Abstract
Background:
There is a growing understanding that depression is associated with systemic inflammation. Statins and aspirin have anti-inflammatory properties. Given these agents have been shown to reduce the risk of a number of diseases characterized by inflammation, we aimed to determine whether a similar relationship exists for mood disorders (MD).
Methods:
This study examined data collected from 961 men (24–98 years) participating in the Geelong Osteoporosis Study. MD were identified using a semistructured clinical interview (SCID-I/NP). Anthropometry was measured and information on medication use and lifestyle factors was obtained via questionnaire. Two study designs were utilized: a nested case-control and a retrospective cohort study.
Results:
In the nested case-control study, exposure to statin and aspirin was documented for 9 of 142 (6.3%) cases and 234 of 795 (29.4%) controls (P < .001); after adjustment for age, exposure to these anti-inflammatory agents was associated with reduced likelihood of MD (OR 0.2, 95%CI 0.1–0.5). No effect modifiers or other confounders were identified. In the retrospective cohort study of 836 men, among the 210 exposed to statins or aspirin, 6 (2.9%) developed de novo MD during 1000 person-years of observation, whereas among 626 nonexposed, 34 (5.4%) developed de novo MD during 3071 person-years of observation. The hazard ratio for de novo MD associated with exposure to anti-inflammatory agents was 0.55 (95%CI 0.23–1.32).
Conclusions:
This study provides both cross-sectional and longitudinal evidence consistent with the hypothesis that statin and aspirin use is associated with a reduced risk of MD.
Keywords: Statins, aspirin, mood disorder, depression, cytokines, epidemiology, immune system, inflammation, prevention
While the underlying pathophysiology of mood disorders (MD) is not fully understood, there is now an extensive body of data showing they are associated with a chronic, low-grade inflammatory response (Berk et al., 2013a). Further support has come from studies of patient groups with immune-related diseases, with which depression is highly comorbid (Dantzer et al., 2008) and studies where infusion of cytokines robustly induces symptoms of depression (DellaGioia and Hannestad, 2010). Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) primarily used in the treatment of hypercholesterolemia and prevention of cardiovascular disease, and aspirin (acetylsalicylic acid), primarily used as an analgesic and/or antiplatelet agent, both have antiinflammatory properties (Dinarello, 2010). Given these agents are now known to reduce the risk of a number of diseases characterized by inflammation and our previous finding of an inverse association with depression in women (Pasco et al., 2010), we aimed to determine whether a similar relationship exists for men.
Data were derived from an age-stratified, random population-based sample of men enrolled in the Geelong Osteoporosis Study, a large, ongoing study located in southeastern Australia (Pasco et al., 2012). Further details of the study have been published elsewhere (Pasco et al., 2012). Briefly, between 2001 and 2006, 1540 men (response 67.0%) were recruited from the Australian Commonwealth electoral rolls for the Barwon Statistical Division. Of the 1540 participants, 978 returned for 5-year follow-up assessment between 2006 and 2011 (81.0% response for those alive and contactable). For the current analyses, participants for whom psychiatric data were not available (n=17) were excluded, resulting in a sample of 961 men aged 24 to 98 years. The study was approved by the Barwon Health Human Research Ethics Committee, and written informed consent was obtained from all participants.
The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Non-patient edition was conducted at the Geelong Osteoporosis Study 5-year follow-up and used to assess lifetime history of MD. Self-reported medication use was documented by questionnaires administered at each visit. Participants were asked to bring a list of medications or containers to assist with accurate recording of details. Anthropometry was measured and information on lifestyle factors was obtained via written questionnaire. Socio-economic status was ascertained using Socio-Economic Index For Areas index scores.
Two study designs were utilized: a nested case-control and a retrospective cohort study. The former identified ‘cases’ as subjects diagnosed with a MD and ‘controls’ were MD-free (ever). Exposure to statins and/or aspirin was recognized if exposure preceded the first-onset MD episode for cases and the 5-year assessment for controls. Multivariable logistic regression was used to calculate odds ratios (ORs) with 95% CI for de novo MD for those exposed to antiinflammatory agents in comparison with those not exposed. The latter design comprised participants without a history of MD at baseline who were followed from baseline until a first episode of MD or until the end of the 5-year follow-up period. The risk for de novo MD associated with exposure to antiinflammatory agents was examined using multivariable Cox proportional hazards regression models. Covariates including age, weight, height, alcohol, smoking, physical activity, socio-economic status, and use of antidepressants, sex hormones, nonsteroidal antiinflammatory drugs, other hyperlipidaemia agents, and other anticoagulants were tested as confounders or effect modifiers in each multivariable model.
Among 961 men, 165 were diagnosed with a MD (cases) and 796 had no history of MD (controls). Twenty-three cases were excluded due to participants having an episode of MD prior to exposure to either statins or aspirin, and one control was excluded because of an unknown medication status. Thus, 142 cases and 795 controls were considered. Participant characteristics are shown in Table 1. At the time of interview, 182 men (19.4%) were exposed to statins; 50 (27.5%) used Simvastatin, 87 (47.8%) used Atorvastatin, 28 (15.4%) used Pravastatin, and 17 (9.3%) used Rosuvastatin. Median duration of use was 6.3 (IQR 2.4–10.2) years. Exposure to statins was documented for 4 of 142 (2.8%) cases and 178 of 795 (22.4%) controls (P<.001); after adjustment for age, the OR for de novo MD was 0.1 (95%CI 0.1–0.4, P≤ .001).
