Abstract
Background: Crops such as maize, sorghum, and millet are being biofortified with provitamin A carotenoids to ensure adequate vitamin A (VA) intakes. VA assessment can be challenging because serum retinol concentrations are homeostatically controlled and more sensitive techniques are resource-intensive.
Objectives: We investigated changes in serum retinol relative differences of isotope amount ratios of 13C/12C (δ13C) caused by natural 13C fractionation in C3 compared with C4 plants as a biomarker to detect provitamin A efficacy from biofortified (orange) maize and high-carotene carrots.
Methods: The design was a 2 × 2 × 2 maize (orange compared with white) by carrot (orange compared with white) by a VA fortificant (VA+ compared with VA−) in weanling male Mongolian gerbils (n = 55), which included a 14-d VA depletion period and a 62-d treatment period (1 baseline and 8 treatment groups; n = 5−7/group). Liver VA and serum retinol were quantified, purified by HPLC, and analyzed by GC combustion isotope ratio mass spectrometry for 13C.
Results: Treatments affected liver VA concentrations (0.048 ± 0.039 to 0.79 ± 0.24 μmol/g; P < 0.0001) but not overall serum retinol concentrations (1.38 ± 0.22 μmol/L). Serum retinol and liver VA δ13C were significantly correlated (R2 = 0.92; P < 0.0001). Serum retinol δ13C differentiated control groups that consumed white maize and white carrots (−27.1 ± 1.2 δ13C‰) from treated groups that consumed orange maize and white carrots (−21.6 ± 1.4 δ13C‰ P < 0.0001) and white maize and orange carrots (−30.6 ± 0.7 δ13C‰ P < 0.0001). A prediction model demonstrated the relative contribution of orange maize to total dietary VA for groups that consumed VA from mixed sources.
Conclusions: Provitamin A efficacy and quantitative estimation of the relative contribution to dietary VA were demonstrated with the use of serum retinol δ13C. This method could be used for maize efficacy or effectiveness studies and with other C4 crops biofortified with provitamin A carotenoids (e.g., millet, sorghum). Advantages include no extrinsic tracer dose, 1 blood sample, and higher sensitivity than serum retinol concentrations alone.
Keywords: biofortification, stable carbon isotope, vitamin A+, carrot, GCCIRMS, vitamin A effectiveness, vitamin A efficacy
Introduction
Biofortifying staple and horticultural foods with provitamin A carotenoids can sustainably ensure adequate vitamin A (VA)8 intakes (1) and mitigate potential hypervitaminosis A risks caused by preformed VA in high-dose supplements and fortified foods (2–4). The bioefficacy of high provitamin A (orange) maize, defined as the production of retinol from consumed provitamin A carotenoids (5), has been demonstrated in gerbil studies (6, 7) and single-meal feeding studies in humans (8, 9). To evaluate health-promoting interventions in humans, efficacy and effectiveness trials are conducted. Efficacy trials are characterized by ideal circumstances that maximize the likelihood of observing a treatment effect: a selected homogeneous population, standardized intervention, and experienced providers or study facilitators. Effectiveness trials are characterized by real-world circumstances designed to determine whether the intervention works as actually used or adopted: a broad heterogeneous population, less standardized treatment protocols, and usual providers (10, 11). A human bioefficacy study determined that orange maize (OM) is an efficacious VA source in children (3), but to our knowledge effectiveness trials have not yet been carried out.
Liver VA concentration is the gold standard for evaluating VA status (12); however, this is only feasible in animal studies or in special cases in humans. Serum retinol concentrations are homeostatically controlled over a wide range of liver reserves (12–14), are affected by infection and inflammation (15, 16), and are nonsensitive indicators of changes in VA status (12). Furthermore, several indicators used for VA assessment, such as serum retinol and dose-response tests, are qualitative because they only distinguish deficiency from adequacy. Studies performed for provitamin A-biofortified maize in humans have used multiple blood draws for postprandial response (9) or stable isotope methods with intrinsically labeled maize (8) and tracer VA doses for isotope dilution (3). These techniques require dosing and multiple blood samples per subject, which may not be practical in large-scale effectiveness studies, particularly in children.
Most plants used for food, including staples such as wheat and rice, are C3 plants; however, there are a few notable crops used for human consumption that are C4 plants (e.g., maize, millet, sorghum, sugar cane) (17). C3 plants discriminate more against 13C during photosynthesis and therefore have lower 13C enrichment than C4 plants (18). 13C content at natural abundance concentrations is often expressed using the δ notation, which refers to Vienna Pee Dee Beleminite (VPDB) and is expressed as δ13C = [Rsample/RVPDB] − 1; r = 13C/12C (19). This value is then typically expressed per mil (‰) by multiplying by 1000. VPDB is relatively enriched compared to most natural materials and has been assigned a δ13C value of 0; therefore, most other natural materials have negative δ13C values. Atmospheric CO2 is relatively stable geographically and topographically and has reported δ13C values ranging from −7.4‰ to −6.7‰ (20). C4 plants typically have δ13C values closer to atmospheric CO2 [e.g., maize (−13.6‰ and −14.0‰) (18), sorghum (−13.8‰ and −14.4‰) (18), and millet (−10.7‰ and −12.0‰) (21)]. C3 plants have lower δ13C [e.g., carrots (29.5‰ ± 0.2‰), bananas (−26.6‰ ± 0.1‰), and mangos (−25.4‰ ± 0.1‰) (17)]. Lipids and other secondary metabolites (including carotenoids) are further reduced in δ13C by ∼5−10‰ (22, 23); however, the difference between C3 and C4 plants is maintained, as noted with lutein obtained from marigold compared with maize (−29.9‰ ± 0.2‰ and −19.8‰ ± 0.3‰, respectively) (23). This difference gives the potential to in vivo metabolites in determining dietary plant origins based on 13C composition. Because no carbon is gained or lost during the cleavage of β-carotene or other provitamin A carotenoids to VA (24), the δ13C of serum retinol can reflect the dietary sources, including preformed and provitamin A (25). The principle of isotope mass balance states that the amount of heavy isotope in a system is a linear combination of its components (19), which could be used to quantitatively estimate the relative contributions of dietary vitamin A sources.
Several C4 crops are being biofortified with provitamin A carotenoids; in addition to maize, sorghum (26) and millet (27) are also targets. C4 plants often have advantages over C3 plants under conditions of drought, heat, and CO2 or nitrogen limitations, and for this reason they are major crops in tropical and subtropical regions (28). Furthermore, they may play a vital role in food and nutrition security under changing climates (28, 29). These biofortified varieties should be confirmed for VA bioefficacy and effectiveness at the population level.
