Abstract
Background:
Low circulating brain derived neurotrophic factor may promote cognitive deterioration, but the effects of neurotrophic and combination drug therapies on serum brain derived neurotrophic factor were not previously investigated in Alzheimer’s disease.
Methods:
We evaluated the effects of Cerebrolysin, donepezil, and the combined therapy on brain derived neurotrophic factor serum levels at week 16 (end of Cerebrolysin treatment) and week 28 (endpoint) in mild-to-moderate Alzheimer’s disease patients.
Results:
Cerebrolysin, but not donepezil, increased serum brain derived neurotrophic factor at week 16, while the combination therapy enhanced it at both week 16 and study endpoint. Brain derived neurotrophic factor responses were significantly higher in the combination therapy group than in donepezil and Cerebrolysin groups at week 16 and week 28, respectively. Brain derived neurotrophic factor increases were greater in apolipoprotein E epsilon-4 allele carriers, and higher brain derived neurotrophic factor levels were associated with better cognitive improvements in apolipoprotein E epsilon-4 allele patients treated with Cerebrolysin and the combined therapy.
Conclusion:
Our results indicate a synergistic action of Cerebrolysin and donepezil to increase serum brain derived neurotrophic factor and delaying cognitive decline, particularly in Alzheimer’s disease cases with apolipoprotein E epsilon-4 allele.
Keywords: Alzheimer’s disease, brain derived neurotrophic factor, Cerebrolysin, combination therapy, apolipoprotein E epsilon-4 allele
Introduction
Reduced brain and cerebrospinal fluid levels of brain derived neurotrophic factor (BDNF) were found to be associated with cognitive deficits in Alzheimer’s disease (AD) patients (Peng et al., 2005; Ginsberg et al., 2006). We recently reported reduced levels of BDNF serum levels in AD patients with apathy and in female AD patients carrying the apolipoprotein E epsilon-4 allele (ApoE4). Interestingly, these patients showed worse cognitive performance than their respective comparison groups, which suggests a negative impact of low circulating BDNF on cognitive functioning at least in these subgroups of AD patients (Alvarez et al., 2014). Although investigations on the influence of peripheral BDNF on cognitive functions in AD patients are inconclusive, recent clinical findings showing that higher BDNF serum levels are associated with a lower risk of developing AD (Weinstein et al., 2014) and that aerobic exercise enhances BDNF plasma levels in AD patients (Coelho et al., 2014), together with experimental evidence indicating that circulating BDNF reflects brain-tissue BDNF (Klein et al., 2011) and activates molecular and cellular substrates relevant for hippocampal functions (Schmidt and Duman, 2010), seem to support the hypothesis that interventions that enhance peripheral BDNF signalling could represent an effective option for delaying AD onset and/or cognitive decline.
Cerebrolysin is a peptidergic drug that demonstrated clinical efficacy in mild to moderate AD patients (Alvarez et al., 2011; Gauthier et al., 2015). This drug contains low-molecular weight peptides and free amino acids and displays neurotrophic properties in experimental and clinical conditions (Alvarez et al., 2011). Cerebrolysin enhanced the levels of insulin-like growth factor-1 (IGF-1) in the sera of AD patients and of diabetic rats (Alvarez et al., 2009; Georgy et al., 2013), increased the maturation of nerve growth factor and the survival of cholinergic neurons in the brain of a transgenic mouse model of AD (Ubhi et al., 2013), and reversed the decrease of serum BDNF in a rat model of Parkinson’s disease (Ahmed et al., 2014).
Taking into consideration that Cerebrolysin reduced the activity of the glycogen synthase kinase-3 beta (GSK3beta) in the brain of hAPP transgenic mice (Rockenstein et al., 2006) and that lithium, a GSK3beta inhibitor, enhanced serum BDNF levels in AD (Leyhe et al., 2009), we hypothesized an increase of serum BDNF in AD patients after treatment with Cerebrolysin. In addition, Cerebrolysin might enhance the BDNF serum increase found in AD patients after chronic treatment with the cholinergic drug donepezil (Leyhe et al., 2008). Thus, in the present study we investigated changes in BDNF serum levels and its correlations with cognitive and clinical responses in AD patients treated with Cerebrolysin, donepezil, or a combination of both drugs in a randomized, double-blind, clinical trial (RCT) (Alvarez et al., 2011).
