Abstract
Study was conducted to evaluate the antioxidative activity of methanolic (ME), ethanolic (EE) and butanolic extracts (BE) of selected gourd vegetables. The antioxidant activity was investigated using different assays namely ferric thiocyanate test (FTC), thiobarbituric acid test (TBA), ferric reducing antioxidant power (FRAP) and DPPH free radicals scavenging test. A densitometric HPTLC analysis was performed for the analysis of phenolic acids and flavonoids. Different extracts of the selected gourd vegetables revealed different antioxidant activity. Different extracts of Lagenaria siceraria, Momordica charantia and Luffa cylindrica revealed significantly higher (p < 0.05) concentrations of total phenols, flavonids, tannins and carotenoids content and also the antioxidant activity in comparison to remaining vegetable extracts. Correlation studies indicated that FRAP test best described the antioxidant activity of phenols, flavonoids and carotenoids (r = 0.854, 0.692 and 0.915 respectively). HPTLC profiles revealed the presence of maximum number of phenolic acids and flavonoids in L. siceraria and M. charantia.
Keywords: Vegetable crops , Antioxidant activity, Phenolic compounds, Carotenoids
Introduction
Fruits, vegetables and other plant based foods contains significant amount of bioactive compounds such as phenolic acid, flavonoids, tannins, alkaloids, saponins, terpenoids etc. that can provide desirable health benefits beyond basic nutrition. These naturally occurring compounds have attracted great attention from the scientific community for their antioxidant properties and their implication in a variety of biological mechanisms at the base of degenerative processes (Kaur and Kapoor 2001). Phenolic compounds have been considered as most important and ubiquitous compounds in the plant kingdom (Naczk and Shahidi 2006), which are beneficial for human health, decreasing the risk of degenerative diseases by reduction of oxidative stress and inhibition of macromolecular oxidation (Pereira et al. 2007). The antioxidant activity of phenolics is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers. Many mechanisms have been proposed to explain biological protective effects of polyphenols, which, for several years, have been often ascribed mainly to their antioxidant capacity. However, this view is now challenged by recent findings, and more complex actions are being investigated. Because of very low bioavailability of polyphenols and difficult translation of in vitro findings regarding antioxidant actions in to in vivo actions, the overall cellular antioxidant activity of the polyphenols appears to be debatable. Considering these findings, in the past few years, the dimensions of research on polyphenols have expanded beyond antioxidant activity unveiling several nutrition-pharma biological activities of these compounds in light of more complex molecular level mechanisms (Visioli et al. 2010). Studies have demonstrated that, besides antioxidant and anti-inflammatory capacities, phenolics may engage with cellular signalling flow, controlling the action of transcription factors and subsequently affecting the expression of those genes involved in cellular metabolism and cellular survival (Chiva-Blanch and Visioli 2012; Giampieri et al. 2014; Forbes-Hernandez et al. 2014). Olive oil phenolics have been found to decrease the cardiovascular mortality (Keys et al. 1986; Covas et al. 2006). The beneficial effects of polyphenols in skin applications as anti-inflammatory agents has been ascribed to the capacity of polyphenols in altering signal transduction and epigenetic regulation of gene expression (Pastore et al. 2011; Pastore et al. 2012; Rhodes et al. 2013). Another indirect way through which polyphenols may exert antioxidative protection is that these bioactive compounds might increase gene transcription of a factor that regulates enzymes involved in the antioxidant function (Na and Surh 2008). The biological effects of polyphenols on metabolically active tissues affect insulin sensitivity, inflammation, lipid metabolism which could be triggered by activation of peroxisome proliferation activated receptors (PPARs) by polyphenols (Cho et al. 2010). Recent studies (Sun et al. 2013) on prostate cancer cells revealed that polyphenols rich tea extracts inhibited cell proliferation, induced apoptosis, and provide reduction of the mitochondrial membrane potential. Apart from polyphenols, saponins are glucosides found in vegetables and their non-sugar moiety has been reported to show anti oxidant property (Chen et al. 2014). The plant terpenoids are a large class of naturally occurring organic chemicals and are used extensively for their aromatic qualities as well as for their antioxidant activity (Papaefthimiou et al. 2014).
Gourd vegetables are consumed worldwide and in India these vegetables have long history of their use in Indian ayurveda also. The health promoting ability of these vegetables is attributed to the antioxidant properties of various phytochemicals present in them (Saha et al. 2011). Studies have revealed the presence of a wide range of secondary metabolites including flavonoids, triterpenoid, saponins and phenolic acids in gourd vegetables possessing distinct biological activities (Rizvi et al. 2009). Gourd vegetables such as L. siceraria, L. cylindrica, Luffa acutangula and Cucurbita pepo have been reported for the presence of secondary metabolites such as alkaloids, flavonoids, glycosides, steroids and saponins, etc. which possess antioxidant activity (Irshad et al. 2010). The antioxidant activity of M. Charantia and Cucurbia maxima has been attributed mainly to their phenolic acids content (Ibrahim et al. 2011; Kubola and Siriamornpun 2008). The extraction mechanism and type and polarity of extracting solvents also have been reported to exert significant effects on extractability of phytochemicals and hence the in vitro antioxidative acitivity of plant extracts (Naczk and Shahidi 2006; Tsuda et al. 1994). The present investigation was undertaken to investigate the polyphenolic compounds and antioxidant potential of methanolic, ethanolic and butanolic extracts of selected gourd vegetables commonly consumed in India.
Materials and methods
The various chemicals and reagents used in the present study were of analytical grade and were purchased from Sigma-Aldrich fine chemicals (U.S.A.), Himedia (India), Fisher scientific (Fair Lawn. New Jersey) and from Merck (Darmstadt, Germany).
Preparation of vegetable extracts
Freshly harvested vegetables of bitter gourd (M. charantia), bottle gourd (L. siceraria), ridge gourd (L. acutangula), sponge gourd (L. cylindrica), pointed gourd (Trichosanthes dioica), pumpkin (C. maxima) and summer squash (C. pepo) were purchased from local market in Rohtak (India). The macerates (500 g) of washed, cleaned and peeled vegetables were separately extracted with 400 ml of methanol, ethanol and n-butanol respectively for 6 days at room temperature with intermediate shaking. After centrifugation and filtration, the filtrates were concentrated in a rotary evaporator at 45 °C under reduced pressure (97.3 kPa) to a constant weight and stored in dark at -20 °C.
