-limiting, tryptophan-degrading enzyme of the kynurenine pathway and is upregulated in response to immune activation. Specifically, IFN-γ is the most potent inducer of IDO activation. However, IFN-γ-independent mechanisms such as prostaglandin E2 or interleukin (IL)-6 in combination with TNF-α or IL-1β are also known inducers of IDO activity (Carlin et al., 1989; Fujigaki et al., 2006; Zunszain et al., 2012). In addition to IDO, activation of the hepatic tryptophan-degrading enzyme tryptophan, 2,3 dioxygenase (TDO) in response to psychological stress, glucocorticoids, or tryptophan itself, also results in kynurenine pathway activation in the liver (Moffett and Namboodiri, 2003). Therefore, IDO/TDO induction has been proposed as a mechanism by which stress and inflammation can precipitate depression via kynurenine pathway activation and tryptophan depletion. A chronic inflammatory state may alter serotonergic neurotransmission via the depletion of tryptophan and increased production of neurotoxic and excitotoxic mediators, which in association with chronic stress and inflammation itself, may have a negative impact on the neurotrophin system and BDNF concentrations in the brain. Stress-induced immune activation is also thought to contribute to the induction of HPA-axis hyperactivity and glucocorticoid resistance (GR), thereby inhibiting the potent antiinflammatory effects of cortisol, which, in turn, contributes further to a dysregulated inflammatory response. Stress-related immune dysregulation and subsequent alterations in monoaminergic neurotransmission, the stress response system and the neurotrophic system, alone or in combination, have a detrimental impact on normal brain functioning, potentially culminating in the manifestation of the behavioral and physiological alterations that currently characterise the depressive condition. GR mechanisms adapted from Barnes (2010, see for review). AP, Activator protein-1; GR, glucocorticoid receptor; GRE, glucocorticoid response elements; iNOS, inducible nitric oxide synthase; NO, nitric oxide; NFκB, nuclear factor kappa B; Th, T helper cells.
Further evidence in support of increased kynurenine pathway activation and subsequent tryptophan depletion in depression stems largely from the study of cytokine-induced depression, which occurs in 30% to 50% of medically ill patients being treated with IL-2 or IFN-α for cancer and Hep C (Musselman et al., 2001; Capuron et al., 2002; Capuron and Miller, 2004). However, it has also been shown that cytokine-induced depression severity is dose dependent; hence, the larger the dose of IFN-α, the greater the depression severity (Schaefer et al., 2002). Therefore, while this is strong evidence in support of the proposed involvement of the kynurenine pathway in depression, idiopathic depression is largely characterized by a low-grade inflammatory phenotype, and it is questionable if the inflammatory profile observed in medically healthy depressed patients is robust enough to induce an elevated kynurenine pathway activation and subsequent tryptophan depletion.
In accordance with this, to date, literature directly associating increased kynurenine pathway activation and idiopathic major depression is scarce. A cross-sectional study by Myint et al. (2007) reports an increased KYN/TRP ratio in the absence of changes in kynurenine and tryptophan alone and decreased neuroprotective kynurenic acid (KYNA) concentrations in the depressed patients relative to controls, while Gabbay et al. (2010) also report an increased KYN/TRP ratio in adolescents with melancholic depression. However, as there was no change in kynurenine, the increase in the KYN/TRP ratio appears to be solely as a consequence of the significant decrease in tryptophan. Yet, increased measures of kynurenine pathway activation were associated with depression severity in the melancholic depressed patients (Gabbay et al., 2010). One study does report an elevated serum concentration of kynurenine and depleted tryptophan and 5HIAA availability in a mixed cohort of bipolar and depressed patients relative to healthy controls (Myint et al., 2013). However, recent reports by Dahl and colleagues (Dahl et al., 2014, 2015) suggest that despite the presence of a dysregulated inflammatory profile in a cohort of unmediated depressed patients at baseline, there was no difference in kynurenine metabolite plasma markers or tryptophan concentrations relative to healthy controls at baseline. Furthermore, in accordance with a recent report by Hughes et al. (2012) of decreased tryptophan availability in a cohort of depressed patients, Maes and Colleagues (Maes et al., 2011; Maes and Rief, 2012) report that while depression and somatic disorders are both characterized by decreased tryptophan concentrations, there was no evidence in support of increased IDO activity in depression.
It has also been suggested that depression is not associated with a decrease in tryptophan per se but rather the downstream catabolites of the kynurenine pathway. This theory is supported by findings by Raison et al. (2010), where they showed that while tryptophan concentrations are depleted in the periphery of Hep C patients being treated with IFN-α, examination of CSF tryptophan concentrations revealed that the concentration of central tryptophan was normal. However, an increased kynurenine concentration observed in both the periphery and CNS and the associated elevations in CSF quinolinic acid (QUIN) and KYNA were found to correlate with the elevated inflammatory profile and depressive symptomology. Consequently, it appears that while tryptophan is maintained in the CNS, a decreased concentration of tryptophan in the periphery may be reflecting changes in kynurenine metabolites in the CNS. Further to this, it has been suggested that it is the kynurenines and perhaps an imbalance between the neurotoxic (QUIN) and neuro-protective (KYNA) catabolites that are responsible for the induction of depressive symptomology (Maes et al., 2011). This is further supported by recent findings showing that while kynurenine metabolite concentrations were unaltered in independent cohorts of depressed and remitted patients, the ratio of KYNA to QUIN was decreased in both patient cohorts relative to controls (Savitz et al., 2015a, 2015b). Furthermore, this reduced KYNA to QUIN ratio was inversely correlated with the number of depressive episodes in the remitted group, whereas in the depressed cohort, while no significant correlation was observed with depression severity, the reduced ratio of KYNA/QUIN was significantly correlated with anhedonia (Savitz et al., 2015b).
Furthermore, a study by Capuron et al. (2009) has shown that while there is considerable symptom overlap between cytokine-induced depression and idiopathic major depression, patients with IFN-α-induced depression display greater somatic symptom severity in the form of psychomotor retardation and weight loss relative to idiopathic depressed patients. This is very interesting in light of the report by Maes and Rief (2012) that concludes that kynurenine pathway activation is more pronounced in comorbid somatisation and depression compared with depression alone and suggest that changes in kynurenine pathway metabolites (tryptophan catabolites) may be more closely associated with somatic symptomology rather than depression per se, although this is in contrast with the above mentioned finding, showing an association between anhedonia and altered kynurenine pathway metabolites (Savitz et al., 2015a, 2015b).