This controlled study was undertaken to determine whether β-carotene efficacy from OM could be demonstrated with the use of shifts in the δ13C of serum retinol from the natural enrichment of maize feeding and comparing these values to the δ13C of liver VA, liver VA concentrations, and serum retinol concentrations. The δ13C was determined with GC combustion isotope ratio MS, which is known for its high degree of precision at natural abundance concentrations (30). Mongolian gerbils are a useful model for human absorption and metabolism of provitamin A carotenoids (31–33). Findings in maize could also extend to millet and sorghum because of their similar 13C enrichment.
Methods
Maize.
The biofortified OM was developed at the International Maize and Wheat Improvement Center in Mexico as part of its HarvestPlus biofortified maize research project (34). Seed was shipped from Mexico to Zambia, and the grain of this OM variety was produced on a commercial farm in Central Province, Zambia.
Orange maize was stored frozen (−20°C to −10°C) after harvest. White maize (WM) is a locally consumed variety in Zambia. Both varieties were hand-carried to the University of Wisconsin to be used in this study.
Carrots.
Carrots from the USDA carrot breeding and genetics program were grown by the University of California Desert Research and Extension Station in sandy loam soil in October and harvested in March. Carrots were refrigerated at 2°C until shipped overnight from California to Wisconsin. Upon arrival, they were returned to 2°C until freeze-dried for feed preparation. The genotypes used (i.e., high carotene mass and B2327) were selected for high β-carotene concentrations.
Gerbil feed.
Gerbil feeds were formulated with assistance from Harlan-Teklad to meet National Research Council-recommended macro- and micronutrient needs (35). Feeds were 50% maize by weight (36) and were modified by adding carrots at 1.5% by weight (Supplemental Table 1). Maize, carrots, or the VA fortificant provided the sources of VA. The retinyl palmitate used as the fortificant was dry vitamin A palmitate (250,000 IU VA/g; DSM Nutritional Products Ltd.) and was added at a concentration to meet ∼50% of estimated utilization rates found in previous studies [2.7 − 5.1 μg retinol activity equivalents (RAEs)/100 g body weight (33, 37)], resulting in a target concentration of 0.25 μg RAE/g feed. All other feed constituents were constant between groups. Treatment groups were differentiated by 3 factors: OM compared with WM, orange carrots (OCs) compared with white carrots (WCs), and VA fortificant (VA+ compared with VA−) in a 2 × 2 × 2 factorial design.
Gerbils and study design.
Gerbils for this study were a random subset of a larger gerbil feeding study (n = 85). Male 28-d-old Mongolian gerbils (Charles River Laboratories) were group housed during VA depletion (2–3/cage) and treatment (2/cage). Animal handling procedures were approved by the University of Wisconsin College of Agricultural and Life Sciences Animal Care and Use Committee. Gerbils were weighed daily for 2.5 wk and thereafter 3 times/wk. Room temperature and humidity were held constant with a 12-h light/dark cycle. Gerbils consumed ad libitum. During the depletion period (days 1–14), all gerbils consumed WM, WCs, and VA− feed. After 14 d, a baseline group kill (n = 5) was performed by exsanguination while the gerbils were under isoflurane anesthesia. The remaining gerbils were weight matched and allocated into 8 groups (n = 5–7/group) for the treatment period (days 15–77). After a 62-d treatment period (day 77), a final kill (n = 50) was performed as described previously.
Carotenoid and retinoid analyses.
All sample analyses for carotenoids and retinoids were performed under gold fluorescent lights to prevent photo-oxidation and isomerization. Feeds were analyzed for carotenoids by a published procedure for extraction (38) and an HPLC system (36). Feeds were analyzed for retinol with the same extraction and a minor modification of the HPLC system for retinol (39); solvent A was acetonitrile:water (92.5:7.5, vol:vol), and solvent B was acetonitrile:methanol:dichloroethane (80:10:10, vol:vol), both with triethylamine (0.05%, vol:vol). Serum retinol was extracted with a modified published procedure (40). Briefly, ∼1 mL serum, 1 mL ethanol, and 25 μL C23 β-apocarotenol in methanol were extracted twice with 1.5-mL hexanes, dried under nitrogen, resuspended in 80 μL methanol, and injected onto the first HPLC system for quantification and primary purification (3). Liver retinol and retinyl esters were analyzed by a modified published procedure (39); retinol and retinyl esters were summed to report total VA. Modifications included using ∼0.5 g liver and C23 β-apocarotenol as the internal standard, resuspending the dried aliquot in 100 μL methanol:dichloroethane (75:25, vol:vol), and using a 25-μL injection volume of reconstituted sample for HPLC.
13C determinations.
Serum retinol was further processed for 13C content by a published procedure (3), including an additional HPLC purification step, drying under vacuum centrifugation, resuspension into hexanes, and injection onto the GC combustion isotope ratio mass MS system (Supplemental Figure 1) (25). A separate aliquot of the liver lipid extract was saponified and extracted (7); the resulting retinol was purified and analyzed similarly to serum retinol.
Maize and carrot total carbon δ13C were determined using an elemental analyzer combined with isotope ratio MS (41). Retinyl acetate from the VA fortificant was saponified and analyzed similarly to liver VA. All feed samples were analyzed in triplicate.
Estimation of maize contribution to dietary vitamin A.
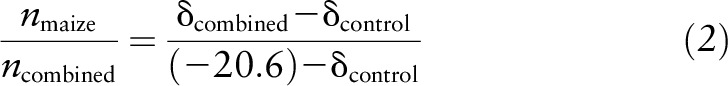
The mass balance (isotope balance) equation (19, 30) was adapted to the population level and solved for the relative contribution of maize to the total VA intake in terms of serum retinol δ13C‰ of the test and control groups (Supplemental Methods):
 |
where n is RAEs expressed as moles and the corresponding subscripts “maize” and “combined” refer to contribution from maize and total VA, respectively. Mean serum retinol δ13C‰ is represented by δ, and the corresponding subscripts refer to treatment groups (control: group consuming no OM; maize: group receiving VA only from OM; combined: group consuming VA sources of control group in addition to OM). Experimental groups were fit to this model to examine whether the calculated proportion of VA coming from maize matched analytical data. WMOCVA−, WMWCVA+, and WMOCVA+ were used as 3 control groups; OMOCVA−, OMWCVA+, and OMOCVA+ were used as the 3 respective test groups that consumed combined sources; and OMWCVA− was used as the maize-only group. A bioconversion of 12 μg β-carotene equivalents:μg RAEs was used (12).
Statistical analysis.