Methods
The present investigation was conducted in 158 patients with mild-to-moderate probable AD included in a RCT (clinicaltrials.gov number NCT00911807) that completed the study and were suitable for the per-protocol analysis. Selection of study participants followed inclusion and exclusion criteria as published previously (Alvarez et al., 2011) and required a Mini-Mental State Examination score between 12 and 25 and the exclusion of clinically significant depression as defined by medical evaluation and/or scores >15 in the 17-item subscale of the Hamilton Depression Scale. Written informed consent was obtained from all patients and caregivers before starting study procedures. This trial was conducted in accordance with the last version of the Declaration of Helsinki and with Spanish and European Union regulations and was reviewed by Independent Ethics Committees of the 3 participating sites.
Study characteristics of this RCT were previously described (Alvarez et al., 2011), and patients were treated with either: (1) Cerebrolysin (n=65) (10mL; 5 i.v. infusions per week during weeks 1–4 and 13–16) plus placebo tablets (once daily, for 28 weeks); (2) donepezil (n=68) (5-mg tablets during weeks 1–4, and 10-mg tablets during weeks 5–28; once daily) plus 40 i.v. placebo infusions (saline; weeks 1–4 and 13–16); or (3) a combined therapy (n=67) with Cerebrolysin (10mL; 40 i.v. infusions as described above) plus donepezil (5mg on week 1–4, and 10mg thereafter; once daily).
Blood samples for laboratory determinations were obtained at baseline, week 16 (end of the period of treatment with Cerebrolysin), and week 28 (study endpoint). Clinical evaluations were done at the same time points by using the AD Assessment Scale-cognitive subscale+ (ADAS-cog+), the Clinical Interview Based Impression of Severity with Caregiver Input, and the Neuropsychiatric Inventory (NPI) as previously described (Alvarez et al., 2011). Apathy (apathy/indifference) and depression (depression/dysphoria) items are scored in the NPI according to the frequency (1–4, occasionally to very frequently) and severity (1–3, mild to severe) of the symptoms. Total apathy and depression scores (frequency x severity) range from 0 to 12 points, and NPI apathy-depression composite scores were obtained by adding the scores of both items (range: 0–24 points). The maximum sum score for the NPI-12 items is 144.
A butterfly-21 INT (Venisystems, Abbott Ireland Ltd., Sligo, Ireland) was inserted into the antecubital vein, and blood samples were taken during the morning using evacuated blood collecting tubes (Venojet, Terumo Europe N.V., Leuven, Belgium). Serum samples were then extracted and stored at -40°C until assays. Serum BDNF levels were measured by using specific enzyme-linked immunosorbent assay kits as in previous studies (Alvarez et al., 2014).
BDNF, IGF-1, platelet count, and ADAS-cg+ data followed a normal distribution (Kolmogorov-Smirnov test), but NPI apathy-depression composite scores did not (1-sample chi-square test). Paired t test and the Wilcoxon test were used for parametric and nonparametric comparisons of paired samples, respectively. Group comparisons were done by chi-square and ANOVA analyses as appropriate. Treatment differences in BDNF responses at weeks 16 and 28 were analysed by ANCOVA using scores of BDNF change from baseline as dependent variable and baseline BDNF levels as covariate with appropriate corrections for age, platelet count, gender, disease severity, (Clinical Interview Based Impression of Severity with Caregiver Input), ApoE4 status (epsilon-4 allele present or absent), treatment with selective serotonin reuptake inhibitors (yes, no), and NPI apathy-depression symptoms (present, absent). Correlations were analyzed by using the Pearson’s lineal correlation test. P < .05 was considered statistically significant.