GR
While prolonged elevations in cortisol may be a consequence of chronic stress, alterations in GR function and sensitivity are also commonly reported in depressed individuals. Evidence in support of decreased glucocorticoid sensitivity, commonly referred to as GR, in depressed patients arises from studies assessing GR response to the synthetic glucocorticoid dexamethasone and the dexamethasone-CRH stimulation test with studies repeatedly reporting impaired glucocorticoid responsiveness and nonsuppression of cortisol secretion, which has been shown to correlate with depression severity and age (Carroll et al., 1981; von Bardeleben and Holsboer, 1991; Holsboer, 2000; Pariante and Miller, 2001; Ising et al., 2007).
Further to this, it is thought that persistent inflammation observed in subgroups of depressed and stressed individuals may have a significant role to play in the onset and maintenance of GR, which in turn may fuel the chronic inflammatory phenotype in a feed forward cascade. In support of this theory, assessment of GR expression on HeLaS3 cells, following stimulation with the inflammatory cytokine TNF-α, revealed an increased expression of the inactive beta (β) isoform relative to the active alpha (α) isoform of the GR. The increased protein expression of GRβ, which is unable to stimulate glucocorticoid inducible genes, was also associated with the development of GR (Webster et al., 2001). Additionally, elevated expression of GRβ has been detected in patients with inflammatory conditions such as arthritis and asthma (Sousa et al., 2000; Chikanza, 2002). Further to this, an elevated mitogen stimulated IL-1β and IL-6 production was positively associated with nonsuppressed plasma cortisol concentrations following the dexamethasone suppression test in a cohort of depressed patients (Maes et al., 1993a, 1993b). In accordance with this, Pariante and colleagues (Pariante et al., 1999) observed that IL-1α reduced GR translocation and decreased dexamethasone-induced GR-mediated gene activity by nearly 50% in a mouse L929 fibroblast cell line, further implicating a role for inflammation in the dysregulation and hyperactivity of the HPA axis.
Under normal physiological conditions, GR activation results in the production of antiinflammatory glucocorticoid-inducible genes and the activation of the hsp90 co-chaperone FK506 binding protein 51 (FKBP5), which acts as a negative regulator of GR activation (Figure 2). Interestingly, healthy controls carrying certain polymorphisms in the FKBP5 gene display an exaggerated increase in the transcriptional expression of FKBP5 upon GR activation in response to psychosocial stress, thereby decreasing the sensitivity of the receptor as evidenced by insufficient cortisol recovery and the persistent activation of the HPA axis (Ising et al., 2008). Additionally, Zimmermann et al. (2011) have shown that homozygotes of the minor allele of the FKBP5 gene are more vulnerable to the development of major depression in the wake of severe adverse stressful life events.
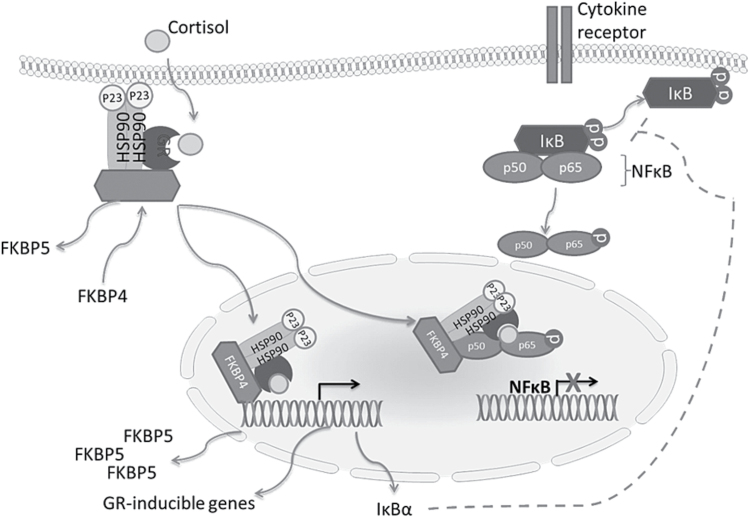
Figure 2.
Glucocorticoid receptor (GR) signalling and suppression of inflammation under normal conditions. The GR is a ligand-activated transcription factor. Upon cortisol binding, it disassociates from its co-chaperone heat shock protein (hsp) complex in the cytosol and translocates to the nucleus. There it interrupts Nuclear Factor-Kappa B (NFκB) signalling through interactions with the NFκB regulator I kappa B alpha (IκB), thereby exerting antiinflammatory effects along with promoting the transcription of glucocorticoid-inducible genes such as glucocorticoid induced leucine zipper (GILZ) and serum and glucocorticoid regulated kinase 1 (SGK1) (Raison and Miller, 2003). The GR can also interact directly with the p65 subunit, preventing NFκB binding and the transcription of inflammatory cytokines. The mechanism of GR activation is tightly regulated by the co-chaperone hsp complex, which effectively controls the sensitivity of the receptor and consequently has been implicated in the pathogenesis of major depression. Specifically, under normal conditions, the hsp90 co-chaperone FK506 binding protein 51 (FKBP5) acts to negatively regulate the GR. When bound to GR, FKBP5 confers a low cortisol binding affinity on the receptor. However, upon cortisol binding and GR activation, FKBP5 is exchanged for FKBP4, permitting the translocation of the GR complex to the nucleus. This action, along with regulating and inducing gene transcription, also upregulates the expression of FKBP5, thereby decreasing the sensitivity of the receptor once again (Binder, 2009). (adapted from Smoak and Cidlowski, 2004).