Values are reported as means ± SDs. Data were analyzed with the use of SAS version 9.4. Outcomes of interest were evaluated with the use of independent 2-sample, 2-tailed t tests or 3- and 1-factor ANOVA to compare treatment groups and to determine differences between groups with the use of the general linear model procedure as appropriate. Feeds were compared with the use of 1-factor ANOVA. Linear regression was also performed with the general linear model procedure. Post hoc letter groupings between treatment groups were determined with the use of least significant differences. Normality of residuals was tested with the Shapiro-Wilk test; homogeneity of variance was tested with Levene’s test. Data failing normality or variance assumptions were analyzed nonparametrically by analyzing ranked data. P < 0.05 was considered significant.
Results
Feed properties.
Carotenoid and retinol equivalent concentrations in the feeds had the expected relations (Table 1). OM provitamin A was predominantly β-carotene (∼96%) with some β-cryptoxanthin (∼3%) and α-carotene (∼1%). Carrot provitamin A was mostly β-carotene (∼65%) but with appreciable α-carotene (∼34%) and minimal β-cryptoxanthin (∼1%). Maize total carbon δ13C was higher than carrots for both OM (−11.0‰ ± 0.2‰ compared with −25.6‰ ± 0.2‰ P < 0.0001) and WM (−11.3‰ ± 0.3‰ compared with −25.3‰ ± 0.1‰ P < 0.0001) varieties; δ13C did not differ within carrot or maize varieties (P ≥ 0.05). The preformed retinyl palmitate used as the fortificant had a δ13C of −27.4‰ ± 1.2‰, which represents only the retinol portion because the sample was saponified before analysis.
TABLE 1.
Feed concentrations of provitamin A carotenoids and preformed VA in maize treatments fed to Mongolian gerbils1
| WCWMVA− | OMWCVA− | WCWMVA+ | OMWCVA+ | OCWMVA− | OCOMVA− | OCWMVA+ | OCOMVA+ | P | |
| β-Carotene,2 μg/g feed | 0.0111 ± 0.0037d | 6.05 ± 0.52c | 0.0125 ± 0.0043d | 7.03 ± 0.26c | 13.7 ± 0.4b | 20.5 ± 0.4a | 13.3 ± 0.2b | 21.3 ± 0.9a | <0.0001 |
| Preformed VA,3 μg RAE/g feed | ND | ND | 0.370 ± 0.116 | 0.198 ± 0.007 | ND | ND | 0.188 ± 0.034 | 0.268 ± 0.156 | |
| Total,4 μg RAE/g feed | 0.000924 ± 0.000304 | 0.505 ± 0.043 | 0.371 ± 0.116 | 0.784 ± 0.023 | 1.14 ± 0.04 | 1.70 ± 0.04 | 1.35 ± 0.12 | 2.05 ± 0.17 | — |
| Maize RAEs/total RAEs5 | 0.536 | 0.999 | 0.002 | 0.685 | 0.000 | 0.326 | 0.000 | 0.284 | — |
Values are means ± SDs, n = 3/feed. Labeled means in a row without a common superscript letter differ, P < 0.05. ND, not detected; OC, orange carrot; OM, orange maize; RAE, retinol activity equivalent; VA, vitamin A; WC, white carrot; WM, white maize.
β-Carotene equivalents in mass were determined by summing [β-carotene isomers + 0.5 × α-carotene + 0.5 × β-cryptoxanthin × 536.9 (molar mass β-carotene)/552.8 (molar mass β-cryptoxanthin)].
VA was not detected in feeds in which VA fortificant was omitted; limit of detection: 0.004 μg RAE/g feed. P value reflects VA+ feeds only. Target concentration: 0.25 μg RAE/g feed; overall mean ± SD: 0.25 ± 0.11 μg RAE/g feed.
Total RAEs were calculated using the bioconversion factor 12 μg β-carotene:μg RAEs (12).
Proportion of VA from maize calculated using overall mean for fortificant VA and also used the bioconversion factor 12 μg β-carotene:μg RAEs (12).
Serum retinol and liver VA concentrations.
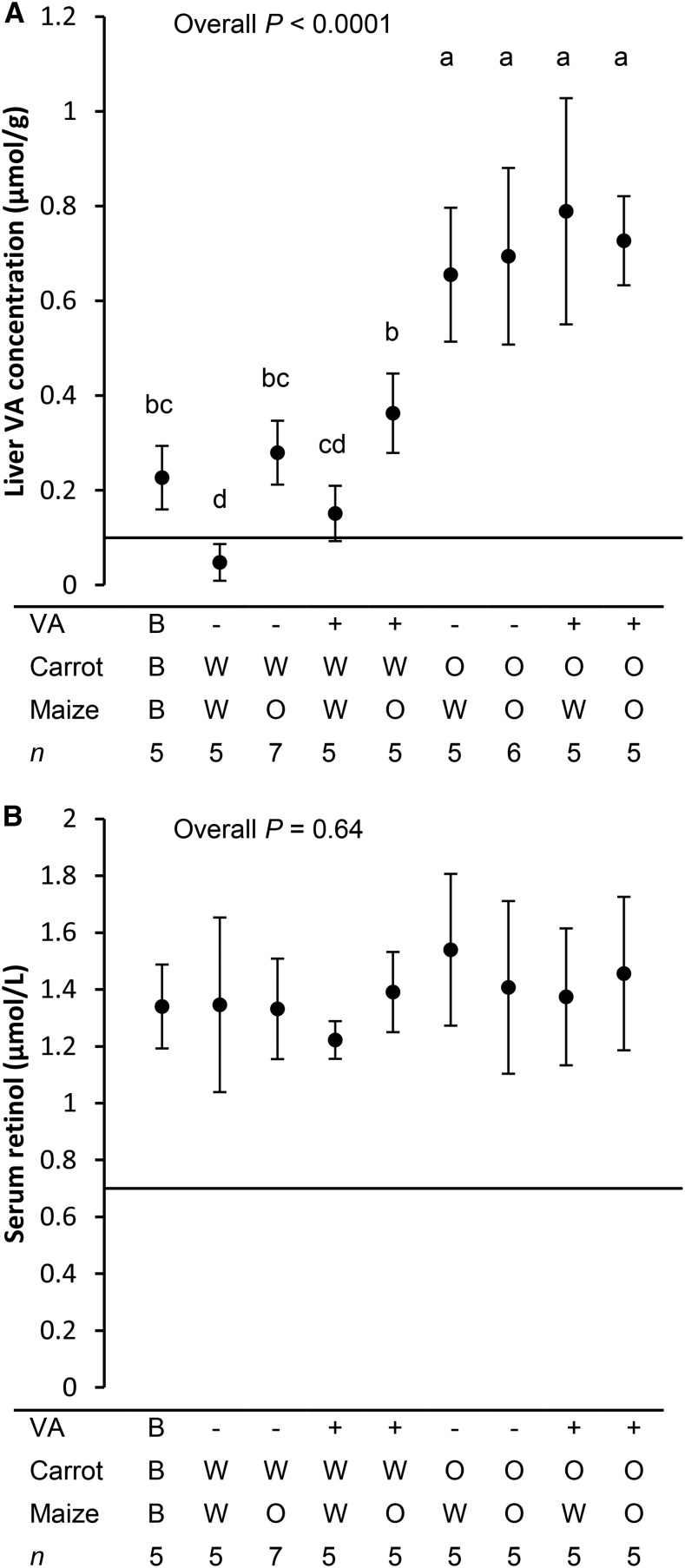
Serum retinol and liver VA (retinol + saponified retinyl ester) concentrations were plotted (Figure 1A, B). Liver VA concentrations were highly dependent on dietary VA. The group mean for the treatment group that consumed the least VA (WMWCVA−) was below the VA deficiency cutoff of 0.1 μmol VA/g liver (14) after treatment. Both provitamin A carotenoid sources increased liver VA concentrations considerably. The mean liver VA concentrations from all groups that consumed OC were not different from each other and were much greater than all groups that consumed WC. Within the WC groups, both groups that consumed OM had higher VA liver concentrations than their respective WM controls. The VA fortificant, which was meant to meet 50% of the estimated requirements of gerbils, did not notably improve VA stores. All groups that consumed the VA fortificant had liver VA concentrations that were similar to their respective controls without the VA fortificant. Nonetheless, the group that consumed appreciable VA from fortificant only (WMWCVA+) had a mean liver VA concentration >0.1 μmol/g and was not significantly different from the baseline group (P = 0.08), which meant they were able to maintain initial VA status. Serum retinol concentrations did not differ between groups despite a wide range of liver VA concentrations, and serum retinol was not correlated to liver VA concentrations (R2 = 0.028).