Phytochemicals screening
Phytochemicals screening of the extracts was performed as described by Sofowora (1996), Trease and Evans (1989) and Harborne (1984).
Determination of total phenols content
The total phenolic content of the extracts was determined using Folin–Ciocalteu reagent (Zhou and Yu 2006) as gallic acid equivalents using regression equation of the standard curve for gallic acid (Y = 4.262× + 0.043; R2 = 0.997).
Determination of total flavonoids
The method as reported by Meda et al. (2005) with some minor modifications was used for determination of flavonoides. The results were expressed as quercetin equivalents using regression equation from standard curve of quercetin as Y = 14.32× + 0.047; R2 = 0.990).
Determination of tannin content (Vanillin–HCl method).
Condensed tannins were determined by slight modification of the vanillin method (Burns 1971). The regression equation Y = 0.5523× + 0.0273; R2 = 0.998 as obtained from the standard curve was used to express the results as mg equivalents of catechin/100 g (dwb).
Determination of β- carotene
The method as reported by Santra et al. (2005) was used for determination of β-carotene. The amount of β- carotene was calculated using regression equation Y = 0.1398× + 0.0463 (R2 = 0.9809) as obtained from the standard curve.
Determination of antioxidant activity of extracts
Ferric thiocyanate (FTC) method
The FTC method was adapted as described by Kikuzaki and Nakatani (1993). The percent inhibition was calculated from the absorbance value on the final day.
Thiobarbituric acid (TBA) method
The samples prepared for FTC method were also used to measure the percent inhibition by TBA method as described by Kikuzaki and Nakatani (1993). Antioxidant activity was recorded on the final day of the FTC assay measuring the percent inhibition.
Ferric reducing antioxidant power (FRAP) method
FRAP assay was adapted as described by Moyer et al. (2002). Based on the measured absorbance, the concentration of FeSO4 was measured (mM FeSo4/100 g dwb) from the regression equation of the standard curve of FeSO4 (Y = 0.5783 × 0.0042; R2 = 0.9991).
Evaluation of the free radical scavenging activity by DPPH assay
DPPH free radical scavenging activity assay used by Chan et al. (2007) was used for determining the DPPH free radical scavenging activity of the extracts. The scavenging activity of each extract on DPPH radical was expressed as IC50 (The effective concentration of the extract required for 50 % scavenging of DPPH).
HPTLC analysis of phenolic acids and flavonoid compounds
A densitometric HPTLC analysis was performed for the analysis of phenolic acids and flavonoids. HPTLC Silica gel plates (60 F254, 10 × 10 cm, Merck) were washed and pre developed with methanol and dried on TLC Plate heater (CAMAG, Switzerland) at 120 °C for 5 min. The standard stock solution (0.1 mg/ml) and sample (100 mg/ml) was prepared in HPTLC grade methanol. These solutions were filtered through 0.2 μ syringe filter before loading in the sample syringe (Hamilton, Bonaduz, Switzerland). The sample (8 μL) was applied with a 100 μL sample syringe using automatic Linomat-5 system (CAMAG, Switzerland). The stock solutions of the reference compounds were prepared in methanol at different concentration levels (2–8 μL). The plates were developed in a vertical glass chamber (CAMAG) until the respective mobile phase i.e. solvent system I (6.4 chloroform: 3.9 hexane: 2.0 methanol: 0.5 formic acid) for detection of gallic acid, caffeic acid, quercetin, apigenin, kampferol and chlorogenic acid; solvent system II (4 chloroform: 1 hexane: 1 methanol: 1 formic acid) for detection of p-coumaric acid, leutolin, myricetin, catechin and ellagic acid and solvent system III (4.5 acetonitrile: 1.0 methanol: 0.5 water) for detection of ferulic acid, benzoic acid, cinnamic acid and vanillic acid) respectively rose to 80 % of the plate height. The developed plates were dried on TLC plate at 120 °C for 5 min and cooled at room temperature. The densitometric evaluation was performed with a TLC scanner 3 (CAMAG) at wavelength 254 nm. The plate image was documented by TLC visualizer documentation system (CAMAG). The quantification and documentation was done by winCAT software. The peak table, peak display and peak densitograms were identified. The concentration of each compound was determined by using calibration curve prepared by plotting the peak area versus concentration of standard compound.
Statistical analysis
Three independent replicates (n = 3) were obtained from each treatment and the results were reported as means ± standard deviation (SD). Analysis of variance was performed by one way ANOVA analysis (SPSS 19.0) followed by Tukey’s HSD post hoc comparison test at p < 0.05. Linear regression analysis between the % inhibition of DPPH and the concentration was done for each sample (n = 3) using a liner function. Pearson correlation coefficients among various antioxidant assays and phytochemicals were performed using SPSS 19.0 software.
Results and discussion
The present study was conducted to evaluate the antioxidant activity of selected gourd vegetables and, in particular, it investigated the influence of different extracting solvents had on polyphenolic compounds extraction and hence antioxidant activity.
Yield of extraction
Three extracting solvents namely methanol, ethanol and butanol were evaluated for their effectiveness to extract antioxidants from fresh gourd vegetables. A significant variation (P < 0.05) in the yields of different extracts ranging from 2.62 to 6.00 g/100 g (fresh weight basis) was observed among the selected vegetables. The yield of ME and EE was the maximum in case of L. acutangula whereas maximum yield of BE was observed in case of L. siceraria. The variation in extract yield depends mainly on the type of solvent, the extraction methods being adopted and the substrate (Sun and Ho 2005). Solvents with low viscosity have low density and high diffusivity that allows them to diffuse easily into the pores of the vegetable cells to leach out the bioactive constituents (Naczk and Shahidi 2006).
Phytochemicals screening
The phytochemicals screening of the selected gourd vegetables showed the presence of considerable amounts of flavonoids, tannins, saponins, terpenoids and alkaloids as shown in Table 1. The results of the study indicated that among all the selected vegetables, M. charantia was the richest in phytochemicals including polyphenolic compounds whereas, these phytochemicals except alkaloids and saponins were absent in C. maxima. Flavonoids and tannins were found to be present in most of the extracts except C. maxima extracts. Tannins were present considerably in ME and EE of M. charantia and L. siceraria whereas; the same extracts of T. dioica showed considerable presence of flavonoids. Previous studies have established the presence of various phytochemicals including flavonoids (Ibrahim et al. 2011; Irshad et al. 2010; Sharma et al. 2012) and phenolic acids (Kubola and Siriamornpun 2008) in gourd vegetables. Flavonoids are considered as most popular natural antioxidants and effective secondary metabolic products as they help to provide protection against oxidation at cellular level by interfering in enzyme activity, chelation of redox active metals and by scavenging free radicals (Gyamfi and Aniya 2002). The results of the present study on phytochemicals screening are also indicative of presence of flavonoids as the most prominent phytochemicals in almost all the selected gourd vegetables.