Serotonin Transporter (SERT)
Further to this, the SERT, a transmembrane protein that removes serotonin from the synapse following its release (Benmansour et al., 1999), also appears to pose a risk for the development of a depressive episode following a stressful life event (Caspi et al., 2003). SERT may also represent a link between the inflammatory hypothesis and reduced serotonergic function in depression. In this regard, studies have demonstrated that inflammatory cytokines, including IL-1β, TNF-α, and IFN-α, increase SERT expression and serotonin reuptake in vitro (Zhu et al., 2006; Tsao et al., 2008). Additionally, a systemic inflammatory challenge with LPS increases SERT expression in the rodent brain (Zhu et al., 2010). A sustained increase in CNS transcriptional expression of IFN-α and SERT in response to a single systemic challenge with the viral mimetic Poly IC was also found to have functional significance, evidenced by an associated decrease in extracellular serotonin concentrations quantified in the patient cohort (Tsao et al., 2006). Furthermore, elevated expression of the inflammatory cytokines IL-1β, TNF-α, IL-6, and IFN-γ on blood leukocytes from depressed patients was associated with an increased transcriptional expression of SERT. However, following chronic selective serotonin reuptake inhibitors treatment, decreased transcriptional expression levels of IFN-γ and SERT were observed (Tsao et al., 2006).
Neurotrophins
Both stress and inflammation can disrupt the expression and function of BDNF (Schaaf et al., 1998; Barrientos et al., 2003). BDNF has a central role in neuronal proliferation, regeneration and survival, neurogenesis, and synaptic plasticity (Tapia-Arancibia et al., 2004). In particular, Barrientos et al. (2003) have shown that alterations in hippocampal-dependent processes may be a consequence of IL-1β mediated decreases in BDNF. In accordance with this, Karege et al. (2005) have shown decreased BDNF expression in suicide victim postmortem hippocampal and prefrontal cortex tissue, while others have repeatedly reported reductions in the circulating concentration of BDNF in depressed patients (Karege et al., 2002; Cattaneo et al., 2010).
Moreover, while numerous studies have suggested a decrease in hippocampal neurogenesis associated with depression (Ekdahl et al., 2003; Kempermann et al., 2008; Boldrini et al., 2009) and furthermore that inflammation and specifically IL-1β has the capacity to mediate this (Goshen et al., 2008; Koo and Duman, 2008; Spulber et al., 2008; Kohman and Rhodes, 2013), the mechanism(s) of action remain to be elucidated. However, recent studies using human hippocampal progenitor cells have yielded novel in vitro findings, indicating that IL-1β medicated kynurenine pathway activation, with specific elevations in the expression of kynurenine monooxygenase and kynureninase, leading to the production of potentially neurotoxic metabolites, have detrimental effects on hippocampal neurogenesis (Zunszain et al., 2012).
Synergy between Stress, Cytokines, and the HPA Axis
Taken together, the synergistic dysregulation and feed forward cascade of multiple biological systems appear to play a significant role in the onset of depressive symptomology (Anisman, 2009) (Figure 3). Stressful life events such as childhood trauma, genetics, and chronic activation of the IRS, alone or in combination, negatively impact on normal functioning and inhibitory control of the stress response system in association with altering the inhibitory feedback mechanisms of glucocorticoids on cytokine secretion, thereby inducing hypercortisolemia (or hypocortisolemia) and subsequently contributing further to the manifestation of a dysregulated inflammatory and neuroendocrine phenotype. These factors potentially culminate in the manifestation of the behavioral and physiological alterations that currently characterize the depressive condition. Furthermore, as discussed by Frodl and O’Keane (2013), preclinical evidence suggests that elevated cortisol concentrations may have an excitotoxic and neurotoxic impact on brain structures strongly associated with depressive symptomology. However, while hippocampal volumetric changes are evident in depressed patients (Frodl et al., 2012), further clinical assessments are required to decipher if putative neuronal alterations are a consequence of a dysfunctional stress response and elevated glucocorticoid and or cytokine concentrations.
An Emerging Role for Microglia?
With increasing evidence of an inflammatory response in major depression, mechanisms of communication between the periphery and the brain, which include the circumventricular organs, active transport, and peripheral afferent nerve fibres (for review, see Connor and Leonard, 1998), provide a means by which large, peripherally produced cytokine molecules (17-51kD), unable to passively diffuse across the blood brain barrier due to their size and structure, can alter neuronal and glial cell function and behavior via cytokine receptor activation in the CNS, thereby potentially impacting on mood and the manifestation of depressive symptomology (Hopkins and Rothwell, 1995; Szelenyi, 2001). Despite this, little is known about the specific inflammatory state in the brain of MDD patients. However, as reviewed by Beumer et al. (2012), advances in brain imaging are opening up new avenues for investigation, with activated microglia found on brain scans of patients with psychiatric disorders. In accordance with this and of particular interest is the recent report by Setiawan et al. (2015), which presents evidence of brain inflammation and specifically microglial activation evidenced by the elevated presence of translocator protein density measured by distribution volume in a patient cohort during a major depressive episode. Furthermore, elevations in translocator protein density measured by distribution volume in the anterior cingulate cortex were robustly correlated with depression severity, suggesting that greater microglial activation in specific brain regions may result in the manifestation of specific depressive symptoms (Setiawan et al., 2015). Interestingly, however, contrary to the above-mentioned reports, preclinical findings by Kreisel et al. (2014) suggest that hippocampal microglial dysregulation, rather than activation, as a consequence of chronic stress may actually be responsible for the development of certain depressive symptoms. More specifically, Kreisel et al. (2014) show that while acute stress induces short-term microglial activation and proliferation, rodents subjected to 5 consecutive weeks of chronic unpredictable stress, in fact, exhibited downregulated microglial functional capacity and apoptosis in addition to depressive-like behavior. Moreover, subsequent immunological challenge, resulting in microglial activation and proliferation, ameliorated or reduced the anhedonic depressive-like behavior observed following CUS, thereby providing a causal link between microglial dysfunction and chronic stress-induced depression symptomatology (Kreisel et al., 2014). Consequently, microglial stimulators may prove to be beneficial in the treatment of some depressive subtypes (Kreisel et al., 2014). The mechanisms responsible for the activation of microglia in psychiatric disease remain elusive. A direct microbe-driven activation of microglia is possible (Kristensson, 2011), but apart from direct microbial/gDNA activation of microglia, inborn errors in the growth and differentiation of myeloid progenitor cells can make the cells vulnerable for hyper-stimulation. The identification of genetic risk factors and the role that genetics might have in these complex relationships is largely unexplored territory to date. As the technology to manipulate the genome has come of age, such questions can be adopted from the clinic and readily explored in animal models.