Results and Discussion
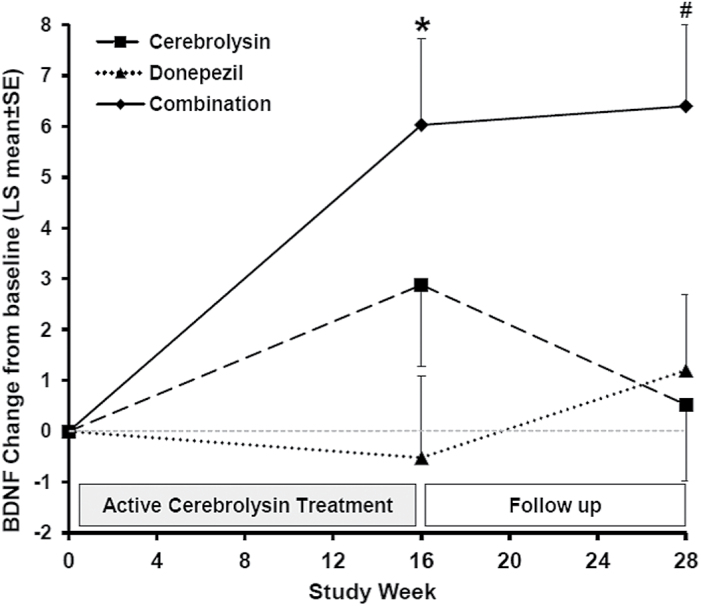
Baseline serum BDNF concentrations and clinical characteristics were similar in all 3 treatment groups (Table 1). A significant increase of serum BDNF values with respect to baseline was observed at week 16 in patients treated with Cerebrolysin alone or in combination with donepezil (P=.023 and P=.007, respectively), but not in patients receiving only donepezil (Table 1). The rise of BDNF was still significant (P=.028) at week 28 in the group of patients treated with the combined therapy, but not in patients on Cerebrolysin monotherapy (Table 1). BDNF changes induced by the combination therapy were significantly higher than those observed in the donepezil (P=.023) and Cerebrolysin (P=.035) groups at weeks 16 and 28, respectively (Figure 1). These results indicate that the significant increase of circulating BDNF induced by Cerebrolysin vanished 12 weeks after stopping its administration and that the concomitant treatment with donepezil augments and prolongs the BDNF response to Cerebrolysin. The effects of Cerebrolysin on peripheral BDNF were not previously investigated, but our results are consistent with the enhanced production of BDNF induced by the drug in brain grafts of neural stem cells (Rockenstein et al., 2015) and in the serum of a Parkinson’s disease rat model (Ahmed et al., 2014). An increase of BDNF serum levels was reported after 15-month treatment with donepezil in a sample of 19 AD patients (Leyhe et al., 2008), but donepezil showed no effect on the decline of serum BDNF levels observed over 2 years in subjects with remitted late-life depression and mild cognitive impairment (Diniz et al., 2014).
Table 1.
Effects of Cerebrolysin, Donepezil, and the Combined Therapy on BDNF Serum Levels in AD Patients: Results and Clinical Characteristics of the Study Groups
| Cerebrolysin | Donepezil | Combined Therapy | |
|---|---|---|---|
| All patients (N) | 52 | 52 | 53# |
| N (%) | N (%) | N (%) | |
| Female gender | 38 (73.1) | 40 (76.9) | 43 (81.1) |
| APOE ε4 allele | 24 (46.2) | 24 (46.2) | 21 (39.6) |
| NPI apathy-depression | 39 (75.0%) | 41 (78.8%) | 43 (81.1%) |
| SSRI treatment | 19 (36.5%) | 18 (34.6%) | 17 (32.1%) |
| Mean±SD | Mean±SD | Mean±SD | |
| Age (y) | 74.65±6.65 | 75.50±7.43 | 72.89±8.13 |
| MMSE (score) | 17.27±4.25 | 17.46±4.27 | 17.75±4.67 |
| NPI apathy-depression (score) | 5.33±4.94 | 4.10±4.16 | 3.75±3.64 |
| Free IGF-1 (ng/mL) | 1.05±0.35 | 0.88±0.25 | 0.96±0.31 |
| Platelets (x109/L) | 229,00±68.01 | 223,79±43.76 | 223,13±57.72 |
| Baseline BDNF (ng/mL) | 13.76±8.55 | 16.62±9.10 | 15.40±10.67 |
| Week 16 BDNF (ng/mL) | 16.27±9.37* | 16.61±10.25 | 19.50±12.15** |
| Week 28 BDNF (ng/mL) | 15.31±9.97 | 17.79±9.89 | 19.07±10.64* |
| Baseline ADAS-cog+ (score) | 41.15±15.55 | 40.51±16.21 | 39.79±17.89 |