Table 1.
Phytochemicals screening of gourd vegetables
| Sample | Flavonoids | Tannin | Saponin | Terpenoids | Alkaloids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | EE | BE | ME | EE | BE | ME | EE | BE | ME | EE | BE | ME | EE | BE | |
| C.pepo | + | + | + | + | + | + | − | + | − | + | + | + | − | + | − |
| C.maxima | − | − | − | − | − | − | − | + | + | − | − | − | + | + | + |
| T.dioica | ++ | ++ | + | + | + | + | + | − | − | + | + | − | + | − | − |
| L.acutangula | + | + | + | − | − | + | + | + | − | + | + | ++ | − | + | − |
| M.charantia | + | + | + | ++ | ++ | + | ++ | ++ | − | ++ | + | + | + | + | + |
| L.siceraria | + | + | + | ++ | ++ | + | + | − | − | − | + | + | − | − | + |
| L.cylindrica | + | + | + | − | + | − | + | + | − | + | + | + | + | + | − |
+ Present moderately, ++ present strongly, − Absent
ME Methanolic extract, EE Ethanolic extract, BE Butanolic extract
Quantitative testing of phytochemicals
A wide variation in contents of different phytochemicals was observed among the selected vegetables (Table 2). The total phenolic content was significantly different among almost all the vegetables in their respective extracts (P < 0.05) and it varied in order of C.pepo > M.charantia > L.siceraria > L.acutangula > L.cylindrica > T.dioica > C. maxima in ME; L.siceraria > M.charantia > C.pepo > L.cylindrica > L.acutangula > T.dioica > C. maxima in EE and L.siceraria > M.charantia > L.cylindrica > C.pepo > L.acutangula > T.dioica > C. maxima in EE. The recovery of total phenolics content was the highest in BE and in different gourd vegetables it varied from 53.1 to 658.9 mg GE/100 g (dwb) whereas; it was the lowest in EE ranging from 56.1 to 463.6 GE/100 g (dwb). Studies have reported total phenols content ranging from 51.6 to 750 mg/100 g in various cucurbits (Lee, You, Hwang, Lee, Lee & Jun., 2012). Phenolic compounds are considered as the main contributor to antioxidant activity in plant extracts due to their higher value in total phenolic content (Hodzic et al. 2009). The polyphenol antioxidant capacity has been taken into account as one of the outstanding mechanisms of action in inhibiting mutagenesis and cancer initiation, by means of their capacity to scavenge ROS, activate antioxidant enzymes, prevent carcinogen- induced DNA adduct formation, enhance DNA repair, and reduce overall oxidative DNA injury (Stoner et al. 2008).
Table 2.
Total phenols, flavonoids, tannins and carotenoids content of different gourd vegetables
| Vegetables crops | Total phenols (mg GE/100 g) | Total flavonoids (mg QE/100 g) | Tannin content (mg CE/100 g) | Carotenoids content (mg β carotene/100 g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | EE | BE | ME | EE | BE | ME | EE | BE | ME | EE | BE | |
| C.pepo | 519.81 ± 2.35 g | 336.25 ± 1.52 e | 272.01 ± 1.57 d | 64.34 ± 1.37 g | 56.83 ± 0.59 f | 45.03 ± 0.90 c | 18.11 ± 4.80 c | 7.72 ± 1.47 b | 6.81 ± 3.36 a | 3.78 ± 0.08 c | 1.19 ± 0.10 b | 2.44 ± 0.08 b |
| C.maxima | 62.53 ± 0.92 a | 56.11 ± 0.69 a | 53.15 ± 0.80 a | 3.72 ± 0.20 a | 3.73 ± 0.22 a | 10.41 ± 0.39 a | 2.94 ± 0.20 a | 1.81 ± 0.76 a | 7.63 ± 1.34 a | 0.50 ± 0.03 a | 0.24 ± 0.05 a | 0.25 ± 0.03 a |
| T.dioica | 163.22 ± 1.53 b | 118.82 ± 1.11 b | 100.54 ± 1.00 b | 30.26 ± 0.44 c | 25.24 ± 0.20 b | 34.95 ± 0.25 b | 11.24 ± 1.29 b | 8.44 ± 0.81 b | 17.3 ± 1.07 c | 3.82 ± 0.06 c | 1.34 ± 0.04 b | 5.11 ± 0.09 d |
| L.acutangula | 228.62 ± 1.77 d | 156.23 ± 2.02 c | 251.12 ± 1.19 c | 32.51 ± 0.41 d | 27.91 ± 0.52 c | 65.36 ± 0.26 d | 3.11 ± 1.71 a | 8.41 ± 2.07 b | 15.5 ± 1.07 bc | 2.43 ± 0.05 b | 2.31 ± 0.10 c | 3.42 ± 0.05 c |
| L.siceraria | 355.17 ± 0.91 e | 463.64 ± 1.37 g | 658.96 ± 2.41 f | 54.32 ± 0.39 f | 84.85 ± 0.35 g | 278.95 ± 0.55 g | 6.64 ± 0.96 ab | 8.13 ± 1.40 b | 10.6 ± 2.03 abc | 7.34 ± 0.03 e | 14.8 ± 0.10 e | 19.41 ± 0.18 f |
| M.charantia | 501.73 ± 2.52 f | 440.79 ± 2.12 f | 451.84 ± 3.49 e | 47.87 ± 0.43 e | 46.93 ± 0.56 e | 104.17 ± 0.57 e | 6.42 ± 1.84 ab | 24.42 ± 1.92 c | 9.69 ± 4.74 ab | 15.7 ± 0.11 f | 20.4 ± 0.13 f | 22.05 ± 0.21 g |
| L.cylindrica | 216.30 ± 2.09 c | 300.06 ± 2.51 d | 275.16 ± 2.62 d | 24.51 ± 0.48 b | 45.44 ± 0.72 d | 146.38 ± 0.75 f | 2.91 ± 1.64 a | 9.25 ± 2.27 b | 5.64 ± 2.37 a | 4.63 ± 0.10 d | 9.94 ± 0.16 d | 12.94 ± 0.17 e |
| Mean | 292.48 | 267.41 | 294.68 | 36.79 | 41.56 | 97.89 | 7.33 | 9.74 | 10.45 | 5.45 | 7.17 | 9.37 |
| F-Statistics at df = 6,14 (p = 0.000) | F = 26,852.9 | F = 25,904.2 | F = 29,559.6 | F = 3072.1 | F = 8496.7 | F = 77,986.6 | F = 16.9 | F = 54.9 | F = 8.6 | F = 14.374 | F = 17,520.2 | F = 15,457.5 |
Values are presented as mean ± SD (n = 3) and referred to the dry weight. Means in columns followed by different letters differed significantly at 5 % level of significance (p < 0.05)
GE Gallic acid equivalent, QE Quercetin equivalent, CE Catechin equivalent
ME Methanolic extract, EE Ethanolic extract, BE Butanolic extract