Implications for Treatment
As the biological basis of depression still remains elusive, treatment strategies are not always effective and multiple trials often necessary to elicit a response. Consequently, in recent years, attention has focused on the identification of biological markers (biomarkers) for depression. Emerging evidence in the last 10 years highlights the importance of peripheral blood as a potential diagnostic tool for many diseases including psychiatric disorders (Tsuang et al., 2005) and especially as it may be used to assess the inflammatory signature in depressed patients. Its importance also arises from studies by Liew et al. (2006), who show that peripheral blood cells share approximately 80% of the transcriptome with 9 non-blood-related tissues; specifically, they found 81.9% of all genes expressed in the brain to be coexpressed in human blood cells. Given that circulating blood cells respond to the macro and micro changes occurring around them and come into close contact with brain regions such as the pituitary and hypothalamus, it has been proposed that peripheral blood cells may act as “surrogates for CNS expression” (Liew et al., 2006; Sullivan et al., 2006). Consequently, whole blood mRNA expression system may be thought of as a proxy measure for mRNA expression in brain (Hepgul et al., 2013). In addition isolated peripheral blood mononuclear cells provide insight into the profile of transcriptional expression in white blood cells that are easily obtained from living patients and enable links to be made between depression severity or clinical staging and the biological profile (Le-Niculescu et al., 2007). As such, changes in specific biological markers may be used to identify predisposing risk factors, distinguish depressive subtypes, predict the onset of depression, progression of the disorder or perhaps treatment response, thereby eradicating self-report systems and subjectivity and facilitating a more targeted and fast-acting approach to treatment (Bell, 2004; Le-Niculescu et al., 2007; Sunde, 2010; Schmidt et al., 2011; Lopresti et al., 2014). However, given the heterogeneity of depression and the large number of biological systems implicated in the aetiology of depressive disorders, to date, the diagnosis of depression is not etiologically or biologically derived (Mossner et al., 2007). However, recent findings by Rapaport et al. (2015) demonstrate the use of a beneficial and promising strategy, which involves using a combination of inflammatory makers to aid in the distinction and identification of responders to antidepressant treatment.
In addition, while numerous facets of the IRS have been assessed in depressed cohorts, there is a lack of in depth studies investigating the expression and function of chemokines and their receptors in depressive disorders. Given the reports associating chemokines with depressive disorders (Rajagopalan et al., 2001; Suarez et al., 2003), future studies should assess PAXgene chemokine expression as a potential biomarker for depression in addition to the assessment of circulating chemokine concentrations. Given their role in the mediation of immune cell trafficking to sites of inflammation or injury, chemokine-medicated immune cell trafficking to the brain in patients with major depression should be explored.
Furthermore, advances in neuroimaging and positron emission tomograph scanning in living patients also hold great promise for the identification of brain specific biomarkers. Given that depression is primarily a disorder of the CNS, assessment of microglial activation states and brain volumetric changes in depressed patients in association with peripheral immune markers may provide a more targeted and comprehensive approach in the search for biomarkers with an ultimate goal to develop personalized treatment strategies for patients with major depressive disorders (Doorduin et al., 2008; van Berckel et al., 2008; Frodl et al., 2012).The primary measure of depression severity is the 21-item Hamilton Rating Scale for Depression (HAM-D) 21 total score which is a routinely used, validated, and standardized assessment tool for major depression (Hamilton, 1960). However, the total score merely provides insight into the global depressive state (Shafer, 2006). Others have demonstrated the use of the HAM-D subscale approach in genetics research on major depressive disorder and in the assessment of antidepressant medication (Seretti et al., 1999; Yu et al., 2002). The majority of research to date has focused on comparisons between depressed patients and healthy controls and correlations with total depression severity; however, the unidimensional scales enable the connection of biological parameters to individual symptom clusters of major depressive disorder, thereby assessing the contribution of each parameter to the individual components of the disorder (Lee et al., 2011). Refined clusters of the HAM-D scale include core depression, anxiety, insomnia, and somatic symptoms (Shafer, 2006). Previous studies have demonstrated that external measures have different association profiles with individual symptom clusters within a test (Williams and Richardson, 1993; Barefoot et al., 2000). Further to this, Faries et al. (2000) reported that core subscales were more efficient at detecting change than the HAM-D total score. Consequently, the use of a more targeted, symptom-wise approach to decipher the underpinning pathophysiological mechanisms of individual symptoms may lead towards the development of specific biomarkers, thereby leading to the development of a more effective treatment strategy.
Strategies that attempt to address wide variation within animal studies by subdividing populations according to behavioral or physiological characteristics, for example, coping styles, will also help to unravel factors underlying susceptibility or vulnerability. Additional research employing stressors with a greater degree of ecological validity that challenge the natural defence and/or adaptive capacity of animals, for example, social stress or influence of early-life stress, serve to increase the face validity and relevance of existing models and practices. The concept that stress may predispose to a premature ageing of the immune response has been proposed (Glaser and Kiecolt-Glaser, 2005) and to explore this further, a greater emphasis on longitudinal investigations is required.
Patients with evidence of an activated IRS are considered less likely to respond to regular antidepressant therapy, and it is hypothesised that treatment resistant patients will respond to conventional treatment in combination with immunosuppressive therapy expected to dampen the inflammatory state of non-responders. Monocyte and microglial activation may be reversed by administration of immunosuppressive drugs, including nonsteroidal antiinflammatories (Muller, 2010; Khan et al., 2014), N-acetylcysteine (Dean et al., 2011), and minocycline (Levkovitz et al., 2010), although such treatments have not been systematically investigated in psychiatric disorders, with further potential for novel immunosuppressive interventions. Use of nonsteroidal antiinflammatories, N-acetylcysteine, minocycline, and novel immunomodulating drugs in animal models (reviewed by McGuinness and Harkin, 2015) could be examined for effectiveness in rectifying behavioral abnormalities. Models will enable in-depth studies on the molecular mechanism of immune mediated behavioral abnormalities and their correction by drug treatment.