| Week 16 ADAS-cog+ (score) | 37.57±16.94** | 37.22±17.33** | 36.02±19.39** |
| Week 28 ADAS-cog+ (score) | 39.47±17.79 | 39.78±18.29 | 37.68±19.33* |
| ApoE4 patients (N) | 24 | 24 | 21 |
| Baseline BDNF (ng/mL) | 10.39±7.08 | 15.24±8.52 | 13.04±9.60 |
| Week 16 BDNF (ng/mL) | 14.67±9.05** | 17.11±8.79 | 19.26±12.55* |
| Week 28 BDNF (ng/mL) | 15.04±10.25** | 18.88±16.56 | 19.61±13.12* |
| Baseline ADAS-cog+ (score) | 43.49±12.97 | 43.94±16.49 | 33.30±12.50 |
| Week 16 ADAS-cog+ (score) | 39.41±15.46* | 42.04±17.04* | 29.04±13.35** |
| Week 28 ADAS-cog+ (score) | 41.76±16.71 | 43.89±17.66 | 31.12±14.10 |
Abbreviations: AD, Alzheimer’s disease; APOE, apolipoprotein E; ADAS-cog+, AD Assessment Scale-cognitive subscale+, BDNF, brain derived neurotrophic factor; IGF-I, insulin-like growth factor-1; MMSE, Mini-Mental State Examination; NPI, neuropsychiatric inventory; SSRI, selective serotonin reuptake inhibitor.
Only samples from patients suitable for the per-protocol analysis data set were assessed.
#Samples were not available for 1 case in the combined therapy group.
*P<.05, **P<.01 vs baseline BDNF levels and baseline ADAS-cog+ scores (paired t test).
Figure 1.
Changes from baseline in brain derived neurotrophic factor (BDNF) serum levels in Alzheimer’s disease (AD) patients treated with Cerebrolysin, donepezil, or a combination of both drugs. LS mean±SE. *P<.05 vs donepezil, and #P<.05 vs Cerebrolysin.
The specific mechanisms through which Cerebrolysin enhances circulating BDNF and donepezil potentiates this effect have to be elucidated, but there is experimental and clinical evidence suggesting that these BDNF responses might be mediated by signaling pathways activated upon GSK3beta inhibition and stimulation of alpha7 nicotinic acetylcholine receptors by Cerebrolysin and donepezil, respectively. Cerebrolysin inhibits GSK3beta as demonstrated in different experimental conditions (Rockenstein et al., 2006; Hartwig et al., 2014), and this inhibition was found to be relevant for the neuroprotective effects of the drug and might account, at least in part, for the increase of serum BDNF induced by Cerebrolysin in AD patients. Inhibition of GSK3beta induced by activation of the phosphoinositide 3 kinase (PI3K)/Akt pathway or by inhibitors such as lithium was shown to enhance BDNF expression in experimental models and AD patients (Leyhe et al., 2009). Given the fact that some protective effects of donepezil against neuronal death are mediated by upregulation of alpha7 nicotinic acetylcholine receptors and inhibition of GSK3beta subsequent to PI3K/Akt activation (Noh et al., 2013), donepezil could potentiate the increase of BDNF in response to Cerebrolysin by inducing additional GSK3beta inhibition through stimulation of such receptors and PI3K/Akt signaling.