The flavonoids content in ME, EE and BE varied from 3.7 to 64.3, 3.7 to 84.8 and 10.4 to 278.9 mg QE/100 g (dwb) respectively and it differed significantly (P < 0.05) among all the vegetables in their respective extracts. C. pepo, L.siceraria and L.cylindrica exhibited the highest concentration of flavonoids in ME, EE and BE respectively. Maximum average concentration of flavonoids (97.8 mg QE/100 g dwb) in the selected vegetables was observed in BE followed by EE and ME. Wide variations in flavonoids content (150–630 mg/100 g) in different extracts of L.cylindrica have been reported (Sharma et al. 2012; Lee et al. 2012). The flavonoids are the main bioactive compounds found in fruits and vegetables and in particular, vegetables flavonoids have been reported to be dominated by glycosidic flavonols (Han et al. 2007). The tannin content among the selected vegetable extracts ranged from 1.8 to 24.4 mg CE/100 g (dwb). Average tannin content of the selected vegetables in different extracts was in order of ME (7.3 mg CE/100 g) < EE (9.7 mg CE/100 g) < BE (10.4 mg CE/100 g). Recent interest in tannins has been stimulated by the potential health benefits arising from the antioxidative activity. Good contribution of tannins in antioxidant activity has been observed by Gomes de Melo et al. (2010). The results showed that the carotenoids content of L. siceraria, M. charantia and L. cylindrica measured as β-carotene was significantly higher when compared to remaining gourd vegetables (P < 0.05) with M. charantia exhibiting the highest content in all types of extracts. The total carotenoids content of the selected gourd vegetables varied from 0.2 to 22.0 mg β-carotene/100 g (dwb) in various extracts. Previous studies have reported wide variations (0.006 to 7.4 mg/100 g) in β -carotene of different gourd vegetables (Dey et al. 2005).
Antioxidant activity of vegetable extracts
The antioxidant capacity might be influenced by several factors and could not be fully described by a single assay. In addition, most natural antioxidants are multifunctional and therefore, a reliable antioxidant evaluation protocol requires different antioxidant activity assessments to take into account various mechanisms of antioxidant action. A number of methods and variations have been developed and applied for the assessment of antioxidant capacity but very often (Niki 2011). Therefore, in the present study also the antioxidant activity was assessed using different assays based on different approaches. The results of total antioxidant activity of different extracts as determined using different assays are as described in Table 3.
Table 3.
Antioxidant activity of different extracts of gourd vegetables determined by FTC, TBA, FRAP & DPPH assays
| Vegetable crops | FTC (% inhibition) | TBA (% inhibition) | FRAP (mM FeSO4/100 g) | DPPH (IC50) (mg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | EE | BE | ME | EE | BE | ME | EE | BE | ME | EE | BE | |
| C.pepo | 30.42 ± 0.73 c | 16.72 ± 1.27 c | 19.03 ± 0.87 b | 40.42 ± 0.44 c | 28.03 ± 0.39 c | 30.15 ± 0.39 c | 973.83 ± 19.56 d | 714.78 ± 16.20 c | 440.13 ± 16.27 b | 0.35 | 0.12 | 0.36 |
| C.maxima | 19.31 ± 0.81 b | 4.89 ± 0.94 a | 5.63 ± 0.66 a | 30.14 ± 0.38 b | 17.72 ± 0.56 a | 18.53 ± 0.39 a | 130.72 ± 6.51 a | 80.14 ± 6.22 a | 39.14 ± 6.32 a | 0.32 | 0.34 | 0.37 |
| T.dioica | 37.54 ± 0.57 e | 16.61 ± 0.55 c | 26.34 ± 0.62 d | 46.69 ± 0.51 e | 28.44 ± 0.39 c | 36.76 ± 0.39 e | 936.14 ± 13.34 d | 466.48 ± 7.34 b | 624.62 ± 8.08 c | 0.18 | 0.20 | 0.29 |
| L.acutangula | 35.42 ± 0.81 d | 9.78 ± 0.64 b | 23.92 ± 0.78 c | 44.74 ± 0.39 d | 22.38 ± 0.54 b | 34.62 ± 0.38 d | 438.47 ± 13.91 b | 480.24 ± 16.86 b | 670.89 ± 15.97 d | 0.30 | 0.27 | 0.65 |
| L.siceraria | 79.64 ± 0.58 g | 77.71 ± 0.70 e | 31.63 ± 0.63 e | 83.82 ± 0.38 g | 81.91 ± 0.35 e | 41.54 ± 0.40 f | 953.21 ± 12.70 d | 1404.51 ± 15.38 d | 2374.24 ± 15.85 g | 0.01 | 0.03 | 0.15 |
| M.charantia | 71.71 ± 0.70 f | 26.03 ± 0.79 d | 76.64 ± 0.62 f | 76.61 ± 0.42 f | 36.24 ± 0.39 d | 80.93 ± 0.46 g | 1532.33 ± 14.34 e | 1711.44 ± 16.46 f | 2130.61 ± 24.5 f | 0.02 | 0.03 | 0.03 |
| L.cylindrica | 14.69 ± 0.58 a | 17.94 ± 0.53 c | 18.63 ± 0.90 b | 26.73 ± 0.38 a | 29.13 ± 0.42 c | 25.72 ± 0.38 b | 751.44 ± 12.78 c | 1607.82 ± 17.17 e | 828.72 ± 18.48 e | 0.14 | 0.20 | 0.17 |
| Mean | 41.24 | 24.24 | 28.83 | 49.87 | 34.83 | 38.32 | 816.59 | 923.63 | 1015.47 | 0.19 | 0.17 | 0.29 |
| F-Statistics at df = 6,14, p = 0.00 | F = 3925.4 | F = 2739.1 | F = 2831.1 | F = 8444.1 | F = 7172.6 | F = 7708.7 | F = 3132.1 | F = 6022.8 | F = 9012.1 | |||
Values are presented as mean ± SD (n = 3) and referred to the dry weight. Means in columns followed by different letters differed significantly at 5 % level of significance (p < 0.05)
FTC % Inhibition of BHT = 88.8 ± 0.28, Vit. E = 74.6 ± 0.16
TBA % Inhibition of BHT = 90.9 ± 0.47, Vit. E = 78.3 ± 0.19
IC50 value of Ascorbic acid = 0.005 mg/ml
ME Methanolic extract, EE Etanolic extract, BE Butanolic extract
FTC and TBA methods
The FTC method was used to measure the peroxide level during the initial stage of lipid (linoleic acid) oxidation. The results of percent inhibition of ME, EE and BE showed that the magnitude of antioxidative potency varied with the type of extract as ME showed maximum percent inhibition (41.2 %) followed by BE (28.8 %) and EE (24.2 %) respectively. This could be because of difference in the type and concentration of antioxidative compounds in these extracts. The results of correlation studies as shown in Table 4 revealed a highly significant and positive correlation of the FTC assay results with phenols content as well as carotenoids content suggesting that phenols and carotenoids were the main compounds responsible for the antioxidative activity of the gourd vegetables as described by FTC method. These results are in agreement with the previous studies which have reported the positive correlation between phenol content and total