There are new possibilities for treatments that target pathways by which the immune system influences the brain such as cytokines or growth factors and their downstream mediators or the activation of relevant CNS immune cell types (eg, microglia) to emerge. Results from trials to determine the efficacy of antiinflammatory drugs such as the use of anti-TNFα in patients with psoriasis (Kannan et al., 2013) with antidepressant potential or the adjunctive use of cyclooxygenase-2 inhibitors for treating depression (Muller et al., 2010) have been encouraging. Drugs acting on the HPA axis, GRs, and postreceptor signalling are being considered as new therapeutic possibilities with the potential to correct dysregulation of the HPA-immune axis in depression (for review, see Maric and Adzix, 2013). Drugs classified as anti-glucocorticoids (GR agonist, GR antagonists, dehydroepiandrosterone-DHEA, steroid synthesis inhibitors) are of interest for their capacity to correct glucocortiocoid-associated inflammation and/or neuronal damage in depression. Many of these trials are still at the early proof-of concept stage and likely to feature in future developments of new treatments for depression with associated dysregulated endrocrine-immune axis function. Moreover, treatments addressing the influence of stress and stress-induced activation of the SAM and HPA axes including behavioral interventions that address psychological and autonomic reactivity to stress such as psychotherapy, exercise, and meditation may have efficacy regarding both treatment and prevention of depression.
Statement of Interest
None.
Acknowledgments
M.H. was supported by a TCD School of Medicine Translational Neuroscience grant. The authors also acknowledge support from the EU FP7-funded MOODINFLAME consortium.
References
- Anisman H. (2009) Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci 34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Anisman H. (2011) Inflaming depression. J Psychiatry Neurosci 36:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC, Brummett BH, Helms NJ, Mark DB, Siegler IC, Williams RB. (2000) Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med 62:790–795. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. (2010) Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 120:76–85. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. (2003) Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 121:847–853. [DOI] [PubMed] [Google Scholar]
- Bawa-Khalfe T, Altememi GF, Mandyam CD, Schwarz LA, Eikenburg DC, Standifer KM. (2007) The presence of beta2-adrenoceptors sensitizes alpha2A-adrenoceptors to desensitization after chronic epinephrine treatment. BMC Pharmacol 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. (2004) Predicting disease using genomics. Nature 429:453–456. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. (1999) Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci 19:10494–10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Dastrange M, Oukka M. (2005) Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A 102:5138–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. [DOI] [PubMed] [Google Scholar]
- Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, Steiner J, Connor TJ, Harkin A, Versnel MA, Drexhage HA. (2012) The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol 92:959–975. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. (2013) Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Cerami A. (1989) The biology of cachectin/TNF-a primary mediator of the host response. Annu Rev Immunol 7:625–655. [DOI] [PubMed] [Google Scholar]
- Binder EB. (2009) The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34:S186–195. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. (2009) Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34:2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, Levrero M, Egyed B, Bosmans E, Meltzer HY, Maes M. (2001) Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res 105:45–55. [DOI] [PubMed] [Google Scholar]
- Borish LC, Steinke JW. (2003) Cytokines and chemokines. J Allergy Clin Immunol 111:S460–475. [DOI] [PubMed] [Google Scholar]
- Bowe WP, Logan AC. (2011) Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future? Gut Pathog 3:1–4749-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce TO. (2008) Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep 10:258–264. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. (2009) Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord 119:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. (2004) Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry 56:819–824. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. (2000) Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol 18:2143–2151. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. (2001) Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology 26:797–808. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. (2002) Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry 7:468–473. [DOI] [PubMed] [Google Scholar]
- Carlin JM, Borden EC, Sondel PM, Byrne GI. (1989) Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol 45:29–34. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA.(1976. a) Urinary free cortisol excretion in depression. Psychol Med 6:43–50. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J.(1976. b) Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychol Med 6:235–244. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, De VignE JP, Young E. (1981) A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry 38:15–22. [DOI] [PubMed] [Google Scholar]
- Caruso C, Candore G, Cigna D, Colucci AT, Modica MA. (1993) Biological significance of soluble IL-2 receptor. Mediators Inflamm 2:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Mcclay J, Mill J, Martin J, Braithwaite A. (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science Signaling 301:386. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Bocchio-Chiavetto L, Zanardini R, Milanesi E, Placentino A, Gennarelli M. (2010) Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int J Neuropsychopharmacol 13:103–108. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA, Pariante CM. (2015) Inflammation and neuronal plasticity: a link between childhood trauma and depression pathogenesis. Front Cell Neurosci 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik C, Erdem M, Cayci T, Ozdemir B, Ozgur Akgul E, Kurt YG, Yaman H, Isintas M, Ozgen F, Ozsahin A. (2010) The association between serum levels of neopterin and number of depressive episodes of major depression. Prog Neuropsychopharmacol Biol Psychiatry 34:372–375. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. (2004) Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82:217–225. [DOI] [PubMed] [Google Scholar]
- Cheng G, Yu A, Malek TR. (2011) T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 241:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikanza IC. (2002) Mechanisms of corticosteroid resistance in rheumatoid arthritis: a putative role for the corticosteroid receptor beta isoform. Ann N Y Acad Sci 966:39–48. [DOI] [PubMed] [Google Scholar]
- Cizza G, Marques AH, Eskandari F, Christie IC, Torvik S, Silverman MN, Phillips TM, Sternberg EM. POWER Study Group (2008) Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biol Psychiatry, 64:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho MM, Souza GE, Pela IR. (1992) Endotoxin-induced fever is modulated by endogenous glucocorticoids in rats. Am J Physiol 263:R423–427. [DOI] [PubMed] [Google Scholar]
- Commins SP, Borish L, Steinke JW. (2010) Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol 125:S53–72. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Leonard BE. (1998) Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci 62:583–606. [DOI] [PubMed] [Google Scholar]
- Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO. (1997) Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 185:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope AP, Londei M, Chu NR, Cohen SB, Elliott MJ, Brennan FM, Maini RN, Feldmann M. (1994) Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest 94:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, Brundin L, Andreassen OA. (2014) The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45:77–86. [DOI] [PubMed] [Google Scholar]