FIGURE 1.
Liver VA (A) and serum retinol (B) concentrations in gerbils after consuming feeds with different combinations of provitamin A carotenoid and preformed VA sources. All values are means ± SDs (n = 5–7). Liver VA residuals were not normally distributed, and variance was not homogeneous; data were analyzed nonparametrically. Horizontal lines at 0.1 μmol/g (A) and 0.7 μmol/L (B) are the deficiency cutoff concentrations. Labeled means without a common letter differ, P < 0.05. B, baseline; O, orange; VA, vitamin A; W, white.
Serum retinol and liver VA δ 13C.
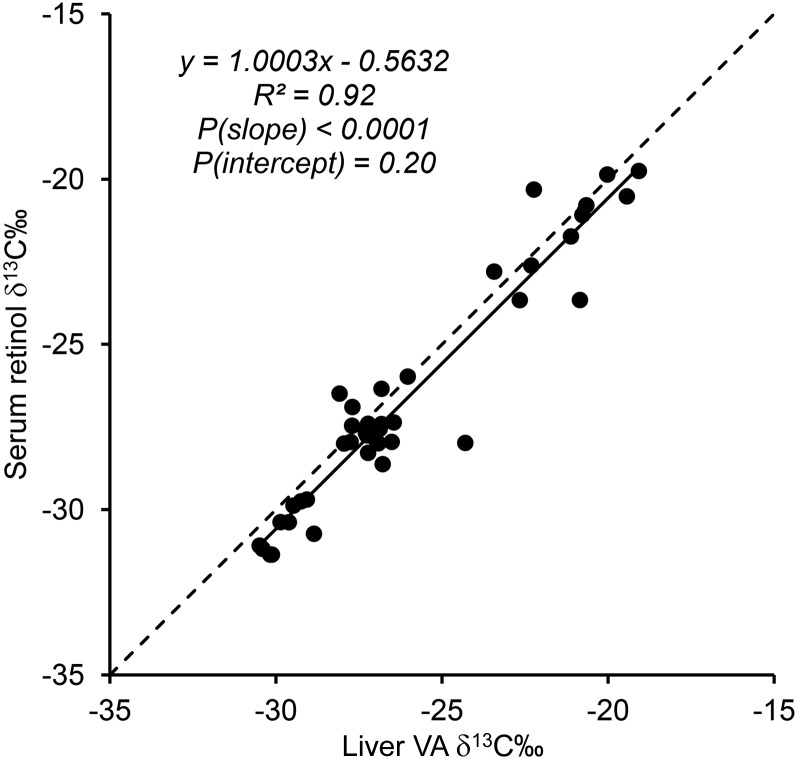
Serum retinol and liver VA δ13C had good agreement (Figure 2), indicating that the accessible serum retinol pool is highly reflective of the major liver VA store.
FIGURE 2.
Serum retinol δ13C‰ plotted against liver VA δ13C‰ for gerbils that consumed diets with varying amounts and sources of provitamin A carotenoids and preformed VA. Only gerbils with values for both outcomes are plotted (n = 40; 3–7/group) along with the best fit line of the data (solid line) and y = x (dashed line). VA, vitamin A.