antioxidant activity (Kubola and Siriamornpun 2008). Although total phenols content of the EE and BE extracts was also comparable to that of ME, but the antioxidant activity of the EE and BE was much lower as compared to ME. Similarly, the flavonoids and carotenoids content of the ME of the selected vegetables was also lower in comparison to the EE or BE but still the antioxidant activity of the ME was higher. These observations in the present study suggested a wide variation in the nature and kind of phenolic and carotenoids compounds recovered in the different solvents and hence differences in the antioxidative potency of the extracts. The effectiveness of phenolics and flavonoids as antioxidants is not only because of their composition or relative amount but also influenced by the degree of polymerization, concentration and interaction of their diverse chemical structures to the colorimetric assays. Polymeric polyphenols are more potent antioxidants than simple monomeric phenols (Moure et al. 2001). Thus, the higher levels of TPC and TFC do not necessarily correspond to the higher antioxidant responses (Parejo et al. 2002).
Table 4.
Pearson correlation coefficients (r) between phytochemicals and antioxidant activity determined by different assays
| Phenols | Flavonoids | Tannins | Carotenoids | FTC | TBA | FRAP | |
|---|---|---|---|---|---|---|---|
| Flavonoids | 0.71** | ||||||
| Tannin | 0.34 | 0.14 | |||||
| Carotenoids | 0.76** | 0.64** | 0.34 | ||||
| FTC | 0.58** | 0.24 | 0.03 | 0.58** | |||
| TBA | 0.57** | 0.22 | 0.04 | 0.57** | 0.99** | ||
| FRAP | 0.85** | 0.69** | 0.38 | 0.92** | 0.56** | 0.56** | |
| DPPH | 0.48* | 0.24 | −0.51 | 0.64** | 0.60** | 0.59** | 0.59** |
*p < 0.05
**p < 0.01,n = 21
During the oxidation process, peroxide is gradually decomposed to malonaldehyde, which was measured by TBA method on the final day of the incubation period. Percent inhibition as measured by TBA method in various types of extracts revealed wide variations among the selected vegetables being highest for ME of L. siceraria (83.8 %) followed by EE and BE of L. siceraria (81.9 %) and M. charantia (80.9 %) respectively. Again the ME of the selected vegetables showed the highest percent inhibition when compared to their EE or BE. As observed in case of FTC method, a highly significant and positive correlation of the TBA results with phenols content as well as flavonoids content indicated that phenols and carotenoids were the main phytochemicals responsible for the antioxidant activity of the gourd vegetables as described by TBA method. A very high positive correlation (r = 0.99, P < 0.01) between FTC and TBA assay showed that the increase in peroxide level caused formation of malonaldehyde compounds (Zin et al. 2002).
Ferric reducing antioxidant power (FRAP) method
As indicated by the results of FRAP assay given in Table 3, significant differences were observed in the FRAP values among most of the vegetables in their respective extracts (P < 0.05). The average FRAP values of the all the selected vegetables, varied from 816.6 to 1015.4 mM FeSO4/100 g being highest in BE followed by EE and ME. The antioxidative activity of all the three types of extracts of L. siceraria, M. charantia and L. cylindrica as determined with FRAP method was significantly higher (P < 0.05) when compared with remaining gourd vegetables in their respective extracts with BE of L.siceraria showing the highest antioxidative activity. The results of correlation studies have indicated highly significant positive correlations of FRAP values with total phenols, flavonoids and carotenoids suggesting that FRAP assay best described the antioxidative activity of gourd vegetables and the antioxidative activity was mainly ascribed to the phenols and carotenoids content. These results were in agreement with observations of Kubola and Siriamornpun (2008) and Butsat and Siriamornpun (2009) who reported a positive correlation of total phenols with the FRAP value.
DPPH radical scavenging activity
The DPPH method is used to estimate the radical scavenging activity of antioxidant compounds. Free radical scavenging activity for DPPH radical was expressed as IC50 value (the concentration required to scavenge 50 % of DPPH). The antioxidant activity as adjudged by IC50 values of different vegetables was in order of L.siceraria > M.charantia > L.cylindrica > T.dioica > L.acutangula > C. maxima > C.pepo in ME; L.siceraria > M.charantia > C.pepo > L.cylindrica > T.dioica > L.acutangula > C. maxima in EE and M.charantia > L.siceraria > L.cylindrica > T.dioica > C.pepo > C.maxima in BE. The free radical scavenging activity of the EE was the highest where as reverse was true for BE. Interpretation of the results of DPPH free radical scavenging power in light of the results as shown in Table 2 showed that radical-scavenging activity differs not only by the concentration of phenolic compounds but also with the nature and kind of phenolic compounds which may vary with degree of hydroxylation and polymerisation (Moure et al. 2001). Statistical correlations as shown in Table 4 revealed significant positive correlations of DPPH results with total phenols and carotenoids indicating that scavenging activity of the gourd vegetables extracts was ascribed mainly to their phenols and carotenoids. Sun and Ho (2005)) also reported a significant correlation between total phenols and scavenging effect of DPPH.