Elevations of peripheral IGF-1 found in AD patients after Cerebrolysin treatment (Alvarez et al., 2009) might also contribute to the increase of serum BDNF levels induced by Cerebrolysin and combination therapy, as it was demonstrated for antidepressants and exercise (Chen and Russo-Neustadt, 2007). Interestingly, although none of the treatment options tested in the present study increased free IGF-1 serum levels significantly (data not shown), we found a significant positive correlation between scores of change in BDNF and free IGF-1 from baseline to week 28 in the combined therapy group (r=0.320; P=.021) and in the sample of all patients treated with Cerebrolysin (r=0.238; P=.015), but not in the group of patients treated with donepezil alone. These results confirm an interaction for the effects of Cerebrolysin on the serum levels of IGF-1 and BDNF in AD patients, but the extent to which changes in each of these neurotrophic factors may influence the other appears rather limited.
Changes in BDNF levels at week 16 and week 28 correlated negatively with baseline BDNF values in the total study population (r=-0.444 and r=-0.488, respectively; P<.001), and these correlations were significant in each of the treatment groups. Overall, BDNF levels and scores of change from baseline showed no significant correlations with measures of clinical efficacy, including cognitive performance (ADAS-cog+), neuropsychiatric symptoms (NPI), and global function (CIBIC+). However, despite that no treatment differences were found for cognition in this trial (Alvarez et al., 2011), significant increases in BDNF levels were accompanied by significant cognitive improvements in Cerebrolysin-treated patients at week 16 and in the combined therapy group at weeks 16 and 28 (Table 1). In addition, in the subgroup of ApoE4 patients, baseline BDNF levels correlated negatively with scores of ADAS-cog+ change from baseline to week 16 and week 28 in patients receiving Cerebrolysin (r=-0.408 and r=-0.400, respectively; P=.005 and P=.006), but were not significant in the group of patients treated with donepezil alone. The present results indicate that ApoE4 patients with higher BDNF levels at baseline experienced greater cognitive improvements (negative scores of ADAS-cog+ change denote cognitive improvement) at the end of the Cerebrolysin treatment period (week 16) and at endpoint (week 28). Since baseline BDNF concentrations were reduced and BDNF responses were enhanced in ApoE4 patients compared with no-ApoE4 cases of all treatment groups (Table 1), it is suggested that drug-induced increases in BDNF may also contribute to improvements in cognition. In fact, enhanced BDNF levels at week 16 were associated with greater cognitive improvements at the same time point (r=-0.406; P=.006) and predicted a better cognitive performance at endpoint too (r=-0.410; P=.005) in ApoE4 patients treated with Cerebrolysin or the combination therapy. In these patients, BDNF increases at week 16 were 2 to 3 times larger and ADAS-cog+ improvements at 16 and 28 weeks were 1.7 to 2.4 points higher than in patients receiving donepezil alone (Table 1). Taken together, our results suggest the influence of ApoE4 on BDNF metabolism are in support of a preventive role of BDNF for delaying cognitive decline and point to the involvement of BDNF in the effects of Cerebrolysin on cognition, at least in AD cases with ApoE4. In this regard, it is worth noting the parallelism that exists between the BDNF responses described here (Table 1, Figure 1) and the combined responder rates (improvements in both ADAS-cog+ and CIBIC+) previously reported (Alvarez et al., 2011), which were similar at week 16 and at endpoint in the combination therapy group (38.8% vs 37.3%) but declined over time in patients on monotherapy either with Cerebrolysin (42.8% vs 31.3%) or donepezil (28.8% vs 21.2%). These results suggest a synergistic effect of the combined therapy to enhance circulating BDNF and delay the clinical deterioration in AD patients.