- Dahl J, Andreassen OA, Verkerk R, Malt UF, Sandvik L, Brundin L, Ormstad H. (2015) Ongoing episode of major depressive disorder is not associated with elevated plasma levels of kynurenine pathway markers. Psychoneuroendocrinology 56:12–22. [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2006) Cytokine, sickness behavior, and depression. Neurol Clin 24:441–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean O, Giorlando F, Berk M. (2011) N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 36:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. (1988) Biology of interleukin 1. FASEB J 2:108–115. [PubMed] [Google Scholar]
- Dinel AL, Joffre C, Trifilieff P, Aubert A, Foury A, Le Ruyet P, Laye S. (2014) Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. J Neuroinflammation 11:155–014-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Talib LL, Mendonca VA, Gattaz WF, Forlenza OV. (2010) Increased soluble TNF receptor 2 in antidepressant-free patients with late-life depression. J Psychiatr Res 44:917–920. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Dierckx RA, Klein HC. (2008) PET imaging of the peripheral benzodiazepine receptor: monitoring disease progression and therapy response in neurodegenerative disorders. Curr Pharm Des 14:3297–3315. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, Van Beveren NJ, Drexhage HA.(2011. a) An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol 14:746–755. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TH, Versnel MA, Berghout A, Nolen WA, Drexhage HA.(2011. b) The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav Immun 25:1206–1213. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. (1992) Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther 261:964–969. [PubMed] [Google Scholar]
- Edgar VA, Cremaschi GA, Sterin-Borda L, Genaro AM. (2002) Altered expression of autonomic neurotransmitter receptors and proliferative responses in lymphocytes from a chronic mild stress model of depression: effects of fluoxetine. Brain Behav Immun 16:333–350. [DOI] [PubMed] [Google Scholar]
- Edgar VA, Silberman DM, Cremaschi GA, Zieher LM, Genaro AM. (2003) Altered lymphocyte catecholamine reactivity in mice subjected to chronic mild stress. Biochem Pharmacol 65:15–23. [DOI] [PubMed] [Google Scholar]
- Elenjov IJ, Chrousos GP. (2002) Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966:290–303. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. (1996) Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians 108:374–381. [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 100:13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. (2006) The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176:6752–6761. [DOI] [PubMed] [Google Scholar]
- Faries D, Herrera J, Rayamajhi J, DeBrota D, Demitrack M, Potter WZ. (2000) The responsiveness of the Hamilton Depression Rating Scale. J Psychiatr Res 34:3–10. [DOI] [PubMed] [Google Scholar]
- Feinstein A. (2011) Multiple sclerosis and depression. Mult Scler 17:1276–1281. [DOI] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, McLoughlin DM, Meaney J, O’Keane V, Connor TJ. (2012) Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry 2:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. (2013) How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 52:24–37. [DOI] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. (2006) The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem 139:655–662. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. (2010) The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry 51:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. (2013) Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 65:1655–1662. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. (2005) Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251. [DOI] [PubMed] [Google Scholar]
- Gillis S, Mizel SB. (1981) T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A 78:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7:254–275. [DOI] [PubMed] [Google Scholar]
- Goleva E, Kisich KO, Leung DY. (2002) A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol 169:5934–5940. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. (2008) Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13:717–728. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. (2005) Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 179:769–780. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. (2015) Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. (2000) Depression and sensitization to stressors among young women as a function of childhood adversity. J Consult Clin Psychol 68:782–787. [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. (2011) The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36:2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. (2012) Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37:137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. (1988) Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. (2012) Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 233:102–111. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710. [DOI] [PubMed] [Google Scholar]
- Hepgul N, Cattaneo A, Zunszain PA, Pariante CM. (2013) Depression pathogenesis and treatment: what can we learn from blood mRNA expression?. BMC Med 11:28–7015-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. (2003) Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J ECT 19:183–188. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Milenovic S, Fulda S, Plumakers B, Sheldrick AJ, Michel TM, Kircher T, Rink L. (2010) Regulatory T cells increased while IL-1beta decreased during antidepressant therapy. J Psychiatr Res 44:1052–1057. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen E, Beutler B. (2004) The interface between innate and adaptive immunity. Nat Immunol 5:971–974. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. (1999) Phylogenetic perspectives in innate immunity. Science 284:1313–1318. [DOI] [PubMed] [Google Scholar]
- Holsboer F. (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. (1995) Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci 18: 83–88. [PubMed] [Google Scholar]
- Horn F, Henze C, Heidrich K. (2000) Interleukin-6 signal transduction and lymphocyte function. Immunobiology 202:151–167. [DOI] [PubMed] [Google Scholar]
- Hughes MM, Carballedo A, McLoughlin DM, Amico F, Harkin A, Frodl T, Connor TJ. (2012) Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain Behav Immun 26:979–987. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. (2007) Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 21:374–383. [DOI] [PubMed] [Google Scholar]
- Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, Kunzel HE, Pfennig A, Uhr M, Holsboer F. (2007) Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biol Psychiatry 62:47–54. [DOI] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. (2008) Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci 28:389–398. [DOI] [PubMed] [Google Scholar]
- Itoh CE, Kizaki T, Hitomi Y, Hanawa T, Kamiya S, Ookawara T, Suzuki K, Izawa T, Saitoh D, Haga S, Ohno H. (2004) Down-regulation of beta2-adrenergic receptor expression by exercise training increases IL-12 production by macrophages following LPS stimulation. Biochem Biophys Res Commun 322:979–984. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. (2005) Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135:1295–1307. [DOI] [PubMed] [Google Scholar]
- Kannan S, Heller MM, Lee ES, Koo JY. (2013) The role of tumor necrosis factor-alpha and other cytokines in depression: what dermatologists should know. J Dermatolog Treat 24:148–152. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. (2002) Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 109:143–148. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. (2005) Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 136:29–37. [DOI] [PubMed] [Google Scholar]
- Kaye J, Gillis S, Mizel SB, Shevach EM, Malek TR, Dinarello CA, Lachman LB, Janeway CA., Jr (1984) Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol 133:1339–1345. [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. (2008) The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry 21:290–295. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. (2002) Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol 5:401–412. [DOI] [PubMed] [Google Scholar]
- Khan D, Fernando P, Cicvaric A, Berger A, Pollak A, Monje FJ, Pollak DD. (2014) Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry 4:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. (2007) Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 31:1044–1053. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS. (2013) Neurogenesis, inflammation and behavior. Brain Behav Immun 27:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. (2002) Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 25:154–159. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. (2008) IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 105:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. (2014) Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19:699–709. [DOI] [PubMed] [Google Scholar]