Table 1.
Characteristics of MD Cases and Controls Included in the Nested Case-Control Study and Baseline Characteristics for Subjects in the Retrospective Cohort Study According to Exposure to the Antiinflammatory Agents Statins and Aspirin
| Case Control Study | Retrospective Cohort Study | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | P-value | Exposed | Not exposed | P-value | |
| n=142 | n=795 | n=210 | n=626 | |||
| Age, y | 50.4 (41.5–59.9) | 61.3 (47.0–75.9) | <.001 | 71.8 (62.7–77.5) | 49.5 (37.5–62.6) | <.001 |
| Height, cm | 176.9±6.7 | 174.6±7.3 | .001 | 172.1±6.9 | 175.9±6.9 | <.001 |
| Weight, kg | 83.7 (75.8–94.0) | 82.3 (74.0–92.4) | .216 | 81.7 (73.2–90.7) | 81.7 (73.9–91.3) | .854 |
| Alcohol, g/d | 17.8 (2.7–35.6) | 11.9 (2.2–28.3) | .047 | 12.1 (1.3–30.0) | 15.3 (3.0–29.8) | .064 |
| Smoking (current) | 22 (15.5%) | 88 (11.1%) | .131 | 7 (3.3%) | 92 (14.7%) | <.001 |
| Physical activity (active) | 103 (72.5%) | 570 (72.2%) | .925 | 164 (78.1%) | 512 (81.8%) | .239 |
| Socio-economic status | .864 | .008 | ||||
| Quintile 1 (most disadvantaged) | 22 (15.5%) | 130 (16.4%) | 42 (20.0%) | 96 (15.3%) | ||
| Quintile 2 | 25 (17.6%) | 167 (21.0%) | 58 (27.6%) | 115 (18.4%) | ||
| Quintile 3 | 28 (19.7%) | 153 (19.3%) | 36 (17.1%) | 125 (20.0%) | ||
| Quintile 4 | 31 (21.8%) | 168 (21.1%) | 34 (16.21%) | 141 (22.5%) | ||
| Quintile 5 | 36 (25.4%) | 177 (22.3%) | 40 (19.1%) | 149 (23.8%) | ||
| Medication use (current) | ||||||
| Antidepressants | 27 (19.0%) | 32 (4.0%) | <.001 | 12 (5.7%) | 13 (2.1%) | .007 |
| Hormone therapy | 1 (0.7%) | 5 (0.6%) | 1.000 | 7 (3.3%) | 7 (1.1%) | .030 |
| Nonsteroidal antiinflammatory drugs | 10 (7.0%) | 57 (7.2%) | .957 | 29 (13.8%) | 50 (8.0%) | .013 |
| Statins | 4 (2.8%) | 178 (22.4%) | <.001 | – | – | |
| Aspirin | 8 (5.6%) | 140 (17.6%) | <.001 | – | – | |
| Aspirin/statins (pooled) | 9 (6.3%) | 234 (29.4%) | <.001 | – | – | |
| MD (de novo) | – | – | 6 (2.9%) | 34 (5.4%) | .130 | |
Abbreviation: MD, mood disorder.
Values are given as mean (± SD), median (IQR), or n (%).
One hundred and forty-eight (15.8%) men had been exposed to aspirin. Median duration of use was 8.4 (3.9–13.0) years. Exposure to aspirin was documented for 8 of 142 (5.6%) cases and 140 of 795 (17.6%) controls (P≤ .001); after adjustment for age and antidepressant use, the OR for de novo MD was 0.4 (95%CI 0.2–0.9, P=0.03). When statin and aspirin users were pooled (n=243, 25.9%), exposure was documented for 9 of 142 (6.3%) cases and 234 of 795 (29.4%) controls (P<.001). Following adjustment for age, exposure to these antiinflammatory agents was associated with reduced risk of de novo MD (OR=0.2, 95%CI 0.1–0.5, P<.001). For all models, no effect modifiers or other confounders were identified.