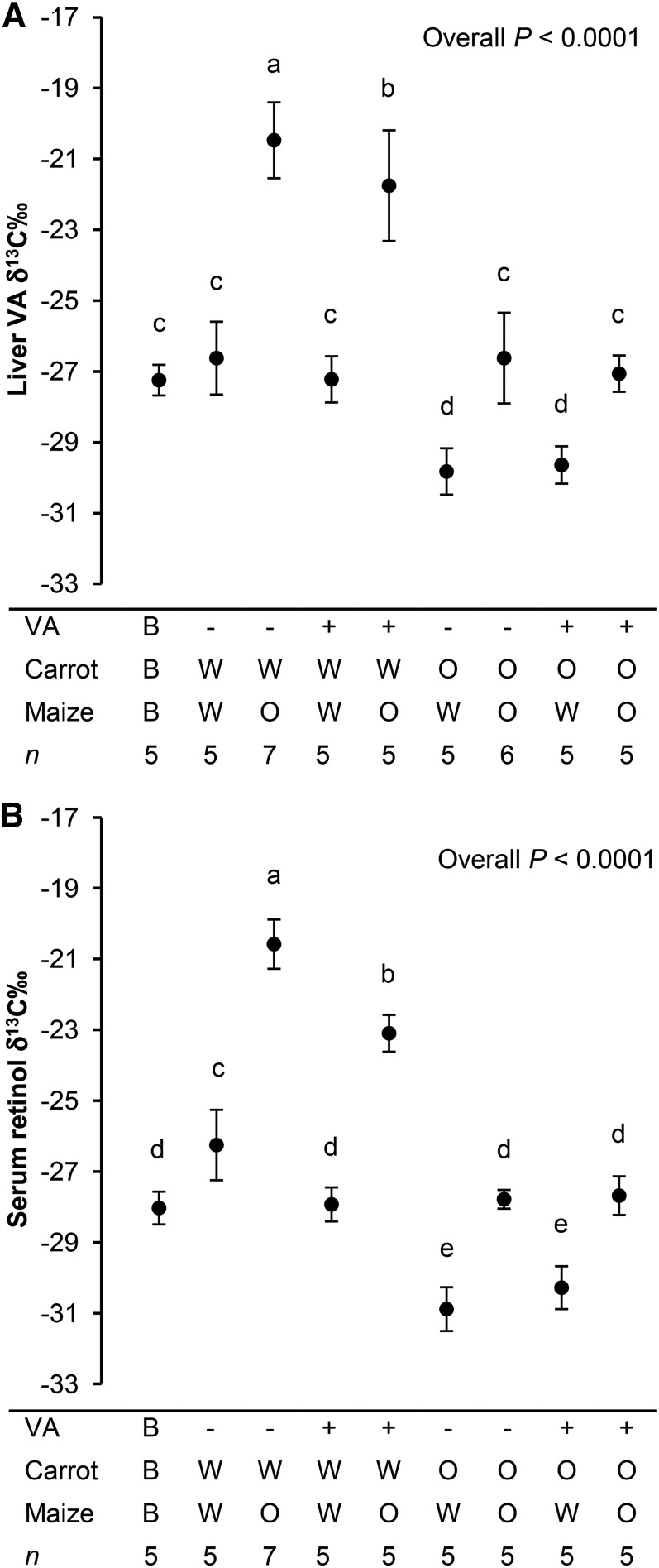
When serum retinol δ13C was analyzed with 3-factor ANOVA, all main effects, the VA × carrot interaction, and the maize × carrot interaction were highly significant (all P ≤ 0.0001). The VA × maize interaction (P = 0.09) and 3-factor interaction were not significant. Because of multiple interactions, 1-factor ANOVA with post hoc analysis was then used to analyze the data, including the baseline group.
Serum retinol and liver VA δ13C by group showed similar responses to treatment (Figure 3A, B). All groups that consumed OM had significantly greater VA δ13C than the corresponding WM controls. By contrast, all groups that consumed OCs had much lower VA δ13C values compared with corresponding WC controls. The group that consumed the VA fortificant as the primary VA source (WMWCVA+) had serum δ13C that was not significantly different than the VA fortificant (−27.9‰ ± 0.5‰ compared with −27.4‰ ± 1.2‰). The group that consumed VA almost entirely (>99.9%) from maize had a serum retinol δ13C of −20.6‰ ± 0.7‰.
FIGURE 3.
Gerbil liver VA δ13C‰ (A) and serum retinol δ13C‰ (B) after consuming feeds with different combinations of provitamin A and preformed VA sources. All values are means ± SDs. Labeled means without a common letter differ, P < 0.05. B, baseline; O, orange; VA, vitamin A; W, white.
Estimation of maize contribution to dietary VA.
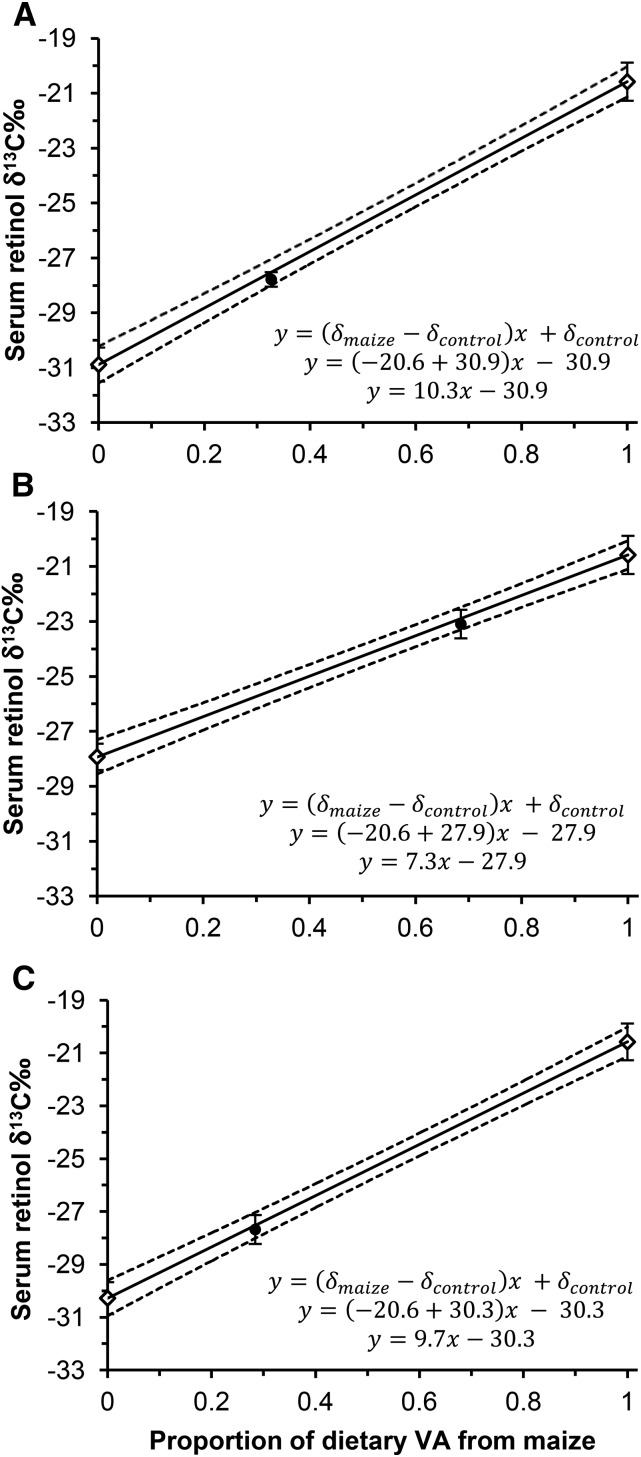
Plots were made with the use of Supplemental Methods Equation 4 of 3 pairs of control and test groups (Figure 4). The proportion of dietary VA predicted from Supplemental Methods Equation 5 in the model agreed well with analytical values; all 3 test group means were within the 95% confidence limits. Supplemental Methods Equation 5 can be simplified to include data from the OMWCVA− group assuming the group mean adequately represents the serum retinol δ13C of gerbils that consumed VA almost entirely from maize (>99.9%). Therefore, the relative contribution of maize to total dietary VA in a population can be estimated by the serum retinol δ13C group means of the control and test (combined) groups:
FIGURE 4.