- Kristensson K. (2011) Microbes’ roadmap to neurons. Nat Rev Neurosci 12:345–357. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Silva J, Jr, Greden J, Dembinski S, Gardner R, Carroll B. (1983) Impaired lymphocyte function in depressive illness. Life Sci 33:241–247. [DOI] [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. (2001) Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol 21:199–206. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. (2000) Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22:370–379. [DOI] [PubMed] [Google Scholar]
- Lee LF, Lih CJ, Huang CJ, Cao T, Cohen SN, McDevitt HO. (2008) Genomic expression profiling of TNF-alpha-treated BDC2.5 diabetogenic CD4+ T cells. Proc Natl Acad Sci U S A 105:10107–10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Yu YW, Chen MC, Chen TJ. (2011) Cortical mechanisms of the symptomatology in major depressive disorder: a resting EEG study. J Affect Disord 131:243–250. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM, Tilders FJ, Dantzer R, Rothwell NJ, Luheshi GN. (1999) Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am J Physiol 276:R652–658. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, McFarland MJ, Mamidipalli S, Ogden CA, Kuczenski R, Kurian SM, Salomon DR, Tsuang MT, Nurnberger JI, Jr, Niculescu AB. (2007) Convergent Functional Genomics of bipolar disorder: from animal model pharmacogenomics to human genetics and biomarkers. Neurosci Biobehav Rev 31:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S. (2010) A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 71:138–149. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, Yang H. (2010) Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord 124:68–75. [DOI] [PubMed] [Google Scholar]
- Liew CC, Ma J, Tang HC, Zheng R, Dempsey AA. (2006) The peripheral blood transcriptome dynamically reflects system wide biology: a potential diagnostic tool. J Lab Clin Med 147:126–132. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. (2012) Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 139:230–239. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Maker GL, Hood SD, Drummond PD. (2014) A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry, 48:102–111. [DOI] [PubMed] [Google Scholar]
- Maes M. (1995) Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 19:11–38. [DOI] [PubMed] [Google Scholar]
- Maes M. (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry 35:664–675. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E.(1993. a) Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry 150:1189–1193. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC. (2008) The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29:117–124. [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. (2011) The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 35:702–721. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R.(1995. a) Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord 34:301–309. [DOI] [PubMed] [Google Scholar]
- Maes M, Rief W. (2012) Diagnostic classifications in depression and somatization should include biomarkers, such as disorders in the tryptophan catabolite (TRYCAT) pathway. Psychiatry Res 196:243–249. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpe S, Meltzer HY, Bosmans E, Suy E, Calabrese J, Cosyns P.(1993. b) Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res 49:11–27. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S.(1995. b) The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology 20:111–116. [DOI] [PubMed] [Google Scholar]
- Maric NP, Adzic M. (2013) Pharmacological modulation of HPA axis in depression – new avenues for potential therapeutic benefits. Psychiatr Danub 25: 299–305. [PubMed] [Google Scholar]
- Mazure CM, Bruce ML, Maciejewski PK, Jacobs SC. (2000) Adverse life events and cognitive-personality characteristics in the prediction of major depression and antidepressant response. Am J Psychiatry 157:896–903. [DOI] [PubMed] [Google Scholar]
- Mcguinness B, Harkin A. (2015) Rodent models of stress-induced depression: the link between stress and immune system related changes. In: Immunology and psychiatry; from basic research to therapeutic interventions (Muller N, Myint A-M, Schwarz MJ, eds), pp33–62. Switzerland: Springer. [Google Scholar]
- Medzhitov R. (2008) Origin and physiological roles of inflammation. Nature 454:428–435. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr (1997) Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 9:4–9. [DOI] [PubMed] [Google Scholar]
- Miller AH. (2010) Depression and immunity: a role for T cells? Brain Behav Immun 24:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA. (2003) Tryptophan and the immune response. Immunol Cell Biol 81:247–265. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Cattaneo A, Belvederi Murri M, Di Forti M, Handley R, Hepgul N, Miorelli A, Navari S, Papadopoulos AS, Aitchison KJ, Morgan C, Murray RM, Dazzan P, Pariante CM. (2011) Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry 72:1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N, Fallgatter AJ, Riederer P. (2007) Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry 8:141–174. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. (2006) The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 11:680–684. [DOI] [PubMed] [Google Scholar]
- Muller N. (2010) COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs 11:31–42. [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. (2001) Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 344:961–966. [DOI] [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, Kim YK. (2005) Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord 88:167–173. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. (2007) Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98:143–151. [DOI] [PubMed] [Google Scholar]
- Myint AM, Bondy B, Baghai TC, Eser D, Nothdurfter C, Schule C, Zill P, Muller N, Rupprecht R, Schwarz MJ. (2013) Tryptophan metabolism and immunogenetics in major depression: a role for interferon-gamma gene. Brain Behav Immun 31:128–133. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. (2004) Neurobiological consequences of childhood trauma. J Clin Psychiatry 65:18–28. [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344. [DOI] [PubMed] [Google Scholar]
- Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. (2013) Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med 19:322–328. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scott LV, Dinan TG. (2006) Antidepressant therapy and C-reactive protein levels. Br J Psychiatry 188:449–452. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW.(2009. a) Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol 182:3202–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C., Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. (2009. b) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65:263–267. [DOI] [PubMed] [Google Scholar]
- Pace TW, Miller AH. (2009) Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci 1179:86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo DL, Quadri SK. (1992) Interleukin-1 inhibits serotonin release from the hypothalamus in vitro. Life Sci 51:1797–1802. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. (2001) Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49:391–404. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. (1999) The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology 140:4359–4366. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. (2003) Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43:60–66. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. (2005) The cholinergic anti-inflammatory pathway. Brain Behav Immun 19:493–499. [DOI] [PubMed] [Google Scholar]
- Pavon L, Sandoval-Lopez G, Eugenia Hernandez M, Loria F, Estrada I, Perez M, Moreno J, Avila U, Leff P, Anton B, Heinze G. (2006) Th2 cytokine response in Major Depressive Disorder patients before treatment. J Neuroimmunol 172:156–165. [DOI] [PubMed] [Google Scholar]
- Pfeffer K. (2003) Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev 14:185–191. [DOI] [PubMed] [Google Scholar]
- Platzer C, Docke W, Volk H, Prosch S. (2000) Catecholamines trigger IL-10 release in acute systemic stress reaction by direct stimulation of its promoter/enhancer activity in monocytic cells. J Neuroimmunol 105:31–38. [DOI] [PubMed] [Google Scholar]