Identification of phenolic acids and flavonoids by HPTLC
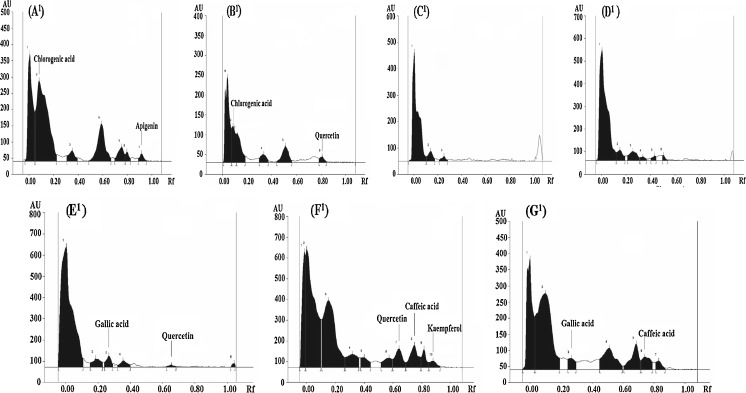
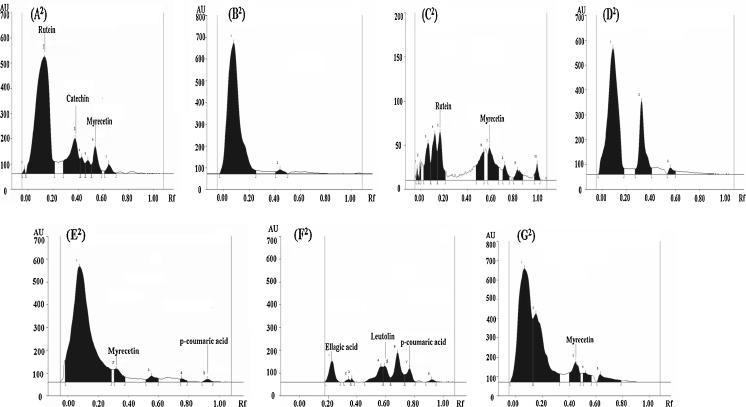
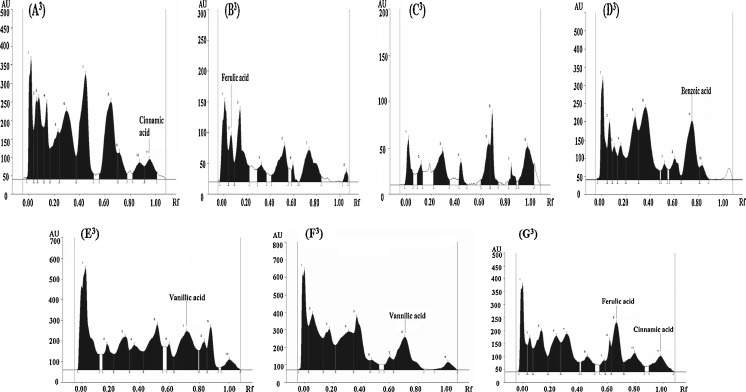
Considering the fact that the average antioxidant activity of ME of the gourd vegetables was reasonably higher, the ME of gourd vegetables were identified and quantified for their phenolic acids and flavonoids by comparing their Rf value. The HPTLC profiles of ME of all the vegetables in solvent system I, II and III are as shown in Figs. 1, 2 and 3 respectively. The distribution of phenolic acids and flavonoids as detected from HPTLC profiles revealed the presence of chlorogenic acid, cinnamic acid, myrecetin, apigenin, rutein and catechin in concentrations of 303.3, 8.5, 53.1, 3.7, 727.8 and 263.8 μg/ml respectively in C. pepo. Rutein, one of the primary flavonoids present in C. pepo, has been studied for beneficial human health effects, such as antioxidant effect and anti-inflammatory effect (Koda et al. 2008). Chlorogenic acid (66.5 μg/ml) and ferulic acid (37.3 μg/ml) were detected as phenolic acids in ME of C.maxima whereas; no flavonoid amongst the studied flavonid compounds was detected in C.maxima extract. This observation was in agreement with the results of qualitative testing which showed absence of flavonoids in C.maxima extracts. However, presence of flavonoids, although in trace amounts, as suggested by the results of quantitative estimation indicated that there could be flavonoid compounds in C.maxima other than those studied. Myrecetin and rutein in concentrations of 28.7 and 0.8 μg/ml respectively were present as flavonoid compounds in T.dioica. Interestingly, L.acutangula showed only benzoic acid as phenolic acid present in significantly high concentration (1817.5 μg/ml). The HPTLC profile of L.siceraria and M.charantia revealed the presence of many of the phenolic acids and flavonoids in comparison to all other gourd vegetables. Caffeic acid, p-coumaric acid, vanillic acid, quercetin and myrecetin in concentrations of 31.1, 19.1, 172.6, 35.5 and 43.1 μg/ml respectively were detected in L.siceraria. Caffeic acid, p-coumaric acid, ferulic acid, ellagic acid, quercetin, apigenin, kaempferol and leutolin were found in M.charantia with ellagic acid and p-coumaric acid being present in maximum (291.2 μg/ml) and minimum (21.13 μg/ml) concentrations respectively. M. charantia showed caffeic acid, vanillic acid, quercetin, kaempferol, and leutolin content of 55.04, 198.81, 76.24, 12.33 and 39.74 microgram/mL, respectively. Ma et al. (2007) reported apigenin as natural flavonoid found in fruits and vegetables. Apigenin has been shown to retard the growth of cancer cells and exhibit the antioxidant activity (Miyoshi et al. 2007). Leutolin, which is categorized under flavones and was present only in M.charantia, has a variety of pharmacological activities including anti hypertensive and antioxidative effect (Qian et al. 2010). In L.cylindrica, a concentration of 26.8, 18.4, 49.6, 8.6 and 35.7 μg/ml was observed for gallic acid, caffeic acid, ferulic acid, cinnamic acid and myrecetin respectively. The previous studies have reported vanillic acid, benzoic acid, caffeic acid, ferulic acid, prottocatechuic acid, myricetin, quercetin, kaempferol, leutolin and apigenin in different fractions of gourd vegetables (Irshad et al. 2010; Kubola and Siriamornpun 2008).