Gerbil serum retinol δ13C‰ plotted against the proportion of dietary VA coming from maize for diets containing varying amounts of VA in the form of preformed vitamin A and provitamin A carotenoids. For all plots, a prediction line with the use of mean serum retinol δ13C‰ from the control group that consumed negligible VA from maize and the maize group that consumed VA exclusively from maize (OMWCVA−, n = 7) was plotted against the analytical determination of maize contribution to dietary VA (open diamonds). Dashed lines: 95% confidence limits; error bars: SDs. The means ± SDs of the corresponding test group were plotted independently to test the predictive ability of Equation 1 (closed circles). (A) Control group: WMOCVA−, n = 5; test group: OMOCVA−, n = 6. (B) Control group: WMWCVA+, n = 5; test group: OMWCVA+, n = 5. (C) Control group: WMOCVA+, n = 5; test group: OMOCVA+, n = 5. OC, orange carrot; OM, orange maize; VA, vitamin A; WC, white carrot; WM, white maize.
 |
Experimental and analytical variation.
Experimental and analytical CVs were very low for δ13C and their corresponding 13C isotopic abundance (13C/total carbon) values (Supplemental Table 2). All δ13C CVs for individual experimental groups were ≤3.4%, and all 13C isotopic abundance CVs for individual experimental groups were ≤0.23%.
Discussion
The analysis of δ13C in serum retinol allowed the determination of provitamin A efficacy from biofortified maize compared with feeds containing minimal VA, provitamin A carotenoids, preformed VA, or a combination of sources when contrasted to an appropriate control group. Furthermore, the relative contribution of maize to total VA intake was quantitatively estimated and verified against analytical determination. The first advantage is high sensitivity to detect provitamin A maize consumption because of low variation within groups that consumed the same feeds. Circulating retinol concentrations are homeostatically controlled outside of severe hypo- or hypervitaminosis A (12, 42, 43) and frequently do not respond to VA interventions (3, 14, 44, 45), both of which were observed in this study. However, they are still often used as a primary outcome to evaluate populations and interventions aimed at improving VA status (46), which may not detect the potential effects of β-carotene or VA interventions that use more sensitive methods (3, 44, 45). A second advantage is that a single blood sample after long-term consumption is required. More sensitive methods used as outcomes in VA studies, such as isotope dilution (3, 44) or appearance in serum (9), use multiple blood draws. This is undesirable—especially when working with children—and can complicate recruitment and follow-up during studies (47). Finally, no external isotopically labeled material or VA analogues, which are used for isotope dilution, tracer, and modified dose-response tests (12, 14, 48), are required. These compounds are often expensive and technically demanding to produce and prepare. Together these advantages show promise for future efficacy or large-scale effectiveness trials to evaluate crop adoption and consumption, especially in populations or settings in which resources are limited and multiple sample collections are not practical.
Variations in natural abundance ratios of stable isotopes (e.g., carbon, nitrogen, sulfur, hydrogen, oxygen) measured from numerous sources (e.g., breath, hair, nails, plasma, RBCs, and specific molecules such as alanine) have been used as biomarkers for dietary origin (49). An early study noted elevated δ13C in breath CO2 after the consumption of sugar (50), and more recent applications have further developed the methodology. For example, δ13C was measured from several tissues to assess sugar intake (51), and serum total carbon δ13C was lower after an intervention to decrease sugar-sweetened beverage intake (52). Nitrogen-15 enrichment varies between plant and animal protein sources, and this natural difference has been correlated with meat and fish intake in numerous studies (49). Reduced serum retinol δ13C was demonstrated in response to increased consumption of C3 vegetables containing provitamin A carotenoids, including carrots and pumpkin (25), which we also demonstrated in this study.
Other studies have applied GC combustion isotope ratio MS to measure per-labeled 13C β-carotene (53) and lutein (54) absorption, enabling the characterization of the appearance and disappearance of carotenoids in plasma after the ingestion of physiological amounts from food. Traditional MS could theoretically be used to apply our proposed method, although instead of a single CO2 peak with 3 mass traces from the combusted retinol to determine the 13C:12C ratio (Supplemental Figure 1), mass distributions of retinol would need to be compared, and adequate precision would first have to be demonstrated.
Although enzymatic isotope effects are established in plants and yield differences in 13C enrichments both in classes of metabolites (i.e., starch compared with lipid) within plants (22) and between plants exhibiting different photosynthetic systems (18), it is relatively unknown whether similar effects can be observed for VA in animals and affect organ partitioning given that numerous enzymes participate in VA metabolism (55). Excellent agreement between serum retinol and liver VA δ13C indicates that in a paradigm of constant long-term consumption, the use of serum retinol δ13C is a suitable alternative to represent that in the major liver store.
Serum retinol and liver VA δ13C of treatment groups agreed with data in the literature. Lipids and carotenoids are reduced in 13C by ∼5–10‰ compared with total carbon (22, 23), which agrees with our results that OC total carbon had a δ13C of −25.6‰, and the treatment group that consumed OCs as the predominant VA source (WMOCVA−) had a mean liver VA δ13C of −29.8‰ (a difference of 4.2‰). OM total carbon had a δ13C of approximately −11.0‰, and the treatment group with the only appreciable VA dietary source as OM (OMWCVA−) had a mean liver VA δ13C of −20.5‰ (a difference of 9.5‰). Furthermore, the liver VA δ13C from these 2 groups corresponded well to lutein obtained from marigold (a C3 plant) compared with maize (−29.9‰ and −19.8‰, respectively) (23). Serum retinol δ13C was similar in the gerbils that obtained VA from the fortificant only (WMWCVA+) to the δ13C of the fortificant itself.
Although natural 13C enrichment is suitable for distinguishing provitamin A carotenoid efficacy from maize compared with a WM control feed in a trial setting, this study revealed limitations on its use as a purely diagnostic tool for VA status. In addition to the baseline group, 4 treatment groups had similar serum retinol and liver VA δ13C despite widely varying liver VA concentrations. These treatment groups either consumed either both OM and OCs or both WM and WCs, and the resulting enrichment was a mixture of the sources. Although WM and WCs are both very low in provitamin A carotenoids, each contributed small amounts to the feed (maize: 5.2 ± 1.1 ng β-carotene/g feed; carrots: 4.5 ± 0.4 ng β-carotene/g feed), which would be reflected in the δ13C values. However, groups that consumed OM had considerably higher serum retinol δ13C and liver VA concentrations than their respective controls that consumed WM. If a population that consumed OM demonstrated elevated serum retinol δ13C, it could be inferred that their VA status is greater than or equal to a control population that consumed WM depending on the initial VA status of the population. δ13C shifts reflect the consumption of provitamin A maize but are not a replacement for evaluating VA status, such as isotope dilution methods (3). Isotope dilution or dose-response tests could be used on a subset of randomly selected individuals to confirm desired VA status in efficacy or effectiveness studies.