- Popmihajlov Z, Smith KA. (2008) Negative feedback regulation of T cells via interleukin-2 and FOXP3 reciprocity. PLoS One 3:e1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. (2005) Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 19:105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554–1565. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. (2001) Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 88:196–8, A7. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D. (2015) Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 21:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. (2001) Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58:445–452. [DOI] [PubMed] [Google Scholar]
- Reiner SL. (2007) Development in motion: helper T cells at work. Cell 129:33–36. [DOI] [PubMed] [Google Scholar]
- Romagnani S. (2000) T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol 85:9–18; quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- Ronaldson A, Gazali AM, Zalli A, Kaiser F, Thompson SJ, Henderson B, Steptoe A, Carvalho L. (2015) Increased percentages of regulatory T cells are associated with inflammatory and neuroendocrine responses to acute psychological stress and poorer health status in older men and women. Psychopharmacology (Berl). Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S, Kema IP, Bosker F, Haavik J, Korf J. (2009) Tryptophan as an evolutionarily conserved signal to brain serotonin: molecular evidence and psychiatric implications. World J Biol Psychiatry 10:258–268. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R.(2015. a) Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R.(2015. b) Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun 46:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. (1998) Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res 813:112–120. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, Grunze H, Lieb K. (2002) Interferon alpha (IFNalpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry 26:731–746. [DOI] [PubMed] [Google Scholar]
- Schewitz LP, Lee RW, Dayan CM, Dick AD. (2009) Glucocorticoids and the emerging importance of T cell subsets in steroid refractory diseases. Immunopharmacol Immunotoxicol 31:1–22. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, Stein M. (1984) Lymphocyte function in major depressive disorder. Arch Gen Psychiatry 41:484–486. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, Duman RS. (2011) Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36:2375–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. (1995) Cytokine production and serum proteins in depression. Scand J Immunol 41:534–538. [DOI] [PubMed] [Google Scholar]
- Seretti A, Cusin C, Lattuada E, Di Bella D, Catalano M, Smeraldi E. (1999) Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry 4:280–283. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AB. (2006) Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 62:123–146. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. (2000) Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A 97:5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. (2008) A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol 18:230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarczyk J, Trojan E, Glombik K, Budziszewska B, Kubera M, Lason W, Popiolek-Barczyk K, Mika J, Wedzony K, Basta-Kaim A. (2015) Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front Cell Neurosci 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. (1991) The macrophage theory of depression. Med Hypotheses 35:298–306. [DOI] [PubMed] [Google Scholar]
- Smoak KA, Cidlowski JA. (2004) Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev 125:697–706. [DOI] [PubMed] [Google Scholar]
- Sommer IE, de Witte L, Begemann M, Kahn RS. (2012) Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry 73:414–419. [DOI] [PubMed] [Google Scholar]
- Song C, Merali Z, Anisman H. (1999) Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 88:823–836. [DOI] [PubMed] [Google Scholar]
- Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. (2000) Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol 105:943–950. [DOI] [PubMed] [Google Scholar]
- Spulber S, Oprica M, Bartfai T, Winblad B, Schultzberg M. (2008) Blunted neurogenesis and gliosis due to transgenic overexpression of human soluble IL-1ra in the mouse. Eur J Neurosci 27:549–558. [DOI] [PubMed] [Google Scholar]
- Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N. (2014) Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int 2014:932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. (2003) The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med 65:362–368. [DOI] [PubMed] [Google Scholar]
- Suberville S, Bellocq A, Fouqueray B, Philippe C, Lantz O, Perez J, Baud L. (1996) Regulation of interleukin-10 production by beta-adrenergic agonists. Eur J Immunol 26:2601–2605. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Fan C, Perou CM. (2006) Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet 141B:261–268. [DOI] [PubMed] [Google Scholar]
- Sunde RA. (2010) mRNA transcripts as molecular biomarkers in medicine and nutrition. J Nutr Biochem 21:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. (2007) Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol 2007:76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenyi J. (2001) Cytokines and the central nervous system. Brain Res Bull 54:329–338. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. (2004) Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol 25:77–107. [DOI] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. (2008) Th17 cells in human disease. Immunol Rev 223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Chen CC, Bai CH, Wu SR. (2006) Cytokines and serotonin transporter in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 30:899–905. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT, Lin CF, Wu HT, Wu SR, Tsai WH. (2008) Interferon-alpha-induced serotonin uptake in Jurkat T cells via mitogen-activated protein kinase and transcriptional regulation of the serotonin transporter. J Psychopharmacol 22:753–760. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Nossova N, Yager T, Tsuang MM, Guo SC, Shyu KG, Glatt SJ, Liew CC. (2005) Assessing the validity of blood-based gene expression profiles for the classification of schizophrenia and bipolar disorder: a preliminary report. Am J Med Genet B Neuropsychiatr Genet 133B:1–5. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64:820–822. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Heijnen CJ, van de Schoot R, Amarouchi K, Maas M, Vermetten E, Geuze E, Kavelaars A. (2011) Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One 6:e29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veru F, Dancause K, Laplante DP, King S, Luheshi G. (2015) Prenatal maternal stress predicts reductions in CD4+ lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: Project Ice Storm. Physiol Behav 144:137–145. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Holsboer F. (1991) Effect of age on the cortisol response to human corticotropin-releasing hormone in depressed patients pretreated with dexamethasone. Biol Psychiatry 29:1042–1050. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller AH. (2004) Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry 9:65–75. [DOI] [PubMed] [Google Scholar]
- Webster JC, Oakley RH, Jewell CM, Cidlowski JA. (2001) Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A 98:6865–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. (2007) Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res 62:207–214. [DOI] [PubMed] [Google Scholar]
- Williams AC, Richardson PH. (1993) What does the BDI measure in chronic pain? Pain 55:259–266. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8:950–957. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. (2002) Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry 7:1115–1119. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. (2009) Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol 21:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31:2121–2131. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, Ising M. (2011) Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry 168:1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. (2001) The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 15:199–226. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. (2012) Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]