Fig. 1.
(A 1 -G 1 ). HPTLC profiles of ME of different gourd vegetables as developed in solvent system I (6.4 chloroform: 3.9 hexane: 2.0 methanol: 0.5 formic acid)
Fig. 2.
(A 2 -G 2 ). HPTLC profiles of ME of different gourd vegetables as developed in solvent system II (4.0 chloroform: 1.0 hexane: 1.0 methanol: 1.0 formic acid)
Fig. 3.
(A 3 -G 3 ). HPTLC profile of ME of different gourd vegetables as developed in solvent system III (4.5 acetonitrile:1.0 methanol: 0.5water)
Conclusion
From the results of the present study it can be concluded that the gourd vegetables especially L. siceraria, M. charantia and L. cylinderica are rich source of phytochemicals like phenols, flavonoides and carotenoids to varying extents. The high antioxidant activity of gourd vegetable extracts could be attributed to their high levels of phenols and flavonoid compounds in particular. Butanol can be recommended as most appropriate solvent for the recovery of phytochemicals and hence increased antioxidant activity from gourd vegetables of L.siceraria., M.charantia and L. cylindrical whereas methanol could be most effective solvent for the vegetables of C. pepo, C. maxima, T. dioica etc. To measure the antioxidant capacity of selected gourd vegetables, we suggest that FRAP assay as most appropriate method. The results have also indicated that the nature of the extracting solvent could also affect to a great extent the kind and concentration of extracted compounds and measurement of the antioxidant activity in vitro. From the observations of quantitative testing and HPTLC profiles of the extracts, it can also be concluded that there could be even wider range of phytochemicals in gourd vegetables. Therefore, more detailed qualitative and quantitative analyses of the phytochemicals in gourd vegetables will be necessary to elucidate the antioxidant activity of gourds. Studies are also required to evaluate the comparative efficacies of various phytochemicals in gourds regarding their antioxidant potential.
Acknowledgments
The authors duly acknowledge the financial support extended in this major research project by University Grants Commission, Government of India.
References
- Burns RE. Method for estimation of tannin in grain sorghum. Agron J. 1971;63(3):511–512. doi: 10.2134/agronj1971.00021962006300030050x. [DOI] [Google Scholar]
- Butsat S, Siriamornpun S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of thai rice. Food Chem. 2009;119(2):606–613. doi: 10.1016/j.foodchem.2009.07.001. [DOI] [Google Scholar]
- Chan E, Lim Y, Omar M. Antioxidant and antibacterial activity of leaves of etlingera species (zingiberaceae) in peninsular Malaysia. Food Chem. 2007;104(4):1586–1593. doi: 10.1016/j.foodchem.2007.03.023. [DOI] [Google Scholar]
- Chen Y, Miao Y, Huang L, Li J, Sun H, Zhao Y, Yang J, Zhou W. Antioxidant activities of saponins extracted from radix trichosanthis: an in vivo and in vitro evaluation. BMC Complement Altern Med. 2014;14(1):86. doi: 10.1186/1472-6882-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiva-Blanch G, Visioli F. Polyphenols and health: moving beyond antioxidants. J Berry Res. 2012;2:63–71. [Google Scholar]
- Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR. Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in ice. Am J Respir Crit Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas MI, Nyyssonen K, Poulsen HE, Kaikkonen J, Zunft HJ, Kiesewetter H. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- Dey S, Behera T, Kaur C (2005) Genetic variability in ascorbic acid and carotenoids content in Indian bitter gourd (Momordica charantia L.) germplasm. Cucurbit Genet Coop Rpt:28–91
- Forbes-Hernandez TY, Giampieri F, Gasparrini M, Mazzoni L, Quiles JL, Alvarez-Suarej JM, Battino M. The effects of bioactive compounds from plant foods on mitochondrial function: a focus on apoptotic mechanisms. Food Chem Toxicol. 2014;68:154–182. doi: 10.1016/j.fct.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Giampieri F, Alvarez-Suarej JM, Battino M. Strawberry and human health: effects beyond antioxidant activity. J Agric Food Chem. 2014;62:3867–3876. doi: 10.1021/jf405455n. [DOI] [PubMed] [Google Scholar]
- Gomes de Melo J, De Sousa ATA, Thijan Nobre de Almeida e Castro V, Lyra de Vasconcelos Cabral D, Do Desterro Rodrigues M, Carneiro do Nascimento S, Cavalcanti de Amorim EL, De Albuquerque UP (2010) Antiproliferative activity, antioxidant capacity and tannin content in plants of semi-arid northeastern Brazil. Molecules 15(12):8534–8542 [DOI] [PMC free article] [PubMed]
- Gyamfi MA, Aniya Y. Antioxidant properties of thonningianin A. Isolated from the african medicinal herb. Thonningia Sanguinea Biochem Pharmacol. 2002;63(9):1725–1737. doi: 10.1016/S0006-2952(02)00915-2. [DOI] [PubMed] [Google Scholar]
- Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8(9):950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- Harborne JB. Phytochemical methods. 2nd. London: Chapman and Hall publications; 1984. p. 288. [Google Scholar]
- Hodzic Z, Pasalic H, Memisevic A, Srabovic M, Saletovic M, Poljakovic M. The influence of total phenols content on antioxidant capacity in the whole grain extracts. Eur J Sci Res. 2009;28(3):471–477. [Google Scholar]
- Ibrahim TA, El-Hefnawy H M, El-Hela AA. Antioxidant potential and phenolic acid content of certain cucurbitaceous plants cultivated in Egypt. Nat Prod Res. 2011;24(16):1537–1545. doi: 10.1080/14786419.2010.489049. [DOI] [PubMed] [Google Scholar]
- Irshad M, Ahmad I, Mehdi SJ, Goel HC, Rizvi MMA. Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. Int J Food Prop. 2010;17(1):179–186. doi: 10.1080/10942912.2011.619025. [DOI] [Google Scholar]
- Kaur C, Kapoor H C. Antioxidants in fruits and vegetables – the millennium’s health. Int J Food Sci Technol. 2001;36(7):703–725. doi: 10.1046/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. J Food Sci. 1993;58(6):1407–1410. doi: 10.1111/j.1365-2621.1993.tb06194.x. [DOI] [Google Scholar]