Liver VA concentrations were not different between all groups that consumed OCs regardless of additional VA from OM or the fortificant, likely reflecting the downregulation of provitamin A bioconversion (56, 57) and a relatively minor impact of the VA fortificant compared with OCs. Despite this, the serum retinol δ13C was still able to distinguish the feeds in which provitamin A carotenoids were obtained from OM or OCs. This is important considering some populations targeted for biofortification have substantial intakes of VA, even if intake varies seasonally (58, 59). Although these reports highlight a need for more sensitive markers of VA status to ensure interventions do not lead to the chronic overconsumption of VA (2, 3), biofortification of staple foods with provitamin A carotenoids can mitigate seasonal gaps in provitamin A consumption and reduce the chances of excessive preformed vitamin A intake caused by the regulation of absorption and bioconversion of provitamin A carotenoids (56, 57). A 62-d treatment period was sufficient to have serum retinol δ13C reflect that in the major body pool (i.e., liver) in this study, but this time requirement in humans likely depends on a number of factors, including the baseline body pool of VA, dietary VA intake, and the rate of VA metabolism. Labeled VA doses mix with body stores within 26 d after administration to adults (60) and 12 d in children (61); however, it will likely take longer for serum retinol δ13C to accurately reflect regular dietary consumption. If the VA pool size increases as the intervention intends, this equilibration time would be shorter.
Acknowledgments
We thank Peter Crump for statistical consultation and Natalia Palacios-Rojas for breeding maize and overseeing the production of grain in Zambia. BMG contributed to the study design, analyzed the samples and the data, and wrote the first draft of the manuscript; IP analyzed the samples and revised the manuscript; LM ran the gerbil study; CRD contributed to the study design, organized the gerbil study, and maintained the mass spectrometer; PS bred the carrots; KVP produced the synthetic maize seed used to grow the grain in Zambia; and SAT designed the study and revised the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: OC, orange carrot; OM, orange maize; RAE, retinol activity equivalent; VA, vitamin A; VPDB, Vienna Pee Dee Beleminite; WC, white carrot; WM, white maize.
References
- 1.Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH. Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull 2011;32:S31−40. [DOI] [PubMed] [Google Scholar]
- 2.Ribaya-Mercado JD, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, Russell RM, Wallace CB. Use of the deuterated-retinol-dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr 2004;80:1291−8. [DOI] [PubMed] [Google Scholar]
- 3.Gannon BM, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalaungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014;100:1541−50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf 2008;7:320−96. [Google Scholar]
- 5.Tanumihardjo SA, Palacios N, Pixley KV. Provitamin a carotenoid bioavailability: what really matters? Int J Vitam Nutr Res 2010;80:336−50. [DOI] [PubMed] [Google Scholar]
- 6.Howe JA, Tanumihardjo SA. Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian gerbils. J Nutr 2006;136:2562−7. [DOI] [PubMed] [Google Scholar]
- 7.Schmaelzle S, Gannon BM, Crawford S, Arscott SA, Goltz S, Palacios-Rojas N, Pixley KV, Simon PW, Tanumihardjo SA. Maize genotype and food matrix affect the provitamin A carotenoid bioefficacy from staple and carrot-fortified feeds in Mongolian gerbils (Meriones unguiculatus). J Agric Food Chem 2013;62:136−43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzhingi T, Gadaga TH, Siwela AH, Grusak MA, Russell RM, Tang G. Yellow maize with high β-carotene is an effective source of vitamin A in healthy Zimbabwean men. Am J Clin Nutr 2011;94:510−9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Nugroho A, Rocheford T, White WS. Vitamin A equivalence of the β-carotene in β-carotene−biofortified maize porridge consumed by women. Am J Clin Nutr 2010;92:1005−12. [DOI] [PubMed] [Google Scholar]
- 10.Flay BR. Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs. Prev Med 1986;15:451−74. [DOI] [PubMed] [Google Scholar]
- 11.Singal AG, Higgins PDR, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol 2014;5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press; 2001. [PubMed] [Google Scholar]
- 13.Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst 1984;73:1439−44. [PubMed] [Google Scholar]
- 14.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94:658S−65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: Report: priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15−17 September 2010. Geneva (Switzerland): WHO; 2012. [Google Scholar]
- 16.Bresnahan K, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr 2014;5:702−11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison DJ, Dodson B, Slater C, Preston T. 13C natural abundance in the British diet: implications for 13C breath tests. Rapid Commun Mass Spectrom 2000;1324:1321−4. [DOI] [PubMed] [Google Scholar]
- 18.Smith BN, Epstein S. Two categories of 13C/12C ratios for higher plants. Plant Physiol 1971;47:380−4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes JM. An introduction to isotopic calculations. Woods Hole (MA): Woods Hole Oceanographic Institution; 2004. [Google Scholar]
- 20.Keeling CD. The concentration and isotopic abundances of carbon dioxide in rural and marine air. Geochim Cosmochim Acta 1961;24:277−98. [Google Scholar]
- 21.McGovern PE, Zhang J, Tang J, Zhang Z, Hall GR, Moreau RA, Nuñez A, Butrym ED, Richards MP, Wang C, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci USA 2004;101:17593−8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleixner G, Danier HJ, Werner RA, Schmidt HL. Correlations between the 13C content of primary and secondary plant products in different cell compartments and that in decomposing Basidiomycetes. Plant Physiol 1993;102:1287−90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y, White WS, Yao L, Serfass RE. Use of high-precision gas isotope ratio mass spectrometry to determine natural abundance 13C in lutein isolated from C3 and C4 plant sources. J Chromatogr A 1998;800:51−8. [DOI] [PubMed] [Google Scholar]
- 24.dela Seña C, Riedl KM, Narayanasamy S, Curley RWJ, Schwartz SJ, Harrison EH. The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J Biol Chem 2014;289:13661−6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C Natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med 2009;234:140−7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Africa Harvest Biotech Foundation International. Africa Biofortified Sorghum Project: five-year progress report [Internet]. [cited 2016 May 6]. Available from: http://issuu.com/africaharvest/docs/abs_project_whole_book_high-res_21may2008?e=1344694/2800147.