Our present findings are in agreement with results of previous studies showing that higher BDNF serum levels were associated with higher Mini-Mental State Examination scores and with a slower rate of 1-year cognitive decline in AD patients, as well as with a reduced 10-year incidence of dementia and AD in the Framingham Heart Study participants (Lee et al., 2009; Laske et al., 2011; Weinstein et al., 2014). The recent report of enhanced peripheral BDNF levels and improved cognitive performance after physical training in MCI patients (Nascimento et al., 2014) is also in support of the associations we found for BDNF and cognitive responses induced by treatment. The present study does not allow us to explain either the mechanisms through which increases in serum BDNF might influence cognition or why the correlations of BDNF with cognitive improvement were present in only ApoE4 patients. Experimental results showing that peripheral/serum BDNF enhances neurogenesis, as well as the expression of BDNF and signaling molecules in the hippocampus of adult mice (Schmidt and Duman, 2010), provide evidence of some potential mechanisms that might account for the positive correlations of serum BDNF and cognitive performance observed in our investigation. On the other hand, the recent finding that some BDNF genetic variants were significantly associated with measures of cognitive decline and hippocampal and/or whole brain atrophy in AD patients (Honea et al., 2013) suggests that interactions of BDNF polymorphisms and ApoE4 might account to some extent for the associations of BDNF and cognition reported here and deserve future investigation.
In summary, results of the present investigation indicate that: (1) Cerebrolysin increases serum BDNF levels in AD patients and the combined therapy with donepezil augments and prolongs this effect; (2) changes in BDNF and IGF-1 induced by Cerebrolysin and the combination therapy at endpoint are related; (3) treatment-induced BDNF responses were higher in ApoE4 carriers; and (4) higher BDNF levels at baseline and week 16 are associated with and predict a better cognitive improvement in ApoE4 patients treated with Cerebrolysin. Future studies are warranted to confirm our findings and overcome limitations of this trial regarding the lack of a placebo-control group, the reduced sample size, and the exclusion of patients with clinically significant depression and prominent neuropsychiatric symptoms. The effects of Cerebrolysin on platelet GSK3beta activity and the potential influence of the interactions of BDNF and APOE polymorphisms on BDNF and cognitive responses are topics to be further investigated in AD patients.
Statement of Interest
The authors declare no conflicts of interest.
X.A. Alvarez was principal investigator in clinical trials and other research projects granted by EVER Neuro Pharma GmbH, a member of EVER scientific advisory board for the EVE-AT-0412 trial, and a speaker in EVER Neuro Pharma GmbH-sponsored symposia. S. Winter is employee of EVER NeuroPharma GmbH. D.F. Muresanu was principal investigator in several clinical trials with Cerebrolysin and is member of the CAPTAIN trial scientific advisory board. H. Moessler is employee of EVER NeuroPharma GmbH. The other authors (I.A., O.I., I.C., J.F., M.A., C.L., E.G., M.G.-F., J.M., E.M.) did not receive from EVER Neuro Pharma GmbH any financial support for activities other than those directly related to the conduction and communication of research studies. No honorarium was received to write this manuscript.
Acknowledgments
The authors thank Fundación Antidemencia Al-Andalus (Córdoba, Spain), and Ever NeuroPharma (Unterach, Austria) for their support with Research Grants for the conduction of the present investigation. X.A.A. also wants to acknowledge the support of the Sixth Framework Programme of the European Union (LSHB-CT-2006–037702).
This work was supported by Research Grants from the Fundación Antidemencia Al-Andalus (Córdoba, Spain), and from Ever NeuroPharma (Unterach, Austria). Sponsors were involved in study design and were aware of the decision to submit the article for publication.