- Koda T, Kuroda Y, Imai H. Protective effect of rutin against spatial memory impairment induced by trimethyltin in rats. Nutr Res. 2008;28(9):629–634. doi: 10.1016/j.nutres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kubola J, Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110(4):881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Lee GO, You YH, Hwang KT, Lee JM, Lee HJ, Jun WJ. Physicochemical characteristics and antioxidant activities of luffa cylindrica (L.) roem. J Korean Soc Food Sci Nutr. 2012;41(6):733–738. doi: 10.3746/jkfn.2012.41.6.733. [DOI] [Google Scholar]
- Ma J, Li Q, Zhao J, Guo Y, Su Q, Ji Z. Effects of apigenin on cell proliferation of human pancreatic carcinoma cell line BxPC-3 in vitro. J Nanjing Med Univ. 2007;21(2):94–98. doi: 10.1016/S1007-4376(07)60023-9. [DOI] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in burkina fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91(3):571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Miyoshi N, Naniwa K, Yamada T, Osawa T, Nakamura Y. Dietary flavonoid apigenin is a potential inducer of intracellular oxidative stress: the role in the interruptive apoptotic signal. Arch Biochem Biophys. 2007;466(2):274–282. doi: 10.1016/j.abb.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, Nunez MJ, Parajo JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J Agric Food Chem. 2002;50(3):519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41(5):1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Niki E. Antioxidant capacity: which capacity and how to assess it? J Berry Res. 2011;1:169–176. [Google Scholar]
- Papaefthimiou D, Papanikolaou A, Falara V, Givanoudi S, Kostas S, Kanellis AK. Genus cistus: a model for exploring labdane-type diterpenes' biosynthesis and a natural source of high value products with biological, aromatic and pharmacological properties. Agric Biol Chem. 2014;2:35. doi: 10.3389/fchem.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C. Comparison between the radical scavenging activity and anti oxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem. 2002;50:6882–6890. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- Pastore S, Lulli D, Potapovich AI, Fidanza P, Kostyuk VA, Dellambra E, de Luca C, Maurelli R, Korkina LG. Differential modulation of stress-inflammation responses by plant polyphenols in cultured normal human keratinocytes and immortalized HaCaT cells. J Dermatol Sci. 2011;63:104–114. doi: 10.1016/j.jdermsci.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Pastore S, Potapovich A, Lulli D, Fidanza P, Kostyuk V, De Luca C, Mikhalchik E, Korkina L. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor (EGFR) system. Antioxid Redox Signal. 2012;16:314–328. doi: 10.1089/ars.2011.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Oliveira I, Sousa A, Valent P, Andrade PB, Ferreira IC, Ferreres F, Bento A, Seabra R, Estevinho L. Walnut (juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol. 2007;45(11):2287–2295. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Qian LB, Wang HP, Chen Y, Chen FX, Ma YY, Bruce IC, Xia Q. Luteolin reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol Res. 2010;61(4):281–287. doi: 10.1016/j.phrs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Rhodes LE, Darby G, Massey KA, Clarke KA, Dew TP, Farrar MD, Bennett S, Watson RE, Williamson G, Nicolaou A. Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid. Br J Nutr. 2013;28:1–10. doi: 10.1017/S0007114512006071. [DOI] [PubMed] [Google Scholar]
- Rizvi MMA, Irshad M, Gamel EH, Salaem BY. Bioefficacies of Cassia fistula: an indian labrum. Afr J Pharm Pharacol. 2009;3:287–292. [Google Scholar]
- Saha P, Mazumder U, Haldar P, Islam A, Kumar RS. Evaluation of acute and subchronic toxicity of lagenaria siceraria aerial parts. Int J Pharm Sci Res. 2011;2(6):1507–1512. [Google Scholar]
- Santra M, Santra D, Rao V, Taware S, Tamhankar S. Inheritance of β-carotene concentration in durum wheat (triticum turgidum L. ssp. Durum) Euphytica. 2005;144(1–2):215–221. doi: 10.1007/s10681-005-5815-6. [DOI] [Google Scholar]
- Sharma D, Rawat I, Goel H. Antioxidant and prebiotic potential of some cucurbits. Res J Medi Plant. 2012;6(7):500–510. doi: 10.3923/rjmp.2012.500.510. [DOI] [Google Scholar]
- Sofowora A. Research on medicinal plants and traditional medicine in africa. The J Altern Complement Med. 1996;2(3):365–372. doi: 10.1089/acm.1996.2.365. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Wang L, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–1674. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Pan S, Miao A, Ling C, Pang S, Tang J, Chen D, Zhao C. Active extracts of black tea (Camellia sinensis) induce apoptosis of PC-3 prostate cancer cells via mitochondrial dysfunction. Oncol Rep. 2013;30:763–772. doi: 10.3892/or.2013.2504. [DOI] [PubMed] [Google Scholar]
- Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90(4):743–749. doi: 10.1016/j.foodchem.2004.04.035. [DOI] [Google Scholar]
- Trease GE, Evans WC. Pharmacognosy. 13th. London: Balliere Tindall; 1989. pp. 176–180. [Google Scholar]
- Tsuda T, Watanabe M, Ohshima K, Yamamoto A, Kawakishi S, Osawa T. Antioxidative components isolated from the seed of tamarind (tamarindus indica L.) J Agric Food Chem. 1994;42(12):2671–2674. doi: 10.1021/jf00048a004. [DOI] [Google Scholar]
- Visioli F, Poli A, Paoletti R. Nutritional intervention helps pharmacology in the management of the metabolic syndrome. Mediterr J Nutr Metab. 2010;3:203–207. doi: 10.1007/s12349-010-0024-9. [DOI] [Google Scholar]
- Zhou K, Yu L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci Technol. 2006;39(10):1155–1162. doi: 10.1016/j.lwt.2005.07.015. [DOI] [Google Scholar]
- Zin ZM, Abdul-Hamid A, Osman A. Antioxidative activity of extracts from mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chem. 2002;78(2):227–231. doi: 10.1016/S0308-8146(01)00402-2. [DOI] [Google Scholar]