- 27.Velu G, Rai KN, Muralidharan V, Kulkarni VN, Longvah T, Raveendran TS. Prospects of breeding biofortified pearl millet with high grain iron and zinc content. Plant Breed 2007;126:182−5. [Google Scholar]
- 28.Sage RF, Monson RK, editors. C4 plant biology. San Diego (CA): Academic Press; 1999. [Google Scholar]
- 29.Technical Centre for Agricultural and Rural Cooperation. Potential for sorghum in food security and economic development among communities in arid and semi-arid lands in Africa [Internet]. [cited 2016 6 May]. Available from: http://knowledge.cta.int/content/download/43787/633290/file/Wambugu+and+Mburu+-+EN+-+Potential+for+sorghum+in+food+security+and+economic+development+-+CTA+K4D+article+July+2014.pdf.
- 30.Brenna JT, Corso TN, Tobias HJ, Caimi RJ. High-precision continuous-flow isotope ratio mass spectometry. Mass Spectrom Rev 1997;16:227−58. [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Boileau A. Review of animal models in carotenoid research. J Nutr 1999;129:2271−7. [DOI] [PubMed] [Google Scholar]
- 32.House W, Apgar J, Smith J. The gerbil: a model for studying the metabolism of beta-carotene and minerals. Nutr Res 1997;17:1293−302. [Google Scholar]
- 33.Lee CM, Lederman JD, Hofmann NE, Erdman JW Jr. The Mongolian gerbil (Meriones unguiculatus) is an appropriate animal model for evaluation of the conversion of β-carotene to vitamin A. J Nutr 1998;128:280−6. [DOI] [PubMed] [Google Scholar]
- 34.Pixley KV, Palacios-Rojas N, Babu R, Mutale R, Surles RL, Simpungwe E. Biofortificaion of maize with provitamin A carotenoids. In: Tanumihardjo SA, editor. Carotenoids in human health. New York: Springer Science and Business Media; 2013. p. 271−92. [Google Scholar]
- 35.National Research Council. Nutrient requirements of laboratory animals. 4th ed Washington (DC): National Academy of Sciences; 1995. [Google Scholar]
- 36.Davis C, Jing H, Howe JA, Rocheford T, Tanumihardjo SA. β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements. Br J Nutr 2008;100:786−93. [DOI] [PubMed] [Google Scholar]
- 37.Bresnahan K, Davis CR, Tanumihardjo SA. Relative vitamin A values of 9-cis- and 13-cis-β-carotene do not differ when fed at physiological levels during vitamin A depletion in Mongolian gerbils (Meriones unguiculatus). Br J Nutr 2014;112:162−9. [DOI] [PubMed] [Google Scholar]
- 38.Howe JA, Tanumihardjo SA. Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp.). J Agric Food Chem 2006;54:7992−7. [DOI] [PubMed] [Google Scholar]
- 39.Tanumihardjo SA, Howe JA. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr 2005;135:2622−6. [DOI] [PubMed] [Google Scholar]
- 40.Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr 2004;134:1186−92. [DOI] [PubMed] [Google Scholar]
- 41.Escaron AL, Green MH, Howe JA, Tanumihardjo SA. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 2009;139:2000−6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes: report of a Joint WHO/UNICEF Consultation. Geneva (Switzerland): WHO; 1996. [Google Scholar]
- 43.WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 44.Ribaya-Mercado JD, Maramag CC, Tengco LW, Dolnikowski GG, Blumberg JB, Solon FS. Carotene-rich plant foods ingested with minimal dietary fat enhance the total-body vitamin A pool size in Filipino schoolchildren as assessed by stable-isotope-dilution methodology. Am J Clin Nutr 2007;85:1041−9. [DOI] [PubMed] [Google Scholar]
- 45.Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple-fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school-aged Thai children. J Nutr 2014;144:519−24. [DOI] [PubMed] [Google Scholar]
- 46.Talsma EF, Brouwer ID, Verhoef H, Mbera GNK, Mwangi AM, Maziya-Dixon B, Boy E, Zimmermann MB, Melse-Boonstra A. Biofortified yellow cassava and vitamin A status of Kenyan children: a randomized controlled trial. Am J Clin Nutr 2016;103:258−67. [DOI] [PubMed] [Google Scholar]
- 47.Boahen O, Owusu-Agyei S, Febir LG, Tawiah C, Tawiah T, Afari S, Newton S. Community perception and beliefs about blood draw for clinical research in Ghana. Trans R Soc Trop Med Hyg 2013;107:261−5. [DOI] [PubMed] [Google Scholar]
- 48.Haskell MJ. The challenge to reach nutritional adequacy for vitamin A : β-carotene bioavailability and conversion—evidence in humans. Am J Clin Nutr 2012;96:1193−203. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien DM. Stable isotope ratios as biomarkers of diet for health research. Annu Rev Nutr 2015;35:565−94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoeller DA, Schneider JF, Solomons NW, Watkins JB, Klein PD. Clinical diagnosis with the stable isotope in 13C in CO2 breath tests: methodology and fundamental considerations. J Lab Clin Med 1977;90:412−21. [PubMed] [Google Scholar]
- 51.Nash SH, Kristal AR, Hopkins SE, Boyer BB, O’Brien DM. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup’ik study population. J Nutr 2014;144:75−80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fakhouri THI, Jahren AH, Appel LJ, Chen L, Alavi R, Anderson CAM. Serum carbon isotope values change in adults in response to changes in sugar-sweetened beverage intake. J Nutr 2014;144:902−5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You C, Parker RS, Goodman KJ, Swanson JE, Corso TN. Evidence of cis-trans isomerization of 9-cis-β-carotene during absorption in humans. Am J Clin Nutr 1996;64:177−83. [DOI] [PubMed] [Google Scholar]
- 54.Yao L, Liang Y, Trahanovsky WS, Serfass RE, White WS. Use of a 13C tracer to quantify the plasma appearance of a physiological dose of lutein in humans. Lipids 2000;35:339−48. [DOI] [PubMed] [Google Scholar]
- 55.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients 2011;3:63−103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, et al. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem 2008;283:4905−11. [DOI] [PubMed] [Google Scholar]
- 57.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production. FASEB J 2010;24:1656−66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr 2012;15:1688−96. [DOI] [PubMed] [Google Scholar]
- 59.Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory JF III, Tanumihardjo SA. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am J Clin Nutr 2015;102:497−504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauberlich H, Hodges R, Wallace D, Kolder H, Canham J, Hood J, Raica N Jr, Lowry L. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 1974;32:251−75. [DOI] [PubMed] [Google Scholar]
- 61.Haskell MJ, Lembcke JL, Salazar M, Green MH, Peerson JM, Brown KH. Population-based plasma kinetics of an oral dose of [2H4]retinyl acetate among preschool-aged, Peruvian children. Am J Clin Nutr 2003;77:681−6. [DOI] [PubMed] [Google Scholar]