References
- Ahmed H, Salem A, Atta H, Ghazy M, Aglan H. (2014) Do adipose tissue-derived mesenchymal stem cells ameliorate Parkinson’s disease in rat model? Hum Exp Toxicol 33:1217–1231. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Sampedro C, Cacabelos R, Linares C, Aleixandre M, García-Fantini M, Moessler H. (2009) Reduced TNF-α and increased IGF-I levels in the serum of Alzheimer’s disease patients treated with the neurotrophic agent cerebrolysin. Int J Neuropsychopharmacol 12:867–872. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Cacabelos R, Sampedro C, Couceiro V, Aleixandre M, Vargas M, Linares C, Granizo E, García-Fantini M, Baurecht W, Doppler E, Moessler H. (2011) Combination treatment in Alzheimer’s disease: results of a randomized, controlled trial with cerebrolysin and donepezil. Curr Alzheimer Res 8:583–591. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Aleixandre M, Linares C, Masliah E, Moessler H. (2014) Apathy and APOE4 are associated with reduced BDNF levels in Alzheimer’s disease. J Alzheimers Dis 42:1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. (2007) Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors 25:118–131. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodorov E, Santos-Galduróz RF. (2014) Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer’s disease. J Alzheimers Dis 39:401–408. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Reynolds CF, 3rd, Begley A, Dew MA, Anderson SJ, Lotrich F, Erickson KI, Lopez O, Aizenstein H, Sibille EL, Butters MA. (2014) Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. J Psychiatr Res 49:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Proaño JV, Jia J, Froelich L, Vester JC, Doppler E. (2015) Cerebrolysin in mild-to-moderate alzheimer’s disease: a meta-analysis of randomized controlled clinical trials. Dement Geriatr Cogn Disord 39:332–347. [DOI] [PubMed] [Google Scholar]
- Georgy GS, Nassar NN, Mansour HA, Abdallah DM. (2013) Cerebrolysin ameloriates cognitive deficits in type III diabetic rats. PLoS One 8:e64847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Wuu J, Counts SE, Mufson EJ. (2006) Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer′s disease. J Neurochem 97:475–487. [DOI] [PubMed] [Google Scholar]
- Hartwig K, Fackler V, Jaksch-Bogensperger H, Winter S, Furtner T, Couillard-Despres S, Meier D, Moessler H, Aigner L. (2014) Cerebrolysin protects PC12 cells from CoCl(2)-induced hypoxia employing GSK3β signaling. Int J Dev Neurosci 38:52–58. [DOI] [PubMed] [Google Scholar]
- Honea RA, Cruchaga C, Perea RD, Saykin AJ, Burns JM, Weinberger DR, Goate AM. (2013) Characterizing the role of brain derived neurotrophic factor genetic variation in Alzheimer’s disease neurodegeneration. PLoS One 8:e76001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14:347–353. [DOI] [PubMed] [Google Scholar]
- Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T. (2011) Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int J Neuropsychopharmacol 14:399–404. [DOI] [PubMed] [Google Scholar]
- Lee JG, Shin BS, You YS, Kim JE, Yoon SW, Jeon DW, Baek JH, Park SW, Kim YH. (2009) Decreased serum brain-derived neurotrophic factor levels in elderly Korean with dementia. Psychiatry Investig 6:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. (2008) Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 258:124–128. [DOI] [PubMed] [Google Scholar]
- Leyhe T, Eschweiler GW, Stransky E, Gasser T, Annas P, Basun H, Laske C. (2009) Increase of BDNF serum concentration in lithium treated patients with early Alzheimer’s disease. J Alzheimers Dis 16:649–656. [DOI] [PubMed] [Google Scholar]
- Nascimento CM, Pereira JR, de Andrade LP, Garuffi M, Talib LL, Forlenza OV, Cancela JM, Cominetti MR, Stella F. (2014) Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res 11:799–805. [DOI] [PubMed] [Google Scholar]
- Noh MY, Koh SH, Kim SM, Maurice T, Ku SK, Kim SH. (2013) Neuroprotective effects of donepezil against Aβ42-induced neuronal toxicity are mediated through not only enhancing PP2A activity but also regulating GSK-3β and nAChRs activity. J Neurochem 127:562–574. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. (2005) Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem 93:1412–1421. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. (2006) Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. J Neurosci Res 83:1252–1261. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Desplats P, Ubhi K, Mante M, Florio J, Adame A, Winter S, Brandstaetter H, Meier D, Masliah E. (2015) Neuro-peptide treatment with Cerebrolysin improves the survival of neural stem cell grafts in an APP transgenic model of Alzheimer disease. Stem Cell Res 15:54–67. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. (2010) Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35:2378–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Vazquez-Roque R, Mante M, Inglis C, Patrick C, Adame A, Fahnestock M, Doppler E, Novak P, Moessler H, Masliah E. (2013) Cerebrolysin modulates pronerve growth factor/nerve growth factor ratio and ameliorates the cholinergic deficit in a transgenic model of Alzheimer’s disease. J Neurosci Res 91:167–177. [DOI] [PubMed] [Google Scholar]
- Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S. (2014) Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol 71:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]