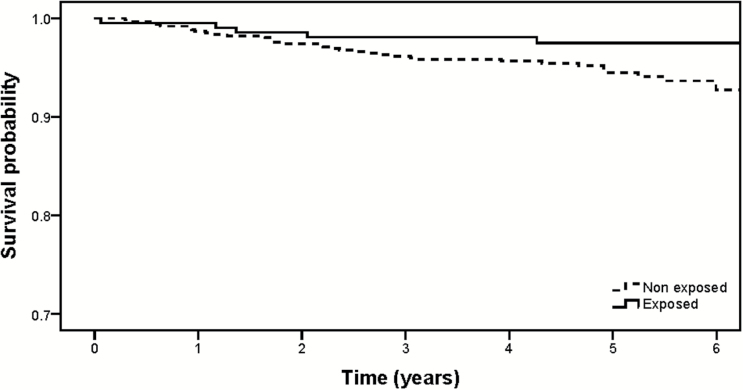
In the retrospective cohort study of 836 men, 210 had been exposed to statins (n=141; 39 [27.7%] used Simvastatin, 60 [42.6%] used Atorvastatin, 37 [26.2%] used Pravastatin, 2 [1.4%] used Rosuvastatin, 2 [14%] used Fluvastain, and 1 [0.7%] was unknown) or aspirin (n=137). Participant characteristics are shown in Table 1. Among the 210 exposed men, 6 (2.9%) developed de novo MD during 1000 person-years of observation, whereas among 626 nonexposed, 34 (5.4%) developed de novo MD during 3071 person-years of observation. The hazard ratio for de novo MD associated with exposure to antiinflammatory agents was 0.55 (95%CI 0.23–1.32, P=.18). A Kaplan-Meier survival plot (Figure 1) shows the probability of remaining free of de novo MD for men exposed and not exposed to the antiinflammatory agents statins and aspirin.
Figure 1.
Survival plot (Kaplan-Meier) showing the probability of remaining free of de novo mood disorder (MD) for men exposed and not exposed to the antiinflammatory agents statins and aspirin.
This study thus provides both cross-sectional and longitudinal evidence consistent with the hypothesis that statin and aspirin use is associated with a reduced risk of MD. Our findings are consistent with both observational and clinical trials of healthy and cardiac populations in suggesting mood-related benefits are associated with statin and aspirin treatment (O’Neil et al., 2012; Berk et al., 2013b). Conversely, others have reported negative or no association (Hyyppa et al., 2003). Given the known antiinflammatory properties of statins and aspirin and the extensive body of evidence to suggest that depression is associated with a chronic, low-grade inflammatory response (Berk et al., 2013a) and oxidative and nitrosative stress (Moylan et al., 2014), a reduction in inflammation is a likely underlying explanatory mechanism.
There are several strengths and weaknesses that need to be taken into consideration. Our ability to incorporate both cross-sectional and longitudinal study designs and identify depression by gold-standard clinical assessment are key strengths, as is our ability to test several potential confounding factors. In regards to limitations, recall bias is possible, as is residual or unrecognized confounding. Furthermore, power limitations prevented subgroup analyses investigating severity and duration of MD, dose, duration, or individual agent effects and are likely to have limited our ability to detect significant differences in the retrospective cohort study given the large effect size.
In conclusion, these data augment the emerging evidence-base suggesting that statins and/or aspirin may play a protective role in the development of depression. Well-conducted, randomized controlled trials investigating the potential of these agents in the treatment and prevention of MDs are now needed.
Statements of Interest
L.J.W. has received Grant/Research support from Eli Lilly, Pfizer, The University of Melbourne, Deakin University and the NHMRC. J.A.P. has received grant/research support from the NHMRC, BUPA Foundation, Deakin University, Barwon Health and the Western Alliance. F.N.J. has received Grant/Research support from the Brain and Behaviour Research Institute, the NHMRC, Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, Eli Lilly, the Meat and Livestock Board, and The University of Melbourne and has been a paid speaker for Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed, Network Nutrition, Angelini Farmaceutica, and Eli Lilly. M.B. has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier, and Woolworths, and has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay, and Wyeth, and served as a consultant to Astra Zeneca, Bioadvantex, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck Merck, and Servier. A.O. has received has received an honorarium from Novartis Pharmaceuticals and grant funding from Meat and Livestock, Australia. M.M., A.L.S., and K.V. have no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC), Australia (projects 299831, 628582, 1026265, and 1021345). L.J.W. is supported by a NHMRC Career Development Fellowship (1064272). A.O. is supported by a NHMRC Early Career Fellowship (1052865). M.B. is supported by a NHMRC Senior Principal Research Fellowship (1059660). The funding providers played no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or in preparation, review, or approval of the manuscript.
References
- Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, Maes M. (2013. a) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, O’Neil A, Davey CG, Sanna L, Maes M. (2013. b) Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. (2010) A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev 34:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. (2010) Anti-inflammatory agents: present and future. Cell 140:935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyyppa MT, Kronholm E, Virtanen A, Leino A, Jula A. (2003) Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 28:181–194. [DOI] [PubMed] [Google Scholar]
- Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, Hayley AC, Pasco JA, Anderson G, Jacka FN, Maes M. (2014) Oxidative and nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45:46–62. [DOI] [PubMed] [Google Scholar]
- O’Neil A, Sanna L, Redlich C, Sanderson K, Jacka F, Williams LJ, Pasco JA, Berk M. (2012) The impact of statins on psychological wellbeing: a systematic review and meta-analysis. BMC Med 10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, Berk M. (2010) Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom 79:323–325. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Nicholson GC, Kotowicz MA. (2012) Cohort profile: Geelong Osteoporosis Study. Int J Epidemiol 41:1565–1575. [DOI] [PubMed] [Google Scholar]